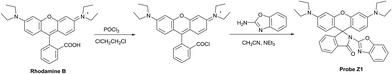
A benzoxazole functionalized fluorescent probe for selective Fe3+ detection and intracellular imaging in living cells†
Zheng
Yang
*ab,
Xinxin
Bai
a,
Siyue
Ma
b,
Xiangrong
Liu
a,
Shunsheng
Zhao
a and
Zaiwen
Yang
a
aSchool of Chemistry & Chemical Engineering, Xi'an University of Science and Technology, Xi'an, 710054, China
bMinistry of Education Key Laboratory of Synthetic and Natural Functional Molecule Chemistry, College of Chemistry & Materials Science, Northwest University, Xi'an, 710069, China
First published on 8th November 2016
Abstract
A rhodamine based 2-aminobenzoxazole functionalized probe was presented for reversible detection of Fe3+ driven by the coordination of Fe3+ to 2-aminobenzoxazole that resulted in strong fluorescence emission and color change. Fluorescent imaging in living L929 and MG-63 cells offered a promising candidate for mapping Fe3+ in biological samples.
The design and development of fluorescent probes for sensitive and selective quantification of fluorescent cellular and subcellular imaging heavy and transition-metal ions in special chemical, environmental and biological samples has evolved into an emerging attractive area of particular interest in analytical chemistry and chemical biology.1,2 Compared with the traditional methods such as atomic absorption spectroscopy and inductively coupled plasma mass spectroscopy which not only require large and expensive instruments, highly trained personnel, and tedious maintenance but also suffer from poor biocompatibility as well as environmental and biological applicability, fluorescence detection enjoys the advantages of simplicity, high spatial resolution, being non-destructive and real time monitoring.3 As a result, numbers of ideal fluorescent probes4,5 are increasing at a high speed, especially for probes based on the spirocyclic ring-opening progress of rhodamine derivatives because of their excellent spectroscopic properties, such as long excitation and emission wavelengths, great photostability, high extinction coefficient and excellent fluorescence quantum yields.6
Amongst a variety of attractive metal ions, ferric iron, which is known for its chemical versatility, industrial importance and as an indispensable trace mineral for humans in the metabolism and energy transfer mechanisms of numerous biological systems,7 plays significant roles in our daily life, industry, environmental science and medicine as well as several crucial biochemical processes at the cellular level. The abnormal levels of Fe3+ have been involved in a series of human diseases, such as anemia and breathing problems, certain cancers, oxidative damage to lipids, proteins, nucleic acids and other cellular components, dysfunction of certain organs, such as the heart, kidneys, pancreas, and liver, and even neurodegenerative diseases, such as Parkinson's disease and Alzheimer's disease.8,9 Therefore, a convenient and rapid method for the analysis of the mechanisms of how Fe3+ affects our metabolism and body health has important consequences in biological concerns.
In recent years, significant emphasis has been placed on the exploitation of facile, highly selective fluorescent probes for Fe3+ and much progress has been made.10–12 Ali et al.13 reported a rhodamine-benzoxazine functionalized probe for Fe3+ in H2O–MeCN (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, v/v) solution at pH 7.2 through 1
7, v/v) solution at pH 7.2 through 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex formation resulting in the fluorescence enhancement. Wang et al.14 synthesized a “double Schiff base” fluorescent turn-on probe for Fe3+ with a low detection limit of 0.27 μmol L−1. Our group has also put considerable efforts in the development of promising fluorescent probes for Fe3+ and made several delightful progresses.15 However, compared with the number of reported Fe3+ probes, the probes for real biological detection are still rare, since the application of some reported probes was sometimes limited by the solubility, sensitivity, selectivity, stability and biocompatibility, especially in biological detection. Therefore, it still remains a significant task for selection of adequate probes with excellent fluorescence properties to be effectually applied in biological fluorescent imaging of Fe3+, particularly for selective sensing allowing for miscellaneous fluorescence signal generation and unknown side-effects in the complex biological systems.
1 complex formation resulting in the fluorescence enhancement. Wang et al.14 synthesized a “double Schiff base” fluorescent turn-on probe for Fe3+ with a low detection limit of 0.27 μmol L−1. Our group has also put considerable efforts in the development of promising fluorescent probes for Fe3+ and made several delightful progresses.15 However, compared with the number of reported Fe3+ probes, the probes for real biological detection are still rare, since the application of some reported probes was sometimes limited by the solubility, sensitivity, selectivity, stability and biocompatibility, especially in biological detection. Therefore, it still remains a significant task for selection of adequate probes with excellent fluorescence properties to be effectually applied in biological fluorescent imaging of Fe3+, particularly for selective sensing allowing for miscellaneous fluorescence signal generation and unknown side-effects in the complex biological systems.
With continuing interest in the development of environmentally and biologically applied fluorescent probes for Fe3+, we herein present another wonderful probe Z1 (Scheme 1) which exhibited superior properties with improved selectivity, sensitivity and biological compatibility compared to the previously reported probes since the selected functional group 2-aminobenzoxazole is considered to have potential excellent bio-medical and optical properties that can not only improve the solubility, selectivity, biocompatibility and cellular affinity but also contribute to the chelation enhanced fluorescence emission and color change with remarkably improved fluorescence stability and sustainability. The wonderful properties make the probe highly suitable for the visualization of Fe3+ in living cells which may provide breakthrough insight into the correlation between Fe3+ and related biological processes.
The absorption and fluorescence detection were conducted in PBS solution (pH = 7.4) during all the photo-property investigation to ensure that the fluorescent and absorption signal changes were caused by the coordination of Fe3+ to the probe since originally the hydrolytic process of Fe3+ can happen in aqueous media and results in the decrease of pH that induces the protonation-induced ring-opening process which may cause great interferences for the detection. This was supported by the pH responses of the probes in EtOH–H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) with pH values from 1.7 to 13.0 (Fig. 1) since both the fluorescence intensity and the absorption exhibited inconspicuous changes in the pH range of 5.0 to 8.0, suggesting that the probes were insensitive to pH in the neutral range and could work under approximate physiological conditions with negligible background fluorescence.
5, v/v) with pH values from 1.7 to 13.0 (Fig. 1) since both the fluorescence intensity and the absorption exhibited inconspicuous changes in the pH range of 5.0 to 8.0, suggesting that the probes were insensitive to pH in the neutral range and could work under approximate physiological conditions with negligible background fluorescence.
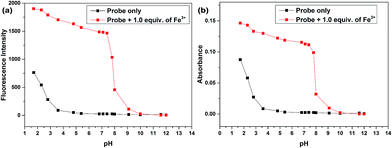 | ||
Fig. 1 Fluorescence intensity (a) and absorption (b) changes of Z1 (20 μmol L−1) in the presence of 1.0 equiv. of Fe3+ in EtOH–H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v, PBS, pH 7.4) solution under different pH conditions. 5, v/v, PBS, pH 7.4) solution under different pH conditions. | ||
The EtOH–H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) mixed solution was used during all the photo-property investigation since the solution investigation suggested that other solvent systems including MeOH–H2O, (CH3)2CHOH–H2O, (CH3)2CO–H2O, THF–H2O, CH3CN–H2O, DMSO–H2O, and DMF–H2O all seemed to be inferior to EtOH–H2O mixed solution because of the lower fluorescence intensities and absorptions upon addition of Fe3+ (Fig. S1–S4†). In addition, when the ethanol content of the solution varies from 0 to 100%, both the fluorescence intensity and absorption increased with the increasing concentration of ethanol in the mixed solvent (Fig. S5 and S6†). Considering the fact that the probe was designed for the detection of Fe3+ in biological samples, a 50% ethanol content seemed to be one of the best choices for fluorescence and absorption detection and a 10% ethanol content should be perfect for biological imaging.
5, v/v) mixed solution was used during all the photo-property investigation since the solution investigation suggested that other solvent systems including MeOH–H2O, (CH3)2CHOH–H2O, (CH3)2CO–H2O, THF–H2O, CH3CN–H2O, DMSO–H2O, and DMF–H2O all seemed to be inferior to EtOH–H2O mixed solution because of the lower fluorescence intensities and absorptions upon addition of Fe3+ (Fig. S1–S4†). In addition, when the ethanol content of the solution varies from 0 to 100%, both the fluorescence intensity and absorption increased with the increasing concentration of ethanol in the mixed solvent (Fig. S5 and S6†). Considering the fact that the probe was designed for the detection of Fe3+ in biological samples, a 50% ethanol content seemed to be one of the best choices for fluorescence and absorption detection and a 10% ethanol content should be perfect for biological imaging.
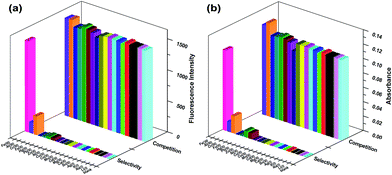
Related metal ions including Li+, Na+, K+, Ag+, Sn2+, Mg2+, Ca2+, Cd2+, Mn2+, Co2+, Cu2+, Ni2+, Zn2+, Pb2+, Hg2+, Fe2+, Al3+, Cr3+ and Fe3+ were used to verify the selective property of Z1 in EtOH–H2O (5/5, v/v, PBS, pH 7.4) solution (Fig. 2, S7 and S8†). Originally, the probe itself displays colourless and emits no fluorescence because the spirocyclic form of rhodamine prevailed. Notably, upon addition of Fe3+, an intense absorption band centered at 542 nm coupled with brilliant pink coloration and concomitantly, a strong emission band appeared around 571 nm, which was reasonably assigned to chelation enhanced fluorescence caused by Fe3+ which resulted in the delocalized xanthene tautomer of rhodamine into the quinoid structure. Although Al3+ and Cr3+ can also cause slight absorption and fluorescent responses, the signals were significantly inferior compared with Fe3+ induced spectroscopic changes, indicating the high specificity of the probe to Fe3+. Time dependent study showed that the Fe3+ induced ring-opening process completed rapidly within 20 s (Fig. S9 and S10†) and showed prolonged stability since both the absorption and fluorescence intensity showed an inconspicuous decrease for several hours. The competition experiments were then performed by adding 5.0 equiv. of the competing ions to the solutions that contained the probe and 1.0 equiv. of Fe3+. The results indicated that the selectivity of the probe did not significantly experience interference from the commonly coexistent metal ions.
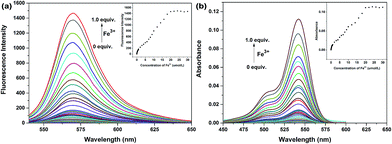
Absorption and fluorescence titrations were then performed (Fig. 3). The results suggested that both the fluorescence intensity and absorption showed a steady and smooth increase with the increasing concentration of Fe3+ and about 1.0 equiv. of Fe3+ was required until a plateau was reached. The fluorescence quantum yield was calculated to be 0.86 by using rhodamine B as a standard. Good linear relationships were observed (Fig. S11 and S12†) between the relative fluorescent and absorption signals and the concentration of Fe3+ in the 0.009–20 μmol L−1 range with a detection limit of 3.0 nmol L−1 in EtOH–H2O (5/5, v/v, PBS, pH 7.4) mixed solution when 20 μmol L−1 of probe was used (Fig. 3, inset), which was much more sensitive to our previously reported Fe3+ probes with benzothiazole and fluorine groups, indicating a better prospect of application.
The stoichiometric ratio investigation through Job's plot established the formation of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
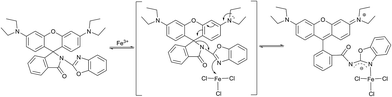
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry between the probe and Fe3+ (Fig. S13 and S14†) with a high association constant of 5.45 × 104 M−1, which indicated the strong coordination of probe Z1 to Fe3+. The mass spectrum manifested the peak at m/z 721.0986 (Fig. S24†), assigned to [Z1 + Fe3+ + 3Cl− + H]+, providing powerful evidence for the binding mode of Z1 with Fe3+. The absorption at 1690 cm−1 in IR analysis did not shift to lower frequency upon addition of Fe3+ (Fig. S23†), indicating that the carbonyl moiety was not involved in the coordination. In addition, the fluorescence intensity gradually decreased with the increasing concentration of ethylenediamine and completely quenched when excess ethylenediamine was added, coupled with the change of pink colour back to colourless (Fig. S15 and S16†). On the other hand, both the colour and the fluorescence appeared again when more Fe3+ was added to this solution (Fig. S17 and S18†). This process could take place several times, thus implying the reversible binding process between probe Z1 and Fe3+. According to our previous work,15 it can be supposed that Fe3+ bonds with the nitrogen atom of the benzoxazole moiety inducing the ring-opening process accompanied by the fluorescent changes (Scheme 2).
1 stoichiometry between the probe and Fe3+ (Fig. S13 and S14†) with a high association constant of 5.45 × 104 M−1, which indicated the strong coordination of probe Z1 to Fe3+. The mass spectrum manifested the peak at m/z 721.0986 (Fig. S24†), assigned to [Z1 + Fe3+ + 3Cl− + H]+, providing powerful evidence for the binding mode of Z1 with Fe3+. The absorption at 1690 cm−1 in IR analysis did not shift to lower frequency upon addition of Fe3+ (Fig. S23†), indicating that the carbonyl moiety was not involved in the coordination. In addition, the fluorescence intensity gradually decreased with the increasing concentration of ethylenediamine and completely quenched when excess ethylenediamine was added, coupled with the change of pink colour back to colourless (Fig. S15 and S16†). On the other hand, both the colour and the fluorescence appeared again when more Fe3+ was added to this solution (Fig. S17 and S18†). This process could take place several times, thus implying the reversible binding process between probe Z1 and Fe3+. According to our previous work,15 it can be supposed that Fe3+ bonds with the nitrogen atom of the benzoxazole moiety inducing the ring-opening process accompanied by the fluorescent changes (Scheme 2).
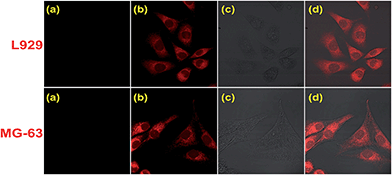
Inspired by the excellent optical properties which showed favorable potential for quantifying Fe3+ in biological systems, we performed the MTT assay in which mitochondrial metabolic enzyme activity is used as an indicator of cell viability to identify the toxicity of the probe. The MCF-7 cell was treated with different concentrations of Z1 and the results (Table. S1†) indicated the strong cell viability and low toxicity of Z1. The practical bio-imaging applications were then conducted by laser scanning confocal microscopy (Fig. 4). Both the cultured mouse fibroblast cells L929 and human osteosarcoma MG-63 cells displayed no intracellular fluorescence when incubated with the probe in culture medium for 30 min at 37 °C (Fig. 4(a)). However, a prominent fluorescence increase was observed in the intracellular area when 1.0 equiv. of Fe3+ was supplemented and incubated in the same growth medium for another 30 min at 37 °C (Fig. 4(b)). It is more encouraging that the cell morphology remained in good condition throughout the imaging experiments which was supported by the bright-field transmission images of cells (Fig. 4(c)). In addition, the overlay (Fig. 4(d)) of fluorescence and bright-field images confirmed that the fluorescent signals were localized in the perinuclear area of the cytosol. The preliminary experiments in living L929 cells and MG-63 cells demonstrated the special potential of the probe for in vivo mapping and imaging of Fe3+, which was supposed to contribute to understand the importance and functional mechanism of Fe3+ in related biological processes.
Some of the recently reported rhodamine based Fe3+ probes (1–11)11d,12d,13–16 are given in Fig. S25† and some salient features including solubility, selectivity, fluorescence quantum yield, sensitivity, stability, and biocompatibility are given in Table S2.† Investigation of these probes indicated that they are all off–on type Fe3+ probes with a moderate detection limit and formation constants and some are applicable for imaging and monitoring Fe3+ ions in living cells. In comparison, probe Z1 has nothing less than those reported probes in the solubility and selectivity but preponderant in sensitivity, stability and fluorescence quantum yield by over 300-fold fluorescence enhancement upon addition of Fe3+ with a large binding constant of 5.45 × 104, a large fluorescence quantum yield of 0.86 and a low detection limit of 3 nmol L−1 through 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry. In addition, the MTT assay demonstrated strong cell viability, good biocompatibility and low toxicity of Z1 and the fluorescent imaging in L929 and MG-63 cells demonstrated that the fluorescent signals were localized in the perinuclear area of the cytosol thus indicating the cytoplasmic binding which makes Z1 a promising candidate for in vivo monitoring of Fe3+.
1 binding stoichiometry. In addition, the MTT assay demonstrated strong cell viability, good biocompatibility and low toxicity of Z1 and the fluorescent imaging in L929 and MG-63 cells demonstrated that the fluorescent signals were localized in the perinuclear area of the cytosol thus indicating the cytoplasmic binding which makes Z1 a promising candidate for in vivo monitoring of Fe3+.
Conclusions
In summary, we developed a novel rhodamine based fluorescent probe with 2-aminobenzimidazole functional groups for specific detection of Fe3+. The probe was facilely synthesized from rhodamine B and displayed special selectivity, sensitivity and fluorescence quantum yield which were superior to those of our previously reported probes with 2-aminobenzothiazole and fluorine functional groups. Preliminary experiments in living L929 cells and MG-63 cells confirmed the membrane permeability and biocompatibility of the probe which would be a promising candidate for intracellular identification of Fe3+ thus providing significant insight into a better understanding of the importance and biological functions of Fe3+ in biological processes and organs.Acknowledgements
We thank Prof. J. L. Li, Prof. S Y. Zhang and Prof. Z. Shi for their great helpful support. We are grateful for the support from the National Natural Science Foundation of China (NSFC 21572177; 21301139; 21272184; 21103137; 21103135; 21073139 and J1210057), the Shaanxi Provincial Natural Science Fund Project (No. 2015JZ003), the Shaanxi Provincial Higher Education Teaching Reform Project (No. 15BY47), the Xi'an City Science and Technology Project (No. CXY1511(3)), Scientific Research Cultivating Fund of Xi'an University of Science and Technology (No. 201619), and the Xi'an University of Science and Technology Teaching Reform Project (No. JG14052).Notes and references
- (a) M. E. Tanenbaum, L. A. Gilbert, L. S. Qi, J. S. Weissman and R. D. Vale, Cell, 2014, 159, 635–646 CrossRef CAS PubMed; (b) M. Grossi, M. Morgunova, S. Cheung, S. D. Cholz, E. Conroy, M. Terrile, A. Panarella, J. C. Simpson, W. M. Gallagher and D. F. O'Shea, Nat. Commun., 2016, 7, 10855 CrossRef CAS PubMed; (c) K. M. Dean and A. E. Palmer, Nat. Chem. Biol., 2014, 10, 512–523 CrossRef CAS PubMed.
- (a) X. Q. Chen, F. Wang, J. Y. Hyun, T. W. Wei, J. Qiang, X. Ren, I. Shin and J. Yoon, Chem. Soc. Rev., 2016, 45, 2976–3016 RSC; (b) Y. H. Tang, D. Lee, J. L. Wang, G. H. Li, J. H. Yu, W. Y. Lin and J. Yoon, Chem. Soc. Rev., 2015, 44, 5003–5015 RSC; (c) X. H. Qian and Z. C. Xu, Chem. Soc. Rev., 2015, 44, 4487–4493 RSC; (d) X. H. Li, X. H. Gao, W. Shi and H. M. Ma, Chem. Rev., 2014, 114, 590–659 CrossRef CAS PubMed; (e) Y. Y. Huang, M. J. Wang, M. Y. She, Z. Yang, P. Liu, J. L. Li and Z. Shi, Chin. J. Org. Chem., 2014, 34, 1–25 CrossRef CAS.
- (a) Y. Liu, F. F. Meng, L. W. He, K. Y. Liu and W. Y. Lin, Chem. Commun., 2016, 52, 7016–7019 RSC; (b) Z. H. Lei and Y. J. Yang, J. Am. Chem. Soc., 2014, 136, 6594–6597 CrossRef CAS PubMed; (c) K. Kitamura, M. Kawaguchi, N. Ieda, N. Miyata and H. Nakagawa, ACS Chem. Biol., 2016, 11, 1271–1278 CrossRef CAS PubMed; (d) Y. C. Su, S. F. Hickey, S. G. L. Keyser and M. C. Hammond, J. Am. Chem. Soc., 2016, 138, 7040–7047 CrossRef CAS PubMed.
- (a) X. G. Liu, Q. L. Qiao, W. M. Tian, W. J. Liu, J. Chen, M. J. Lang and Z. C. Xu, J. Am. Chem. Soc., 2016, 138, 6960–6963 CrossRef CAS PubMed; (b) H. Kim, R. Kumar, W. Kim, J. H. Lee, M. Suh, A. Sharma, C. Kim, C. Kang and J. S. Kim, Chem. Commun., 2016, 52, 7134–7137 RSC; (c) X. Zhou, Y. Y. Zeng, L. Y. Chen, X. Wu and J. Yoon, Angew. Chem., Int. Ed., 2016, 55, 4729–4733 CrossRef CAS PubMed; (d) F. Liu, J. Du, D. Song, M. Y. Xu and G. P. Sun, Chem. Commun., 2016, 52, 4636–4639 RSC; (e) N. Narayanaswamy, S. Narra, R. R. Nair, D. K. Saini, P. Kondaiah and T. Govindaraju, Chem. Sci., 2016, 7, 2832–2841 RSC.
- (a) M. Zhao, H. Li, H. Li, Q. L. Qiao, C. Cao and Z. C. Xu, RSC Adv., 2015, 5, 86355–86358 RSC; (b) P. Qi, D. Zhang, Y. Sun and Y. Wan, Anal. Methods, 2016, 8, 3339–3344 RSC; (c) Q. L. Qiao, M. Zhao, H. J. Lang, D. Q. Mao, J. N. Cui and Z. C. Xu, RSC Adv., 2014, 4, 25790–25794 RSC; (d) X. H. Sun, Y. F. Xu, W. P. Zhu, C. S. He, L. Xu, Y. J. Yang and X. H. Qian, Anal. Methods, 2012, 4, 919–922 RSC; (e) L. L. Song, Z. H. Lei, B. Y. Zhang, Z. P. Xu, Z. Li and Y. J. Yang, Anal. Methods, 2014, 6, 7597–7600 RSC; (f) S. Paul, S. Goswami and C. Das Mukhopadhyay, New J. Chem., 2015, 39, 8940–8947 RSC.
- (a) X. Q. Chen, T. Pradhan, F. Wang, J. S. Kim and J. Yoon, Chem. Rev., 2012, 112, 1910–1956 CrossRef CAS PubMed; (b) R. W. Ramette and E. B. Sandell, J. Am. Chem. Soc., 1956, 78, 4872–4878 CrossRef CAS; (c) M. Beija, C. A. Afonso and J. M. Martinho, Chem. Soc. Rev., 2009, 38, 2410–2433 RSC.
- (a) B. D'Autreaux, N. P. Tucker, R. Dixon and S. Spiro, Nature, 2005, 437, 769–772 CrossRef PubMed; (b) S. R. Lynch, Nutr. Rev., 1997, 55, 102–110 CrossRef CAS PubMed; (c) D. Touati, Arch. Biochem. Biophys., 2000, 373, 1–6 CrossRef CAS PubMed.
- (a) J. B. Schulz, S. Boesch, K. Burk, A. Durr, P. Giunti, C. Mariotti, F. Pousset, L. Schols, P. Vankan and M. Pandolfo, Nat. Rev. Neurol., 2009, 5, 222–234 CrossRef PubMed; (b) D. Galaris, V. Skiada and A. Barbouti, Cancer Lett., 2008, 266, 21–29 CrossRef CAS PubMed.
- (a) D. Kaur, S. Rajagopalan and J. K. Andersen, Brain Res., 2009, 1297, 17–22 CrossRef CAS PubMed; (b) E. S. Carlson, R. Magid, A. Petryk and M. K. Georgieff, Brain Res., 2008, 1237, 75–83 CrossRef CAS PubMed.
- (a) Y. J. Ding, H. Zhu, X. X. Zhang, J. Zhu and C. Burda, Chem. Commun., 2013, 49, 7797–7799 RSC; (b) R. Kagit, M. Yildirim, O. Ozay, S. Yesilot and H. Ozay, Inorg. Chem., 2014, 53, 2144–2151 CrossRef CAS PubMed; (c) S. Paul, A. Manna and S. Goswami, Dalton Trans., 2015, 44, 11805–11810 RSC; (d) S. Goswami, S. Das, K. Aich, D. Sarkar, T. K. Mondal, C. K. Quah and H. Fun, Dalton Trans., 2013, 42, 15113–15119 RSC.
- (a) S. Li, D. Zhang, X. Y. Xie, S. G. Ma, Y. Liu, Z. H. Xu, Y. F. Gao and Y. Ye, Sens. Actuators, B, 2016, 224, 661–667 CrossRef CAS; (b) C. C. Wang, D. Zhang, X. Y. Huang, P. G. Ding, Z. J. Wang, Y. F. Zhao and Y. Ye, Talanta, 2014, 128, 69–74 CrossRef CAS PubMed; (c) J. H. Wang, D. Zhang, Y. Q. Liu, P. G. Ding, C. C. Wang, Y. Ye and Y. F. Zhao, Sens. Actuators, B, 2014, 191, 344–350 CrossRef CAS; (d) G. C. Li, J. Tang, P. G. Ding and Y. Ye, J. Fluoresc., 2016, 26, 155–161 CrossRef CAS PubMed.
- (a) Z. Q. Yan, L. Hua and J. M. You, Anal. Methods, 2016, 8, 5738–5754 RSC; (b) Y. J. Hu, J. L. Wang, L. P. Long and X. M. Xiao, Luminescence, 2016, 31, 16–21 CrossRef CAS PubMed; (c) N. R. Chereddy, M. V. N. Raju, P. Nagaraju, V. R. Krishnaswamy, P. S. Korrapati, P. R. Bangal and V. J. Rao, Analyst, 2014, 139, 6352–6356 RSC; (d) S. Lohar, A. Banerjee, A. Sahana, A. Banik, S. K. Mukhopadhyay and D. Das, Anal. Methods, 2013, 5, 442–445 RSC.
- H. A. Molla, R. Bhowmick, A. Katarkar, K. Chaudhuri, S. Gangopadhyay and M. Ali, Anal. Methods, 2015, 7, 5149–5156 RSC.
- X. Han, D. E. Wang, S. Chen, L. L. Zhang, Y. D. Guo and J. Y. Wang, Anal. Methods, 2015, 7, 4231–4236 RSC.
- (a) S. Y. Ma, Z. Yang, M. Y. She, W. Sun, B. Yin, P. Liu, S. Y. Zhang and J. L. Li, Dyes Pigm., 2015, 115, 120–126 CrossRef CAS; (b) Z. Yang, M. Y. She, B. Yin, J. H. Cui, Y. Z. Zhang, W. Sun, J. L. Li and Z. Shi, J. Org. Chem., 2012, 77, 1143–1147 CrossRef CAS PubMed; (c) M. Y. She, Z. Yang, B. Yin, J. Zhang, J. Gu, W. T. Yin, J. L. Li, G. F. Zhao and Z. Shi, Dyes Pigm., 2012, 92, 1337–1343 CrossRef CAS.
- (a) M. P. Yang, W. F. Meng, Q. L. Ding, N. Su, X. J. Liu, M. Zhang and B. Q. Yang, New J. Chem., 2015, 39, 4790–4795 RSC; (b) Y. M. Liu, J. Y. Zhang, J. X. Ru, X. Yao, Y. Yang, X. H. Li, X. L. Tang, G. L. Zhang and W. S. Liu, Sens. Actuators, B, 2016, 237, 501–508 CrossRef CAS; (c) X. F. Bao, X. W. Cao, X. M. Nie, Y. Xu, W. H. Guo, B. J. Zhou, L. Y. Zhang, H. Liao and T. Pang, Sens. Actuators, B, 2015, 208, 54–66 CrossRef CAS; (d) S. H. Hou, Z. G. Qu, K. L. Zhong, Y. J. Bian and L. J. Tang, Tetrahedron Lett., 2016, 57, 2616–2619 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ay02660e |
| This journal is © The Royal Society of Chemistry 2017 |