Open Access Article
Open Access ArticleCeO2 nanowires self-inserted into porous Co3O4 frameworks as high-performance “noble metal free” hetero-catalysts†
Xiao
Wang
,
Shuna
Zhao
,
Yibo
Zhang
,
Zhuo
Wang
,
Jing
Feng
,
Shuyan
Song
* and
Hongjie
Zhang
*
State Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, P. R. China. E-mail: songsy@ciac.ac.cn; hongjie@ciac.ac.cn
First published on 28th October 2015
Abstract
Recently, mixed metal oxides have attracted tremendous interest because of their great importance for fundamental studies and practical applications in the catalytic field to replace expensive noble metals. Herein, we report the designed synthesis of novel CeO2–Co3O4 mixed metal oxides with complex nanostructures using uniform short CeO2 nanowires self-inserted into ZIF-67 nanocrystals as precursors followed by a thermal annealing treatment. Interestingly, such a synthetic strategy can be easily extended to fabricate other CeO2 nanowires inserted into metal oxide nanoframeworks such as NiCo2O4 and ZnCo2O4. Choosing the NO reduction reaction by CO as the catalytic model, the as-obtained CeO2–Co3O4 hybrids exhibited enhanced catalytic performance, which could be attributed to the strong two-phase interaction between each component.
Introduction
In hetero-catalytic reactions, ceria (CeO2)-based nanomaterials have been used as efficient catalysts due to their high oxygen storage capability, good redox properties, and low cost as well as being green materials.1–4 Many efforts have been made to develop CeO2-based noble metal hybrid nanocatalysts with remarkably activity and long-term stability for three-way catalytic reactions (TWCs) to eliminate toxic automobile exhaust fumes, low-temperature water–gas shift (WGS) reactions, oxygen sensors, oxygen permeation membrane systems and fuel cells, but they suffer from such drawbacks as high cost, limited availability and relatively low selectivity at high temperatures which limit their widespread application.5–8 The development of CeO2-based “noble metal-free” nanocatalysts is an urgent and important problem to be solved. A widely accepted viewpoint for the successful fabrication of “noble metal-free” catalysts is to hybridize together multiple components with a strong synergistic effect.9–12 Otherwise, it is found that the as-formed two-phase interface area can strongly affect the catalytic process, such as the adsorption–desorption rate of the substrate molecules and the stability of the intermediate state.13–18 In recent years, more attention has been focused on the development of a large number of CeO2-based mixed metal oxides including CeO2–CuO,9–11 CeO2–Cu2O,12,13 CeO2–Co3O4,14,15 CeO2–ZnO,16 CeO2–NiO18et al.Generally speaking, the hybrid nanostructure and morphology of the building blocks play important roles in determining the catalytic performance, which can cause great differences in the two-phase interface state and the interface area as well as the anti-sintering ability. In particular, one-dimensional and porous nanomaterials have received continuous attentions owing to their combination of carrier directed motion, anisotropism, high surface area, low density, and high loading capacity.19–23 The presence of nanoscale porous interiors with a functional one-dimensional component endows them with technological significance in catalytic applications. However, the synthesis of CeO2-based mixed metal oxides with necessary structure is still problematic because of the difficulties in simultaneously manipulating different materials with distinct physical and chemical properties during the conventional synthesis processes. Therefore, it is still a great challenge to create novel and complex CeO2-based metal oxides.
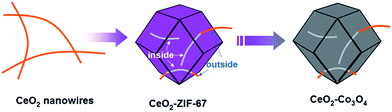
It is noticed that the thermolysis of metal–organic frameworks (MOFs) route under certain atmosphere is an efficient way to produce highly porous transition metal oxide nanostructures.24–28 The sacrificial MOF templates determine directly the shape and size of the resultant porous structures by engaging themselves as consumable reactants for shell construction.29,30 In addition, the growth process of the MOF precursor is easily controlled to obtain MOF nanocrystals with desired morphology, size and composition on a variety of substrates. Inspired by recently achieved progress, herein we demonstrate a novel approach for the effective synthesis of short ultrathin CeO2 nanowires inserted highly porous Co3O4 frameworks with different Ce/Co molar ratios, namely, the CeO2–Co3O4 hybrid nanocomposite. This strategy involves the following two separate steps: the facile synthesis of CeO2 nanowires inserted into ZIF-67 hetero-precursors; and subsequent thermolysis treatment. Besides, the synthetic strategy can be extended to prepare other complex CeO2 nanowires inserted into ZnCo2O4 and NiCo2O4 hybrid nanocomposites. Such unique hybrids take into account the components, hybrid nanostructures and the unique morphologies of the building blocks. More importantly, the as-obtained mixed metal oxides have shown superior performance when evaluated as catalysts in the chemical reduction of NO by CO.
Experimental
Synthesis of ultra-thin CeO2 nanowires
The synthetic process is accordance with the report by Yu's group. Typically, 2 mmol Ce(Ac)3 is dissolved in a water/ethanol mixture (1/1) and heated in an oil bath. Until the temperature reached 140 °C, 20 mL ammonia is added very fast to the reaction solution. 12 hours later, the products are purified by centrifugation and washed three times with water and dried at room temperature overnight.Synthesis of CeO2–ZIF-67 precursors
20 mg of the as-obtained CeO2 nanowires are dispersed in 20 mL absolute methanol. Under vigorous stirring, 4 mmol 2-methylimidazole powder is added. 30 min later, 5 mL of a Co(NO3)2 methanol solution (0.2 M) is injected into the mixture. The reaction only needs one hour. Finally, the products are purified by centrifugation and washed with methanol and dried for further application.Synthesis of CeO2–ZIF-67 (Ni, Co or Zn, Co)
The synthetic strategy is very similar to CeO2–ZIF-67. The only difference is using Ni, Co (or Zn, Co) mixed metal ions to replace the Co ions.Synthesis of CeO2–Co3O4 nanoframeworks
The dried precursors are further annealed at 400 °C for 60 min at a heating rate of 1 °C min−1.Characterization
The X-ray diffraction patterns of the products were collected on a Rigaku-D/max 2500 V X-ray diffractometer using Cu-Kα radiation (λ = 1.5418 Å), with an operation voltage and current maintained at 40 kV and 40 mA. Transmission electron microscopy (TEM) images were obtained with a TECNAI G2 high-resolution transmission electron microscope operating at 200 kV. The catalytic performances of the catalysts were monitored on-line by a mass spectrogram.Catalytic test: NO reduction
50 mg of catalyst was put in a stainless steel reaction tube. The experiment was carried out under a flow of reactant gas mixture (2000 ppm CO, 2000 ppm CO, balance Ar) at a rate of 50 mL min−1.Results and discussion
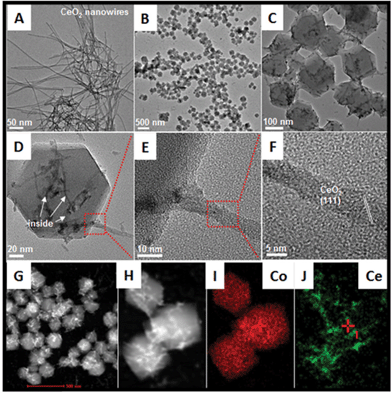
The whole synthetic process is schematically illustrated in Fig. 1. First of all, the ultrathin CeO2 nanowires are obtained via the previously reported method (as shown in the Experimental part; the corresponding TEM images are shown in Fig. 2A). Subsequently, a certain amount of CeO2 nanowires was dispersed in methanol by ultrasound treatment followed by the addition of 2-methylimidazole to the mixture and stirring for 30 min. The preliminary mixing of CeO2 nanowires and 2-methylimidazole together is necessary. This is because the 2-methylimidazole ligand can coordinate with Ce ions on the surface, which is a prerequisite for the successful surface growth of ZIF-67 nanocrystals.The structure of the as-formed inserted hybrid nanostructures was characterized by powder X-ray diffraction (XRD), transition electron microscopy (TEM), energy-dispersive X-ray spectroscopy (EDX), high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) and thermogravimetric analysis (TGA). The XRD pattern of the CeO2–ZIF-67 hybrids is shown in Fig. S1.† The diffraction peak of CeO2 is relatively weak, and it is difficult to distinguish directly the presence of CeO2. The small particle size and poor crystallization are considered to be the main reasons. Furthermore, the diffraction peaks between 10° to 20° match well with the ZIF-67 crystals.23 Further investigation by TEM imaging was also conducted and is shown in Fig. 2B and C, indicating that the as-obtained precursors are uniform and monodisperse sub-300 nm polyhedra. Interestingly, the enlarged TEM image in Fig. 2D confirms that the original long nanowires have disappeared and several short and ultra-thin ones are formed and inserted in situ into the ZIF-67 nanocrystals. Some of the short nanowires are enclosed completely and some of them are partly embedded in the polyhedra leaving parts of the nanowires exposed outside. Such a self-inserted hybrid nanostructure is easily observed in Fig. 2E. Additionally, the HRTEM image in Fig. 2F illustrates that the lattice spacing of 0.31 nm corresponds well with the characteristic (111) planes of fluorite phase CeO2. A further STEM image is shown in Fig. 2G, in which the inserted one-dimensional nanostructures are easy to distinguish owing to their brighter contrast. HAADF-STEM-mapping image has also been used to analyze further the distribution of Ce and Co elements in the precursors. As shown in Fig. 2H–J, the polyhedra are composed by only Co element; and Ce element exhibits random one-dimensional distribution, both inserted and outside the polyhedra. The formation process of such unique hybrids is supposed to occur by the pre-mixing of CeO2 and 2-methylimidazole molecules making parts of the 2-methylimidazole molecules coordinate with Ce ions on the surface of CeO2 nanowires. The addition of Co2+ into the reaction solution causes the surface nucleation of ZIF-67 nanocrystals. However, the rigid CeO2 nanowires are not stable enough, such that during the growth process of ZIF-67 nanocrystals, CeO2 nanowires are cut into several short pieces.
In addition, the rapid growth rate of ZIF-67 nanocrystals causes the as-formed short CeO2 nanowires to embed or insert successfully into the ZIF-67 nanocrystals. From these results, some important advantages of the unique hybrid nanostructures should be noted: (1) using MOFs as the precursors greatly simplifies the difficulty in hybridization of CeO2 and other kinds of transition metal oxides. The surface nucleation and in situ self-inserted growth behavior cause the two different components to be strongly coupled together, and more importantly, such a synthesis is a clean strategy, in that the whole process does not use of any additive agent; (2) the organic part can be removed completely without any residue via a simple heating treatment; (3) this is the first time to combine one-dimensional CeO2 and a three-dimensional transition metal oxide (precursor) together, which is considered very interesting for the efficiency of hybridization for the optimization of the catalytic performances.
Furthermore, TG analysis is also conducted in order to determine a suitable annealing temperature. With the data shown in Fig. S2,† an obvious weight-loss peak has appeared at about 400 °C, which matches well with the previous reports.31 Moreover, simply changing the feeding molar ratio of Ce and Co can cause the component-evolution of the hybrids. With the TEM images shown in Fig. S3,† doubling the molar ratio of Ce/Co leads to the growth of ZIF-67 nanocrystals be much looser (CeO2–ZIF-67-1). On the opposite, halving the molar ratio of Ce/Co causes more intensive ZIF-67 nanocrystals formed with CeO2 nanowires inserted (CeO2–ZIF-67-3). The corresponding inductively coupled plasma atomic emission spectrometry (ICP-AES) of the three samples are also taken and shown in Table S1.† The final mixed metal oxides with different Ce/Co molar ratios are named as CeO2–ZIF-67-1 (CeO2–Co3O4-1), CeO2–ZIF-67-2 (CeO2–Co3O4-2) and CeO2–ZIF-67-3 (CeO2–Co3O4-3).
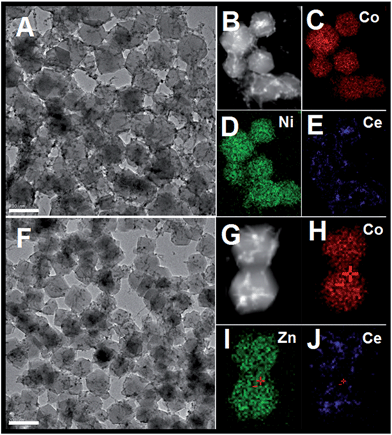
In addition, such a synthesis strategy can be further extended to the fabrication of one-dimensional CeO2 inserted mixed metal–organic frameworks (ZIF-67 [Zn, Co] or ZIF-67 [Ni, Co]). As shown in Fig. 3, the as-obtained precursors have similar morphologies to CeO2–ZIF-67 (Co). The smooth surface of the MOFs and the inserted hybrid nanostructure are easy to distinguish. Further HAADF-STEM-mapping analyses have also confirmed the as-obtained hybrids are composed of Ce, Zn (Ni) and Co elements, that the polyhedra is composed by Ni and Co (or Zn and Co) elements, and the Ce elements are in the form of one-dimensional nanostructures.
Subsequently, the precursors are annealed at 400 °C for 60 min to accomplish the oxidation process. The XRD data is shown in Fig. 4E. Compared with the data of CeO2–ZIF-67 precursors, the weak diffraction peaks of ZIF-67 are totally absent in the XRD pattern and the (220), (311), (400) and (440) peaks are unambiguously ascribed to a typical Co3O4 nanostructure. Importantly, a small diffraction peak at 28.7° is observed clearly, which can be attributed to the (111) planes of CeO2. TEM observations have been used to obtain further insight into the structural information of annealed samples. As shown in Fig. 4A, the products maintain well the structural morphology of CeO2–ZIF-67 even after the high-temperature annealing process, but the particle sizes are decreased significantly to about 200 nm. The increased surface roughness and the tiny holes on the surface of CeO2–Co3O4 can be ascribed to the liberation of CO2 and H2O during the heating treatment. CeO2 components kept their original one-dimensional nanostructures, which could be easily distinguished by their different contrast and growth-orientation as marked by the red arrows in Fig. 4A. More interestingly, parts of the un-embedded CeO2 could be also found outside the Co3O4 porous nanostructures with the data shown in Fig. 4B. The HRTEM images in Fig. 4C and D illustrate that the lattice spacing of 0.31 nm and 0.23 nm corresponds well with the characteristic (111) planes of the fluorite phase of CeO2 and the (220) planes of Co3O4, respectively. The X-ray photoelectron spectroscopy (XPS) curves (Fig. S4†) show that the two peaks at 780 eV and 791 eV correspond well to the Co 2p5/2 and 2p3/2 spin orbit peaks, respectively, while the peaks at 881.9 eV and 900.2 eV can be assigned to Ce 3d5/2 and 3d3/2 spin orbit peaks, respectively. The specific surface area of the as-obtained CeO2–Co3O4-2 sample calculated from the BET curve (Fig. S5†) reaches about 64.76 m2 g−1. In addition, the IR spectra of the 2-methylimidazole molecule and the final CeO2–Co3O4-2 sample are also investigated and shown in Fig. S6.† No observed peaks could be found in the final products, indicating that no functional groups were left after the heating treatment.
 | ||
| Fig. 4 (A and B) TEM images of CeO2–Co3O4 hybrid nanoframeworks; (C and D) the corresponding HRTEM images; (E) XRD spectra of CeO2–ZIF-67 precursors and CeO2–Co3O4 products. | ||
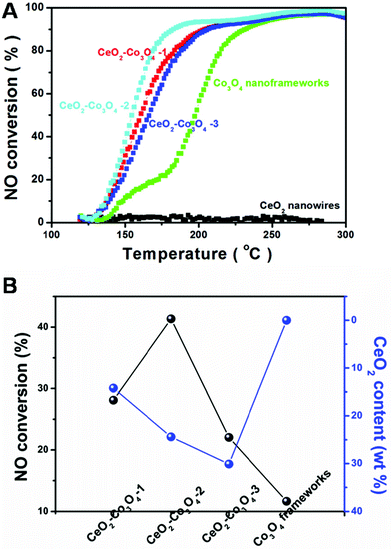
The catalytic reaction: CO + NO → CO2 + N2 has received increased attention, this is because the reaction realizes the goal of reduction of NO and oxidation of CO at the same time. However, compared with the traditional model reaction, CO oxidation, the above catalytic reaction, reduction of NO by CO, is much harder to accomplish. This requires the catalyst to have higher activity, stability and selectivity. The evaluation of catalytic activity was performed in a fixed-bed reactor coupled online with a mass spectrometer. Fig. 5A shows the typical conversion ratio of NO to N2 as a function of reaction temperature over the five catalysts with different molar ratios of Ce/Co with a feed gas containing 2000 ppm CO. 2000 ppm NO (balanced with Ar) was allowed to pass through the reactor at a gas hourly space velocity (GHSV) of 120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 mL (g−1 h−1). As illustrated in Fig. 5, it seems obvious that the pure CeO2 nanowires only exhibit limited catalytic activity. Even when increasing the reaction temperature to about 300 °C, the conversion is still almost 0%. A porous Co3O4 nanoframework exhibits a much higher catalytic performance. At 200 °C, over 50% NO is converted. Interestingly, all of the three kinds of hybrid nanostructure are more active than pure CeO2 or Co3O4. CeO2–Co3O4-2 has the highest catalytic properties. The conversion reaches 38.7%, 93.8% and 96.9 at 150 °C, 200 °C and 250 °C, respectively.
000 mL (g−1 h−1). As illustrated in Fig. 5, it seems obvious that the pure CeO2 nanowires only exhibit limited catalytic activity. Even when increasing the reaction temperature to about 300 °C, the conversion is still almost 0%. A porous Co3O4 nanoframework exhibits a much higher catalytic performance. At 200 °C, over 50% NO is converted. Interestingly, all of the three kinds of hybrid nanostructure are more active than pure CeO2 or Co3O4. CeO2–Co3O4-2 has the highest catalytic properties. The conversion reaches 38.7%, 93.8% and 96.9 at 150 °C, 200 °C and 250 °C, respectively.
For comparison, increasing or decreasing the Ce/Co ratio causes activity loss. At the reaction temperature of 150 °C (or 200 °C), CeO2–Co3O4-1 and CeO2–Co3O4-3 can only convert 30.7% (or 88.8%) and 23.8% (or 87.7%) of NO, respectively. This is a typical component-dependent catalytic performance. It is well-known that the CeO2 dispersion and the interaction between cobalt oxide and ceria play an important roles in determining the catalytic activity. Therefore, a certain proportion of CeO2 in Co3O4 is the most suitable to exhibit the best catalytic activity. A similar phenomenon usually happens in other kinds of CeO2-based mixed metal oxides as catalysts in heterogeneous catalytic reactions.32–34 In order to display intuitively the relationship between the catalytic activity and the component, Fig. 5B illustrates the component-dependent conversion at 150 °C, indicating that the as-obtained hybrid samples exhibit component-dependent catalytic performances. It should be also noted that limited N2O could be detected during the whole test catalyzed by the hybrid component, illustrating the unique catalytic selectivity. Further aging tests have also been taken by monitoring the NO conversion catalyzed by CeO2–Co3O4-2 sample at 180 °C for 9 continuous hours. As shown in Fig. S7,† the as-obtained CeO2–Co3O4-2 samples keep their catalytic performance; 83% conversion could also be achieved 9 hours later. This result suggests the high stability of the CeO2–Co3O4 hybrid nanostructure.
In order to clarify the redox relationship and their synergistic effects for improved catalytic performance, we further tested the H2-TPR profiles of the five nanocatalysts. As shown in Fig. 6, two broad TPR peaks observed at 375 °C and 600 °C for CeO2 can be attributed to the reduction of the surface capping oxygen and the bulk oxygen of ceria, respectively.12,35 For pure Co3O4 nanoframeworks, the two reduction peaks at about 337 °C and 497 °C match well with the previous reports by Venezia's group.36 For comparison, the mixed metal oxides show relatively high reducibility with respect to pure CeO2 and Co3O4 due to the significant interaction between the two phases. Moreover, a noticeable new reduction peak below 250 °C appears in all three hybrids. With the increase of Co3O4 content in the mixed metal oxide, such new reduction peaks become stronger and stronger, indicating that the synergistic effect is continuously enhanced. Comparing CeO2–Co3O4-2 with CeO2–Co3O4-3, although the peak position and intensity of the new peak are similar, the obvious blue-shifts of the following two peaks have been observed very clearly. Combined with the above analyses, H2-TPR profiles confirmed firmly that the CeO2–Co3O4-2 sample has the highest activity, which is in accordance with the catalytic results.
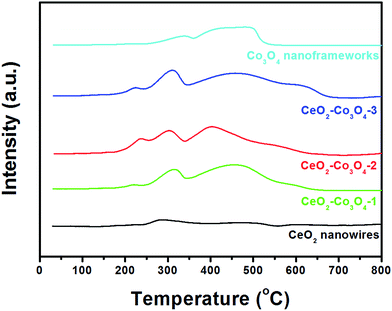 | ||
| Fig. 6 H2-TPR profiles of CeO2 nanowires, CeO2–Co3O4-1, CeO2–Co3O4-2, CeO2–Co3O4-3, Co3O4 nanoframeworks. | ||
By referring to the Eley–Rideal mechanism and the Langmuir–Hinshelwood mechanism,37–40 the possible advantages of such a hybrid nanostructure in the reduction of NO by CO have been supposed as:
(1) Co3O4 can react with CeO2 to form CoO during the catalytic reaction. In other words, the interaction between Co3O4 and CeO2 can greatly increase the stability of CoO and elongate the Co–O bond on the CeO2–Co3O4 interface.37
(2) CeO2 has a high oxygen storage capacity and well-known redox properties (couples of Ce4+/Ce3+), which produce lots of active oxygen available for the oxidation process. The presence of mobile oxygen and weak Co–O bonds in cobalt oxide were reflected in the relatively low ΔH for O2 vaporization.40
(3) Such a self-inserted hybrid nanostructure gives the final products a large specific surface area and a strongly coupled interface.
Conclusions
In conclusion, a novel low-cost and facile synthesis strategy has been successfully developed to create unique hybrid nanostructures composed by one-dimensional CeO2 nanostructures inserted into porous Co3O4 frameworks. The synthesis strategy involves the in situ formation of CeO2 nanowires self-inserted into ZIF-67 precursors and subsequent thermal annealing in air. During the annealing process, porous cages are formed and several short CeO2 nanowires have been inserted into the three-dimensional Co3O4 nanocages. Such a synthesis strategy could be extended to the synthesis of CeO2 inserted into NiCo2O4 and ZnCo2O4 hybrid nanocomposites. When evaluated as hetero-catalysts for the NO reduction by CO, they exhibited component-dependent catalytic properties. The CeO2–Co3O4-2 has shown the highest catalytic performance, much better than even the current reported Ce–Cu mixed metal oxide, which is also synthesized via a MOFs-assistant method.3 This is the first time that the goal of optimization of the catalytic performance of CeO2-based mixed metal oxides has been realized by taking account of the components of the hybrid nanostructure as well as the size and shape of each component. It is believed that the developed synthesis and the obtained unique hybrid nanostructures have opened a new window to design new kinds of “noble metal free” nanocatalysts.Acknowledgements
The authors are grateful for financial aid from the National Natural Science Foundation of China (Grant No. 21221061, 51372242, 91122030, 21401186 and 21210001), the Hong Kong, Macao and Taiwan Science and Technology Cooperation Special Project of Ministry of Science and Technology of China (No. 2014DFT10310), the Program of Science and Technology Development Plan of Jilin Province of China (No. 20140201007GX), the National Key Basic Research Program of China (No. 2014CB643802) and the Jilin Province Youth Foundation (20130522122JH).Notes and references
- Y. Liang, H. Wang, P. Diao, W. Chang, G. Hong, Y. Li, M. Gong, L. Xie, J. Zhou, J. Wang, T. Z. Regier, F. Wei and H. Dai, J. Am. Chem. Soc., 2012, 134, 15849 CrossRef CAS PubMed.
- S. Y. Song, X. Wang and H. J. Zhang, NPG Asia Mater., 2015, 7, e179 CrossRef CAS.
- S. Li, N. Wang, Y. Yue, G. Wang, Z. Zu and Y. Zhang, Chem. Sci., 2015, 6, 2495 RSC.
- Y. Wang, Y. Shao, D. Matson, J. Li and Y. Lin, ACS Nano, 2010, 4, 1790 CrossRef CAS PubMed.
- J. Wang, H. Zhong, Y. Qian and X. Zhang, Angew. Chem., Int. Ed., 2013, 52, 5248 CrossRef CAS PubMed.
- H. Zhong, J. Wang, Y. Zhang, W. Xu, W. Xing, D. Xu, Y. Zhang and X. Zhang, Angew. Chem., Int. Ed., 2014, 53, 14235 CrossRef CAS PubMed.
- X. Wang, D. P. Liu, S. Y. Song and H. J. Zhang, J. Am. Chem. Soc., 2013, 135, 15864 CrossRef CAS PubMed.
- X. Y. Li, X. Wang, D. P. Liu, S. Y. Song and H. J. Zhang, Chem. Commun., 2014, 50, 7198 RSC.
- W. Liu, X. Liu, L. Feng, J. Guo, A. Xie, S. Wang, J. Zhang and Y. Yang, Nanoscale, 2014, 6, 10693 RSC.
- A. Jia, S. Jiang, J. Lu and M. Luo, J. Phys. Chem. C, 2010, 114, 21605 CAS.
- H. Yen, Y. Seo, S. Kaliaguine and F. Kleitz, Angew. Chem., Int. Ed., 2012, 51, 12032 CrossRef CAS PubMed.
- X. Wang, D. Liu, J. Li, J. Zhen and H. Zhang, NPG Asia Mater., 2015, 7, e158 CrossRef CAS.
- H. Bao, Z. Zhang, Q. Hua and W. Huang, Langmuir, 2014, 30, 6427 CrossRef CAS PubMed.
- J. Zhen, X. Wang, D. Liu, Z. Wang, J. Li, F. Wan, Y. Wang and H. Zhang, Nano Res., 2015, 8, 1944 CrossRef CAS.
- J. Zhen, X. Wang, D. Liu, S. Song, Z. Wang, Y. Wang, J. Li, F. Wang and H. Zhang, Chem.–Eur. J., 2014, 20, 4469 CrossRef CAS PubMed.
- Q. Xie, Y. Zhao, H. Guo, A. Lu, X. Zhang, L. Wang, M. Chen and D. Peng, ACS Appl. Mater. Interfaces, 2014, 6, 421 CAS.
- F. Wang, X. Wang, D. Liu, J. Zhen, J. Li, Y. Wang and H. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 22216 CAS.
- Y. Liu, X. Sun, Z. Zhou and Y. Lei, J. Mater. Chem. A, 2014, 2, 14038 CAS.
- D. D. Zhang, S. W. Eaton, Y. Yu, L. Dou and P. D. Yang, J. Am. Chem. Soc., 2015, 137, 9230 CrossRef CAS PubMed.
- R. Wu, J. F. Zhang, Y. M. Shi, D. L. Liu and B. Zhang, J. Am. Chem. Soc., 2015, 137, 6983 CrossRef CAS PubMed.
- L. Zhang, H. B. Wu and X. W. Lou, J. Am. Chem. Soc., 2013, 135, 10664 CrossRef CAS PubMed.
- S. Y. Song, X. Wang, S. L. Li, Z. Wang, Q. Zhu and H. J. Zhang, Chem. Sci., 2015, 6, 6420 RSC.
- H. Hu, B. Y. Guan, B. Y. Xia and X. W. Lou, J. Am. Chem. Soc., 2015, 137, 5590 CrossRef CAS PubMed.
- X. Z. Song, S. Y. Song, S. N. Zhao, Z. M. Hao, M. Zhu, X. Meng, L. L. Wu and H. J. Zhang, Adv. Funct. Mater., 2014, 24, 4034 CrossRef CAS.
- M. Zhu, X. Z. Song, S. Y. Song, S. N. Zhao, X. Meng, L. L. Wu, C. Wang and H. J. Zhang, Adv. Sci., 2015, 2, 1500012 Search PubMed.
- H. X. Zhong, J. Wang, Y. W. Zhang, W. L. Xu, W. Xing, D. Xu, Y. F. Zhang and X. B. Zhang, Angew. Chem., Int. Ed., 2014, 53, 14235 CrossRef CAS PubMed.
- J. Wang, H. X. Zhong, Y. L. Qin and X. B. Zhang, Angew. Chem., Int. Ed., 2013, 52, 5248 CrossRef CAS PubMed.
- W. Zhang, Z. Y. Wu, H. L. Jiang and S. H. Yu, J. Am. Chem. Soc., 2014, 136, 14385 CrossRef CAS PubMed.
- J. Tang, R. R. Salunkhe, J. Liu, N. L. Torad, M. Imura, S. Furukawa and Y. Yamauchi, J. Am. Chem. Soc., 2015, 137, 1572 CrossRef CAS PubMed.
- J. J. Zhou, P. Wang, C. X. Wang, Y. T. Goh, Z. Fang, P. B. Messersmith and H. W. Duan, ACS Nano, 2015, 9, 6951 CrossRef CAS PubMed.
- G. Huang, F. F. Zhang, X. C. Du, Y. L. Qin, D. M. Yin and L. M. Wang, ACS Nano, 2015, 9, 1592 CrossRef CAS PubMed.
- Y. Gao, K. Xie, W. Wang, S. Mi, N. Liu, G. Pan and W. Huang, Catal. Sci. Technol., 2015, 5, 1568 CAS.
- F. Zhao, M. Gong, G. Zhang and J. Li, J. Rare Earths, 2015, 33, 604 CrossRef CAS.
- S. Hu, F. Zhou, L. Wang and J. Zhang, Catal. Commun., 2011, 12, 794 CrossRef CAS.
- Y. Zuo, X. Huang, L. Li and G. Li, J. Mater. Chem. A, 2013, 1, 374 CAS.
- L. F. Liotta, H. Wu, G. Pantaleo and A. M. Venezia, J. Mater. Chem. A, 2013, 3, 2085 Search PubMed.
- J. Luo, M. Meng, X. Li, X. Li, Y. Zha, T. Hu, Y. Xie and J. Zhang, J. Catal., 2008, 254, 310 CrossRef CAS.
- V. Shapovalov and H. Metiu, J. Catal., 2007, 245, 205 CrossRef CAS.
- G. Sedmak, S. Hocevar and J. Levec, J. Catal., 2003, 213, 135 CrossRef CAS.
- Q. Yang, F. Gu, Y. Tang, H. Zhang, Q. Liu, Z. Zhong and F. Su, RSC Adv., 2015, 5, 26815 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: XRD data of pure CeO2 nanowires; TG curve of CeO2–ZIF-67-2 precursor; XPS and N2 adsorption–desorption isotherm data of CeO2–Co3O4-2 sample and ICP data. See DOI: 10.1039/c5sc03430b |
| This journal is © The Royal Society of Chemistry 2016 |