Facile fabrication of heterostructured cubic-CuFe2O4/ZnO nanofibers (c-CFZs) with enhanced visible-light photocatalytic activity and magnetic separation†
Chuchu Lua,
Zhimin Baoa,
Chuanxiang Qin*a,
Lixing Daia and
Aiping Zhub
aCollege of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou, Jiangsu 215123, People's Republic of China. E-mail: qinchuanxiang@suda.edu.cn; Fax: +86-0512-65883354; Tel: +86-0512-65883354
bCollege of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou, Jiangsu 225002, People's Republic of China
First published on 14th November 2016
Abstract
A novel p–n heterojunction photocatalyst of cubic-CuFe2O4/ZnO nanofibers (c-CFZs) was fabricated through a simple and economical technique of electrospinning technique combined with coprecipitation method. The products were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and vibrating sample magnetometer (VSM). The prepared cubic-CuFe2O4 (c-CuFe2O4) nanofibers revealed lengths of several micrometers and were composed of many c-CuFe2O4 nanoparticles about 15–20 nm in size. After coprecipitation, the surface of c-CuFe2O4 nanofibers was densely covered with ZnO particles. The photocatalytic activity was evaluated by monitoring the degradation of the dye model Rhodamine B (RhB) under visible-light irradiation. Compared with pure ZnO and c-CuFe2O4, the c-CFZs exhibited a better photocatalytic performance. The enhanced activity could be attributed to the extended absorption in the visible light region resulting from c-CuFe2O4 and the effective separation of photo-generated carriers driven by the photo-induced potential difference generated at the p–n heterojunction photocatalyst of c-CFZs. Furthermore, these nanofibers could be recollected easily with a magnet after the photocatalytic process, avoiding secondary pollution of treated water effectively.
1. Introduction
Population growth and economic development have contributed to increased global environmental pollution and energy supply demand. Factories dump about 300–400 million tons of heavy metals, solvents and other wastes into the global waters each year, causing severe environmental problems.1 Hence, it is urgent and essential to remove organic pollutants by researching neoteric, efficient and environmentally friendly materials. Prospectively, a novel and green photocatalysis technology has been known as a promising way, which can harness solar energy as a source of energy to treat the degradation of sewage, the production of hydrogen and other aspects. This technology has become a fast developing research area over the past few decades, as it possesses such advantages as low cost, non-toxic products, complete mineralization and so on. Some notable photocatalytic semiconductors, for example, TiO2,2 ZnO,3 CdS,4 SnO2 (ref. 5) and SrTiO3 (ref. 6) have better photocatalytic performance. Among the traditional materials, the anatase TiO2 and wurtzite ZnO are the proverbially used metal oxides as photocatalysts due to their electronic band structure.2 However, ZnO has higher quantum efficiency, which is believed to have higher efficiency in the photocatalytic performance than TiO2 in many cases.3–5ZnO semiconductor has a large band gap (∼3.2 eV) and allows it absorbing UV light for the required band gap excitation and charge carrier generation, limiting its light harvesting efficiency, since UV light only contributes ∼4% of solar energy. Therefore, considerable effort has been applied to extend the photo-response of ZnO into visible-light region (∼43% of solar energy).6 One promising way is coupling two different semiconductors to form p–n heterojunction. ZnO is a typical n-type semiconductor, then it can be combined with p-type semiconductor to form a p–n heterojunction photocatalyst. It can not only broaden the wavelength range of semiconductors, but also control the recombination of photo-generated carriers by electric field. Practically, several semiconductor heterojunctions possessing good photocatalytic activities have be published, such as Bi2S3/ZnO,7 CdWO4/ZnO,8 CulnSe2/ZnO,9 Dy2O3/ZnO,10 and SnO2/ZnO.11
Furthermore, the photocatalytic reactions are usually carried out either in a slurry-type or in an immobilized-type reactor. Then the recyclable catalysts are of great significance in sustainable process management. Copper ferrite (containing tetragonal and cubic CuFe2O4), which is one kind of high thermal stable and magnetic material with effective catalytic activity, has been used extensively in photocatalysis and solar water-splitting.12–14 When acting as p-type semiconductor, CuFe2O4 can couple with n-type semiconductors to form p–n heterojunction composite catalysts (CuFe2O4/CeO2,15 CuFe2O4/CdS16 and CuFe2O4/TiO2 (ref. 17)). Uddin et al. reported that the loading of TiO2 on CuFe2O4 enhanced the photocatalytic activity in the visible light range.17 Since the magnetic materials possess relatively large saturation magnetization, high coercive force and rather high uniaxial magneto-crystalline anisotropy, chemical stability as well as corrosion resistivity, some of them are used as the core.18 Karunakaran et al. reported that the magnetically separable bactericidal CuFe2O4-embedded Ag-deposited ZnO nanosheets showed better photocatalytic activity than pure CuFe2O4 and ZnO.19 Nevertheless, there are hardly any results about photocatalytic activity of cubic-CuFe2O4 nanofibers-deposited ZnO particles. Hence, CuFe2O4 was selected as a basal material here and combined with ZnO (n-type semiconductor) to achieve the purpose of magnetic separation after photocatalytic reactions.
Herein, we firstly reported the facile preparation of p–n heterojunction cubic-CuFe2O4/ZnO nanofibers (c-CFZs). The nanofibers were prepared by electrospinning technique combined with coprecipitation method. Rhodamine B (RhB) was employed to test the photo-degradation ability of c-CFZs, and the results suggested that c-CFZs showed higher photocatalytic activity than pure ZnO and cubic-CuFe2O4 (c-CuFe2O4). Furthermore, a possible enhanced photocatalytic activity mechanism was proposed.
2. Experimental section
2.1 Materials
Iron nitrate nonahydrate (Fe(NO3)3·9H2O), copper nitrate trihydrate (Cu(NO3)2·3H2O), citric acid monohydrate (C6H8O7·H2O), polyvinylpyrrolidone (PVP, Mw = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), sodium hydroxide (NaOH), zinc nitrate hexahydrate (Zn(NO3)2·6H2O), and ethanol were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All materials were used as received without further purification. Deionized water was obtained from local sources.
000), sodium hydroxide (NaOH), zinc nitrate hexahydrate (Zn(NO3)2·6H2O), and ethanol were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All materials were used as received without further purification. Deionized water was obtained from local sources.
2.2 Catalyst preparation
The cubic-CuFe2O4/ZnO nanofibers (c-CFZs) had been fabricated by electrospinning technique and coprecipitation method, as illustrated in Fig. 1.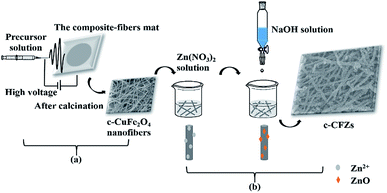 | ||
| Fig. 1 Schematic representation for the fabrication of c-CuFe2O4/ZnO nanofibers (c-CFZs) by electrospinning technique (a) and coprecipitation method (b). | ||
(a) Firstly, 0.080 g Fe(NO3)3·9H2O, 0.096 g citric acid monohydrate and 0.024 g Cu(NO3)2·3H2O were dissolved in the solution containing 4 mL DMF and 6 mL ethanol. Secondly, 1.5 g PVP was added into the above solution and stirred about 6 h to form a transparent light-yellow solution. Thirdly, the viscous solution was loaded into a plastic syringe equipped with a stainless steel needle for electrospinning. The applied voltage, receiving distance and solution flow rate were 10.20 kV, 18 cm and 0.5 mL h−1, respectively. The collecting composite-fibers mat was dried overnight in a blast oven at 30 °C. Finally, the composite-fibers mat was further annealed for 4 h at 600 °C (Fig. S1 and S2†) to produce ultimate cubic-CuFe2O4 (c-CuFe2O4) nanofibers.
(b) First, the obtained c-CuFe2O4 nanofibers (2.5 mmol) were put into 25 mL Zn(NO3)2·6H2O solution (2.5 mmol). Then, 25 mL NaOH solution (0.2 mol L−1) was added dropwise with continuous stirring at 60 °C for 2 h. After aging at room temperature for 4 h, the c-CFZs were collected by magnet, washed several times with ethanol and deionized water, respectively. Finally, the obtained c-CFZs were dried at 60 °C for 12 h. Furthermore, the ZnO was obtained in the absence of c-CuFe2O4 following the similar procedure as control sample.
2.3 Characterization
The phase of prepared nanofibers was analyzed by X-ray diffractometer (XRD) using Cu Kα radiation. The morphologies of these nanofibers were studied by scanning electron microscopy (SEM) coupled with an energy-dispersive X-ray (EDX) and transmission electron microscopy (TEM). The sample structure was further investigated by Almega Dispersive Raman with an He–Ne laser (532 nm) at room temperature. The magnetization properties (coercivity Hc, specific saturation magnetization Ms, and specific remanent magnetization Mr) were performed at room temperature by vibrating sample magnetometer (VSM). The optical properties were obtained by UV-vis ultraviolet/visible diffuse reflectance spectrophotometer (DRS). The recombination of the photogenerated electron–hole pairs in semiconductor was revealed by fluorescence emission spectra over a wavelength range of 220–780 nm.2.4 Photocatalytic activity measurement
The photocatalytic activity of c-CFZs were evaluated by the degradation of rhodamine B (RhB) as a model pollutant dye solution under visible-light irradiation of a 500 W Xe lamp with a 420 nm cut-off filter at ambient temperature. The photocatalyst (30 mg) was dispersed in the reactor containing 100 mL of RhB solution (10 mg L−1). Before irradiation, the suspension was magnetically stirred in the dark for 30 min to ensure an adsorption–desorption equilibrium. At a certain time interval, 4 mL solution was pumped and magnetic recovered & centrifuged to remove solid catalysts. The absorbance of solution at 554 nm was measured to determine the concentration of RhB solution using an UV-vis spectrophotometer (200–800 nm). In order to analysis the degradation of RhB, C/C0 vs. time was applied to reflect the degradation of RhB, where C was the absorbance of RhB solution after degradation, C0 was the absorbance of RhB solution before degradation.3. Results and discussion
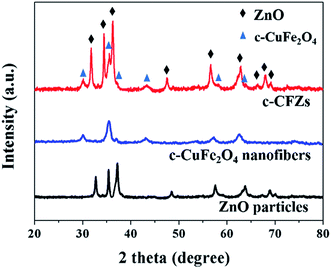
Fig. 2 showed the XRD patterns of ZnO particles, c-CuFe2O4 nanofibers, and c-CFZs. The characteristic peaks at 2θ = 31.7°, 34.4°, 36.2°, 47.5°, 56.6°, 62.8°, 66.3°, 67.9°, and 69.1° were attributable to the ZnO (JCPDS: 36-1451). In the same way, the characteristic peaks at 2θ = 30.1°, 35.6°, 37.1°, 43.0°, 57.1°, and 62.7° were attributable to the cubic phase of CuFe2O4 (JCPDS: 25–0283). The peaks of c-CFZs were marked with ZnO and c-CuFe2O4, resulting from the possible structure that ZnO particles were densely coated on the surface of the c-CuFe2O4 nanofibers, which was verified by the following SEM analysis.Fig. 3 showed the typical surface topography of c-CuFe2O4 nanofibers and c-CFZs. As shown in Fig. 3(a and b), the prepared c-CuFe2O4 nanofibers revealed several micrometers in length and were composed of many nanoparticles about 15–20 nm in size. It followed the formation principle of the inorfil, while the inorganic ions crystallised and grew along the axis direction of the fibers in the process of calcination. Fig. 3(c and d) showed that ZnO particles were densely coated onto the surface of c-CuFe2O4 nanofibers, thereby forming c-CFZs. It was clear that during the formation of c-CFZs, the dispersed c-CuFe2O4 nanofibers in suspension served as heterogeneous seeded around which ZnO particles grew, abiding by a well-known heterogeneous seeded growth process.20–22 The SEM results suggested that c-CFZs had been successfully achieved via electrospinning technique and coprecipitation method.
Then the chemical composition and element distribution of c-CFZs were carried out by EDX elemental mapping in scanning mode. The EDX spectrum of c-CFZs showed obvious signals of Fe, Cu, Zn, and O elements (excepting C, Si, and Au elements which came from the test conditions), demonstrating that the c-CFZs were composed of Fe, Cu, Zn, and O elements. Moreover, the componential ratio of Cu/Fe/O and Zn/O was in agreement with the stoichiometric value required in the chemical formulae of CuFe2O4 and ZnO. For further confirmation, EDX mapping of c-CFZs was conducted. As shown in Fig. 4(b–e), the four constituent elements Fe, Cu, Zn, and O elements could be detectable entirely, and they were homogeneously distributed in the composite. These results implied that the existence of c-CuFe2O4 and ZnO in the c-CFZs.
TEM and HR-TEM images provided further insight into the microstructure of the products. The microstructure of c-CuFe2O4 nanofibers (Fig. 5(a)) and c-CFZs (Fig. 5(b)) were consistent with SEM observation. It demonstrated that the fibers were composed of two kinds of substances, containing c-CuFe2O4 nanofibers and ZnO particles. Fig. 5(c and d) displayed the magnified HR-TEM images of c-CFZs. It was evident that there were two sets of distinct lattice fringes in the pattern. The inter-planar spacing of 0.24 and 0.48 nm corresponded well to the (101) facet of ZnO and (111) facet of c-CuFe2O4, respectively. SAD pattern (Fig. 5(f)) displayed discontinuous diffraction rings (Fig. S1 and S5†). Compared with Fig. 5(e), the diffraction points came from the ZnO. It was confirmed that the c-CFZs were polycrystalline, containing both c-CuFe2O4 and ZnO. These results strongly demonstrated that the ZnO particles were assembled on the surface of c-CuFe2O4 nanofibers.
 | ||
| Fig. 5 TEM images, magnified HR-TEM and SAD pattern of c-CuFe2O4 nanofibers (a, d, and e), ZnO particles coved above c-CuFe2O4 nanofibers (c) and c-CFZs (b and f). | ||
The sample structure was further investigated by Raman spectra. In Fig. 6, the band at 676 cm−1 corresponded to the c-CuFe2O4.23 The band at 1320 cm−1 could be identified as α-Fe2O3 presented in the sample, which was corresponding to the Raman spectra of α-Fe2O3 in the Cu/Fe samples but with a low intensity.24 Therefore, the intense feature at 1320 cm−1 was due to a two-magnon scattering arose from the interaction of two magnons created on antiparallel close spin site.25 The band at 437 cm−1 (E2 (high) mode) corresponded to the wurtzite ZnO,26,27 and non-polar E2 mode was only Raman active. However, it did not observe any significant peak at 586 cm−1 (E1L mode), suggesting that ZnO had high quality crystal without oxygen vacancies over the surface of spheres.28 As for the c-CFZs, both the characteristic Raman peaks of c-CuFe2O4 and ZnO located at similar regions. However, after the formation of heterojunction between c-CuFe2O4 and ZnO, the relative peaks distinctly decreased, which could be attributed to the ZnO coated on the surface of c-CuFe2O4 nanofibers. Hence, these results further confirmed the existence of ZnO and c-CuFe2O4 in the c-CFZs.
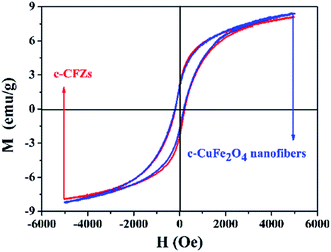
Fig. 7 showed the magnetic hysteresis loop of c-CuFe2O4 nanofibers and c-CFZs at room temperature. It could be obtained that Ms of c-CuFe2O4 nanofibers slightly was greater than that of the c-CFZs, and Hc was almost unanimous. As a rule, Ms of the sample primarily depended on the content of magnetic component Ms = Φ × ms (Φ and ms were the volume fraction of magnetic materials and the saturation magnetization of a single magnetic material, respectively).29 Ms of the c-CFZs was relatively low because the content of magnetic component in the c-CFZs was less than that of the c-CuFe2O4. The Hc value of the c-CFZs was close to that of c-CuFe2O4. While Hc was influenced by some factors, such as the crystallite size, lattice, crystal surface and internal defects, and the material anisotropy constant.30,31 The magnetic property of the c-CFZs was not effected obviously when the c-CuFe2O4 nanofibers were covered of ZnO particles. And the c-CFZs samples could be separated easily in suspend solution.
The optical properties of ZnO particles, c-CuFe2O4 nanofibers and c-CFZs were detected by UV-DRS. According to the spectra shown in Fig. 8(a), c-CuFe2O4 nanofibers displayed the obvious photo-absorption ability in both UV light and visible light regions, while ZnO particles exhibited a strong absorption mostly in UV light region but weakly in visible light. As the c-CFZs presented better visible light absorption abilities than pure ZnO, indicating that the c-CFZs exhibited a wide photo-absorption region, making them potential prospect in visible light photocatalysis application. Besides, for a crystalline semiconductor, the band gap energy could be determined by the equation αhν = A(hν − Eg)n/2,32 where α, h, ν, Eg, and A were the absorption coefficient, the Planck constant, the light frequency, the band gap, and a constant, respectively. There, the electronic transitions could be direct allowed when n was 1, while indirect allowed transitions when n was 4.32,33 As an indirect transition semiconductor, the n value of c-CuFe2O4 here should be 4.14,34 While the n value of ZnO was 1 owing to its direct transition feature.35 Based on the experimental data, the band gap of c-CuFe2O4 and ZnO were estimated to be 1.56 eV and 3.11 eV from the plots (Fig. 8(b and c)), respectively, which were quite similar to the reported data.14,36
Fluorescence emission spectra studies provided insight into the recombination of the photogenerated electron–hole pairs in semiconductor.37 The fluorescence was monitored using an excitation wavelength of 500 nm and the results spectra were shown in Fig. 9. The emission spectrum of CuFe2O4 showed a relatively high intensity, indicating that electrons and holes of CuFe2O4 was easily recombined. The relative of the c-CFZs was lower than that of CuFe2O4, suggesting that the addition of ZnO suppressed the recombination of electrons and holes and improved photocatalytic activity.
The photocatalytic activities of the as-prepared samples were evaluated by degradation of RhB solution as the model pollutant under visible-light irradiation (λ > 420 nm). Fig. 10(a) showed the variation in RhB solution concentration over the photocatalytic degradation reaction under visible light irradiation. Comparison to the photo-degradation of the other samples, the expose of RhB solution to visible light did not stimulate obvious self-photo-degradation. After irradiation for 6 h, only 6.71% of RhB was degraded by the pure ZnO because ZnO had almost no photo-absorption ability under the visible light. Meanwhile, 30.52% of RhB was degraded by the pure c-CuFe2O4 for its narrow band gap. The p–n heterojunction of c-CFZs exhibited a significant influence on the photocatalytic activity (Fig. S3 and S4†). In the presence of c-CFZs, the photocatalytic activity of the hybrid product was enhanced to 86.92%, possibly arising from the visible light response of c-CuFe2O4 and p–n heterojunction formed between c-CuFe2O4 and ZnO. Such heterostructured photocatalyst was beneficial for the reduction of photo-electron–hole pair recombination in CuFe2O4 with the introduction of ZnO. Moreover, the sample could be separated from the solution by applying an external magnetic field after photocatalysis reaction, which avoiding the secondary pollution (insets in Fig. 10(a)). In addition, the kinetic constant k of a photocatalysis was expressed by the pseudo-first-order reaction rate equation of ln(C0/C) = kt,38 where t was the irradiation time and k is the rate constant. Compared the pure photocatalysts, the better linear fit of ln(C0/C) vs. time shown in Fig. 10(b) indicated the photo-degradation was dominated controlled by the pseudo-first-order process. The calculated curves of k for the reaction in the presence of ZnO, c-CuFe2O4 and c-CFZs were 0.012, 0.055, and 0.129 h−1, respectively. This suggested that the photo-degradation of p–n c-CFZs heterojunction was stable and c-CFZs had an optical photocatalytic performance.
 | ||
| Fig. 10 Degradation profiles of RhB solution different products under visible light irradiation (a). Kinetic linear simulation curves of RhB solution photocatalytic degradation with the products (b). | ||
Based on the above results, it was evident that the enhanced photocatalytic activity of the hybrid photocatalyst involving ZnO particles and c-CuFe2O4 nanofibers, could because of the synergistic effects of visible-light sensitization and p–n junction structure. The c-CuFe2O4 was a typical p-type semiconductor with a narrow band gap,39 while ZnO was an typical n-type semiconductor with a large band gap.40 The conduction band (CB) and valence band (VB) potentials of the two semiconductor at the point of zero charge could be calculated by eqn (1) and (2) listed as follows:41
| EVB = X − Ee + 0.5Eg | (1) |
| ECB = EVB − Eg | (2) |
In a conventional photocatalytic system, the excited electrons and holes migrated randomly to the photocatalyst surface to react with the absorbed reactants, meanwhile they underwent the recombination in the bulk or on the surface of the photocatalyst.43,44 The recombination rate of the photo-induced electrons and holes may strongly influence the activity of a photocatalyst. However, serious recombination of the free electrons and holes would lead to the poor quantum efficiency in the photocatalytic process. Whereas in a photocatalytic system involving a p–n heterojunction, the driving force which came from an interior electric field could separate electron–hole pairs efficiently, so the electrons and holes would be driven in different directions and the recombination could be reduced.45–48 Pure c-CuFe2O4 and ZnO had the nested band structure before contact, which could not facilitate separation of photo-generated electron–hole pairs. As a p-type semiconductor, the Fermi level (EF) of c-CuFe2O4 lay close to the VB, whereas ZnO was an n-type semiconductor with the EF close to its CB. When c-CuFe2O4 was in contact with ZnO to form the p–n heterojunction (Fig. 11), the Fermi energy level of c-CuFe2O4 rose up, whereas the Fermi energy level of ZnO descended until the EF of the c-CuFe2O4 and ZnO reached an equilibrium. At equilibrium, the inner electric field induced the region of c-CuFe2O4 and ZnO to be negative charged and positively charged, respectively. And that the conduction band of ZnO was more positive than that of c-CuFe2O4. The holes flowed into the negative field and the electrons moved to the positive field, prompting the migration of the photo-generated carriers. The semiconductor of c-CuFe2O4 could be easily excited under visible-light irradiation, and thus the photoelectrons and holes were generated. The excited electrons in CB of the p-type c-CuFe2O4 transferred to that of the n-type ZnO, and simultaneously the holes remained in the VB of the p-type c-CuFe2O4. Therefore, the p–n heterojunction formed in the heterostructured p-c-CuFe2O4/n-ZnO would effectively separate the photo-generated electron–hole pairs, further enhancing the photocatalytic performance. Then these effectively separated photo-generated electrons and holes would transfer to the photocatalyst surface quickly and generate more ·O2 and ·OH.49,50
In addition, c-CuFe2O4 could act as a sensitizer to absorb the visible light. Under the effect of the higher photon energy, the electrons in the VB of c-CuFe2O4 could be excited up to a higher potential edge (−1.21 eV) under visible light.51 The reformed CB edge potential of c-CuFe2O4 became more active, and the electrons could react with oxygen to produce ·O2. As a result, the heterostructured p-c-CuFe2O4/n-ZnO would promote separation of photo-generated electron–hole pairs and reduce its recombination, improving the photocatalytic activity of c-CFZs.
Considering the photostability of the nanocomposite, the test of RhB decolorization using the used catalyst was shown in Fig. 12(a). It was distinct that although the catalysis rate of repeated cycles was slightly lower than that of the first cycles, the decomposition of RhB solution was still remain 82.67% over the reused c-CFZs in the third test. The photocatalytic rate was decrease, which came from the change of ZnO in the photocatalytic experiment. The consequence was consistent with the result of the XRD results in Fig. 12(b). When the used c-CFZs were detected by the XRD again, there was no detectable difference of c-CuFe2O4, while the main crystal peak of ZnO had changed after photocatalytic experiment. The slightly reduction of the catalytic efficiency of the c-CFZs was mainly ascribed to the little photocatalytic loss of ZnO.
 | ||
| Fig. 12 Cycling runs (a), XRD patterns acquired before and after the photocatalytic process (b) of c-CFZs. | ||
4. Conclusions
The novel p–n heterojunction photocatalyst of c-CFZs had been successfully fabricated for the first time through electrospinning technique and following coprecipitation method. SEM and TEM results confirmed that the ZnO particles were densely coated on the surface of c-CuFe2O4 nanofibers. The visible-light-driven photo-degradation activities of c-CFZs were demonstrated to be superior to the pure c-CuFe2O4 and ZnO. The enhanced photo-activity could be attributed to the p–n heterojunction formed between c-CuFe2O4 and ZnO, which greatly promoted efficient separation of the photo-generated carriers and charge transfer as well as controlling recombination of the charge carriers. In addition, the sample could be separated from the solution by applying an external magnetic field after photocatalysis reaction, which avoiding the secondary pollution.Acknowledgements
The authors thank financial support from Jiangsu Provincial Natural Science Foundation of China (No. BK20161214), Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and Jiangsu Key Laboratory of Environmental Material and Environmental Engineering.References
- J. Xiao, Y. Xie and H. Cao, Chemosphere, 2015, 121, 1–17 CrossRef CAS PubMed.
- M. Lazar, S. Varghese and S. Nair, Catalysts, 2012, 2, 572–601 CrossRef CAS.
- S. K. Kansal, M. Singh and D. Sud, J. Hazard. Mater., 2008, 153, 412–417 CrossRef CAS PubMed.
- Y. Li, W. Xie, X. Hu, G. Shen, X. Zhou, Y. Xiang, X. Zhao and P. Fang, Langmuir, 2010, 26, 591–597 CrossRef CAS PubMed.
- C. Tian, Q. Zhang, A. Wu, M. Jiang, Z. Liang, B. Jiang and H. Fu, Chem. Commun., 2012, 48, 2858–2860 RSC.
- Z. Wang, Y. Liu, B. Huang, Y. Dai, Z. Lou, G. Wang, X. Zhang and X. Qin, Phys. Chem. Chem. Phys., 2014, 16, 2758–2774 RSC.
- S. Balachandran and M. Swaminathan, Dalton Trans., 2013, 5338–5347 RSC.
- E. T. D. Kumar, K. Thirumalai, R. Aravindhan, M. Swaminathan, J. R. Rao and B. U. Nair, RSC Adv., 2015, 5, 60926–60937 RSC.
- M. Bagheri, A. R. Mahjoub and B. Mehri, RSC Adv., 2014, 4, 21757–21764 RSC.
- G. A. S. Josephine and A. Sivasamy, Environ. Sci. Technol. Lett., 2014, 1, 172–178 CrossRef CAS.
- M. T. Uddin, Y. Nicolas, C. Olivier, T. Toupance, L. Servant, M. M. Müller, H.-J. Kleebe, J. Ziegler and W. Jaegermann, Inorg. Chem., 2012, 51, 7764–7773 CrossRef CAS PubMed.
- H. Yang, J. Yan, Z. Lu, X. Cheng and Y. Tang, J. Alloys Compd., 2009, 476, 715–719 CrossRef CAS.
- Y. Ding, L. Zhu, N. Wang and H. Tang, Appl. Catal., B, 2013, 129, 153–162 CrossRef CAS.
- P. Jing, J. Li, L. Pan, J. Wang, X. Sun and Q. Liu, J. Hazard. Mater., 2015, 284, 163–170 CrossRef CAS PubMed.
- L. Zou, Q. Wang, X. Shen, Z. Wang, M. Jing and Z. Luo, Appl. Surf. Sci., 2015, 332, 674–681 CrossRef CAS.
- N. Nasrallah, M. Kebir, Z. Koudri and M. Trari, J. Hazard. Mater., 2011, 185, 1398–1404 CrossRef CAS PubMed.
- M. R. Uddin, M. R. Khan, M. W. Rahman, A. Yousuf and C. K. Cheng, React. Kinet., Mech. Catal., 2015, 116, 589–604 CrossRef CAS.
- C.-J. Li, J.-N. Wang, X.-Y. Li and L.-L. Zhang, J. Mater. Sci., 2010, 46, 2058–2063 CrossRef.
- C. Karunakaran, P. Vinayagamoorthy and J. Jayabharathi, RSC Adv., 2016, 6, 1782–1791 RSC.
- L. Carbone, C. Nobile, G. M. De, F. D. Sala, G. Morello, P. Pompa, M. Hytch, E. Snoeck, A. Fiore and I. R. Franchini, Nano Lett., 2007, 7, 2942–2950 CrossRef CAS PubMed.
- W. Chiu, P. Khiew, M. Cloke, D. Isa, H. Lim, T. Tan, N. Huang, S. Radiman, R. Abdshukor and M. A. A. Hamid, J. Phys. Chem. C, 2010, 114, 8212–8218 CAS.
- A. Fiore, R. Mastria, M. G. Lupo, G. Lanzani, C. Giannini, E. Carlino, G. Morello, G. M. De, Y. Li and R. Cingolani, J. Am. Chem. Soc., 2009, 131, 2274–2282 CrossRef CAS PubMed.
- M. Balaji, P. C. Lekha, D. P. Padiyan, M. Balaji, P. C. Lekha and D. P. Padiyan, Vib. Spectrosc., 2012, 62, 92–97 CrossRef CAS.
- X. Lin, Y. Zhang, L. Yin, C. Chen, Y. Zhan and D. Li, Int. J. Hydrogen Energy, 2014, 39, 6424–6432 CrossRef CAS.
- D. L. A. D. Faria, S. V. Silva and M. T. D. Oliveira, J. Raman Spectrosc., 1997, 28, 873–878 CrossRef.
- C. Dong, X. Xiao, G. Chen, H. Guan and Y. Wang, Mater. Chem. Phys., 2015, 155, 1–8 CrossRef CAS.
- L. Li, H. Yang, G. Qi, J. Ma, X. Xie, H. Zhao and F. Gao, Chem. Phys. Lett., 2008, 455, 93–97 CrossRef CAS.
- N. Tripathy, R. Ahmad, H. Kuk, D. H. Lee, Y. B. Hahn and G. Khang, J. Photochem. Photobiol., B, 2016, 161, 312–317 CrossRef CAS PubMed.
- F. Sauzedde, A. Elaïssari and C. Pichot, Colloid Polym. Sci., 1999, 277, 846–855 CAS.
- L. Li, K. Chen, H. Liu, G. Tong, H. Qian and B. Hao, J. Alloys Compd., 2013, 557, 11–17 CrossRef CAS.
- C. R. Vestal and Z. J. Zhang, Nano Lett., 2003, 3, 1739–1743 CrossRef CAS.
- P. Ju, P. Wang, B. Li, H. Fan, S. Ai, D. Zhang and Y. Wang, Chem. Eng. J., 2014, 236, 430–437 CrossRef CAS.
- J. Cao, B. Xu, B. Luo, H. Lin and S. Chen, Catal. Commun., 2011, 13, 63–68 CrossRef CAS.
- N. Helaili, G. Mitran, I. Popescu, K. Bachari, I.-C. Marcu and A. Boudjemaa, J. Electroanal. Chem., 2015, 742, 47–53 CrossRef CAS.
- M. H. Habibi and M. H. Rahmati, Spectrochim. Acta, Part A, 2014, 133, 13–18 CrossRef CAS PubMed.
- W. Yu, T. Liu, S. Cao, C. Wang and C. Chen, J. Solid State Chem., 2016, 239, 131–138 CrossRef CAS.
- T. Cai, M. Yue, X. Wang, Q. Deng, Z. Peng and W. Zhou, Chin. J. Catal., 2007, 28, 10–16 CrossRef CAS.
- V. tengl and S. Bakardjieva, J. Phys. Chem. C, 2010, 114, 19308–19317 Search PubMed.
- W. Zhao, Y. Jin, C. H. Gao, W. Gu, Z. M. Jin, Y. L. Lei and L. S. Liao, Mater. Chem. Phys., 2014, 143, 952–962 CrossRef CAS.
- J. Pan, Y. Sheng, J. Zhang, P. Huang, X. Zhang and B. Feng, ACS Appl. Mater. Interfaces, 2015, 7, 7878–7883 CAS.
- Q. Yuan, C. Lang, X. Miao, H. Jie, S. L. Luo, C. T. Au, S. F. Yin, Q. Yuan, X. Miao and H. Jie, Chem. Eng. J., 2014, 255, 394–402 CrossRef CAS.
- S. Huang, Y. Xu, M. Xie, H. Xu, M. He, J. Xia, L. Huang and H. Li, Colloids Surf., A, 2015, 478, 71–80 CrossRef CAS.
- C. Chen, W. Ma and J. Zhao, Chem. Soc. Rev., 2010, 39, 4206–4219 RSC.
- A. Fujishima, Surf. Sci. Rep., 2008, 63, 515–582 CrossRef CAS.
- Y. Chen, J. C. Crittenden, S. Hackney, A. L. Sutter and D. W. Hand, Environ. Sci. Technol., 2005, 39, 1201–1208 CrossRef CAS PubMed.
- F. Ye, A. Ohmori and C. Li, J. Mater. Sci., 2003, 39, 353–355 CrossRef.
- J. Zhang, J. Sun, K. Maeda, K. Domen, P. Liu, M. Antonietti, X. Fu and X. Wang, Environ. Sci. Technol., 2011, 4, 675–678 CAS.
- C. Jing, L. Xin, H. Lin, S. Chen and X. Fu, J. Hazard. Mater., 2012, 239–240, 316–324 Search PubMed.
- Y. Xiang, P. Ju, Y. Wang, Y. Sun, D. Zhang and J. Yu, Chem. Eng. J., 2016, 288, 264–275 CrossRef CAS.
- Z. Zhang, C. Shao, X. Li, C. Wang, M. Zhang and Y. Liu, ACS Appl. Mater. Interfaces, 2010, 2, 2915–2923 CAS.
- A. Kezzim, N. Nasrallah, A. Abdi and M. Trari, Energy Convers. Manage., 2011, 52, 2800–2806 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra23970f |
| This journal is © The Royal Society of Chemistry 2016 |