3D cuboidal vanadium diselenide embedded reduced graphene oxide hybrid structures with enhanced supercapacitor properties†
Subba R.
Marri‡
a,
Satyajit
Ratha‡
b,
Chandra Sekhar
Rout
*b and
J. N.
Behera
*a
aSchool of Chemical Science, National Institute of Science Education and Research (NISER), Jatni 752050, India. E-mail: jnbehera@niser.ac.in
bSchool of Basic Sciences, Indian Institute of Technology, Bhubaneswar 751013, India. E-mail: csrout@iitbbs.ac.in; csrout@gmail.com
First published on 29th November 2016
Abstract
The electrochemical supercapacitor performance of a VSe2–reduced graphene oxide (RGO) hybrid has been reported for the first time. The hybrid was synthesized via a one-step hydrothermal route at different concentrations of graphene oxide, i.e. 0.15, 0.3, and 0.75 wt%. Enhanced supercapacitor performances were observed in the case of the hybrid obtained at 0.3 wt% of GO. It showed a specific capacitance of ∼680 F g−1 at a mass normalised current of 1 A g−1 which was ∼6 and ∼5 fold higher than those of bare VSe2 and bare RGO, respectively. Furthermore, a high energy density of ∼212 W h kg−1, power density of ∼3.3 kW kg−1, and ∼81% retention of the initial capacitance even after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of charge–discharge were observed.
000 cycles of charge–discharge were observed.
The growing interest toward clean, safe and effective energy storage technologies and the graphene revolution have collectively, paved the way for the realization and development of two-dimensional layered materials of interest.1 These materials not only mimic the layered structure of graphene but also possess sophisticated physicochemical properties to their advantage. In this context, extensive research has been carried out on layered transition metal chalcogenides (TMCs).2 Over the years and recently, these uniquely composed materials have shown outstanding activities and promising results in areas like spintronics,3 sensing,4 optics,5 and energy storage.6 MoS2, WS2, and VS2etc. are some of the well-known TMCs which have been studied extensively due to their unique physical and chemical properties.7,8 According to reports, both VS2 and VSe2 are metallic in nature while most of the other TMCs are either of semiconducting nature or insulators with a few of them possessing superconducting properties as well.9 Much like graphene, these materials offer greater flexibility and robustness, which is advantageous to the concept of efficient energy storage and conversion.
Graphene based supercapacitors store charge following the Helmholtz double layer mechanism known as electric double layer capacitance (EDLC) but they don't possess sufficient specific capacity (energy density) required in practical portable electronic devices.10 Layered TMCs, on the other hand, follow a fast surface redox (faradic) reaction based charge storage principle and have much higher capacitance to mass ratio. However, they lack long term stability which is essential for supercapacitor devices.10 Graphene has peculiar chemical and physical properties such as high surface area,11 excellent tensile strength,12 and chemical stability, etc.13 It not only acts as an excellent supporting framework which promotes growth and nucleation of other materials but also provides greater stability to them and enhances their physicochemical properties. Due to such robustness and flexibility, TMCs are often hybridized with graphene to form composites which are highly stable and deliver much performance. The presence of graphene as an active matrix/substrate results in much better ion transportation and mechanical adherence. This is evident from a number of reports on such hybrids, i.e. MoS2/graphene,14 WS2/graphene,9 SnS2/graphene,15 and VS4/graphene, etc.,16 where graphene hybrids of TMCs excelled as compared to their pristine forms. As discussed earlier, of the TMCs reported to date, only VS2 and VSe2 show metallic behavior.9 It has been observed that in contrast to TMCs with a semiconducting nature, those having metallic properties show ultra-high conductivity and thus are highly suitable materials for supercapacitor electrodes. VSe2 has been reported to have versatile electronic properties beneficial for the realization of futuristic nano-devices.17 In addition to its exceptional activities towards intercalation reactions, VSe2 has also been reported to show promising energy storage properties as an active component in the cathode material of Li-ion batteries.18,19 Hybridization of these highly conducting TMCs with graphene would further enhance their overall supercapacitor performances. While the graphene hybrid of VS2 proved to be a better alternative for enhanced energy storage,20 improved performance is expected from a VSe2/RGO hybrid in contrast to pristine VSe2. Recently, Wang et al. observed an interesting lithium storage property in a VSe2/graphene hybrid prepared via the hydrothermal route.21 VSe2 is made up of two Se atoms sandwiching one V atom between them forming layer structures which are stacked via weak van der Waals forces. It has a hexagonal crystal structure with space symmetry group P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m1.22 Although, like most of the TMCs, it may crystallize either in 2H or 1T phase,23 yet, in both the cases, the metallic nature is preserved.22 With its unique physical properties, owing to strong electron correlation between the adjacent V atoms and charge density wave induced structural instability,24 VSe2 acquires prominence amongst layered TMCs and therefore holds immense potential, which remains to be exploited in energy storage and conversion applications.
m1.22 Although, like most of the TMCs, it may crystallize either in 2H or 1T phase,23 yet, in both the cases, the metallic nature is preserved.22 With its unique physical properties, owing to strong electron correlation between the adjacent V atoms and charge density wave induced structural instability,24 VSe2 acquires prominence amongst layered TMCs and therefore holds immense potential, which remains to be exploited in energy storage and conversion applications.
Even though several synthetic routes,24–26 exfoliation techniques,24 and physical characterization have been detailed in previous reports, and despite having a resemblance with the other renowned TMCs like MoS2 and WS2 (at least from a structural point of view),27 reports on the energy storage/conversion performance of VSe2 and/or its graphene hybrids are rather scarce.21,26 Furthermore, to the best of our knowledge, there have been no reports on the supercapacitor performance of VSe2. In this context, we have demonstrated, for the first time, a facile one-step hydrothermal method for the synthesis of vanadium diselenide–reduced graphene oxide (VSe2/RGO) hybrids at different concentrations of graphene oxide (GO) and their detailed supercapacitor performances in a typical coin-cell arrangement.
VSe2/RGO hybrids were synthesized at three different concentrations, i.e. 0.15, 0.3, and 0.75 wt% of GO and henceforth are labelled V/G_0.15, V/G_0.3 and V/G_0.75, respectively. Field emission scanning electron microscopy (FESEM) images of the V/G_0.3 hybrid are provided in Fig. 1a and b, which clearly show the cuboids of VSe2 embedded in RGO. The growth mechanism of the VSe2 cuboids in the presence of graphene oxide is explained in the ESI† with a schematic illustration (Fig. S1). Images obtained from transmission electron microscopy (TEM) are elucidated in Fig. 1c and d. The inset in Fig. 1d shows the SAED pattern for VSe2. Energy dispersive X-ray spectroscopy (EDX) on TEM was also performed for V/G_0.3 (see Fig. S2, ESI†) showing the presence of both RGO and VSe2. Fig. S3a and b (ESI†) show the morphologies of the VSe2 sample at low and high magnification, respectively. Fig. S3c (ESI†) shows the X-ray diffraction spectra of VSe2 suggesting prominent phase growth along the (011) direction. All the diffraction peaks obtained were indexed as per the standard JCPDS file number: 89-1641. No visible discrepancy could be observed in the case of the diffraction spectra collected from VSe2, V/G_0.15, V/G_0.3, and V/G_0.75 (Fig. S4, ESI†). In the diffraction spectra of VSe2/RGO hybrids, the peak corresponding to RGO (at 2θ = ∼26°) is absent, which can be correlated with both the decrease in the interplanar spacing of GO and the high crystalline nature of VSe2. Though visibly similar diffraction spectra were obtained in the case of all the hybridized samples, i.e. V/G_0.15, V/G_0.3, and V/G_0.75, their morphologies were however found to be different from each other. The FESEM images of V/G_0.15 and V/G_0.75 are provided in Fig. S5a and b (ESI†), respectively. Raman spectra of V/G_0.3 and VSe2 are illustrated in Fig. S6 (ESI†). The inset shows the characteristic D and G bands of RGO confirming its presence in the hybrid. BET analyses were also done to measure the variation in surface area in the case of VSe2 and V/G_0.3. The results are plotted in Fig. S7 (ESI†). The average surface area in the case of VSe2 was found to be ∼7 m2 g−1, whereas in the case of V/G_0.3, a two fold increase in the value (∼15 m2 g−1) was observed. Valence band spectra for V, Se, and C elements in the V/G_0.3 hybrid are shown in Fig. S8 (ESI†). For vanadium, two clear peaks at ∼516 and ∼523.5 eV were observed (Fig. S8a, ESI†) corresponding to the 2p3/2 and 2p1/2 levels, respectively.28,29 The presence of 2p1/2 suggests mild oxidation of vanadium in the hybrid. The 3d core level spectra of Se resulted in three peaks positioned at ∼53, ∼54, and ∼55 eV,25,30 respectively after a deconvolution process as shown in Fig. S8b (ESI†). The C 1s spectra (Fig. S8c, ESI†) show a single distinct peak at ∼284 eV corresponding to the C–C bond energy.16 The absence of any other peaks suggests a high quality thermal reduction of GO during the hydrothermal process.
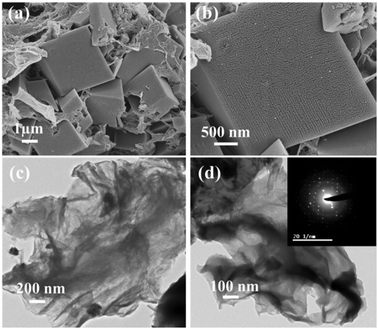 | ||
| Fig. 1 FESEM images of V/G_0.3 at (a) low and (b) high magnification; (c) low and (d) high magnification TEM images of V/G_0.3 with the inset showing the SAED pattern for VSe2. | ||
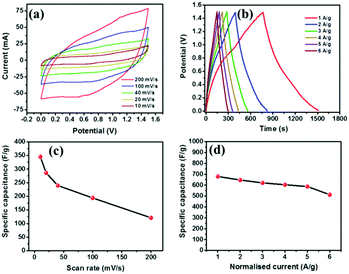
Electrochemical supercapacitor performances of all the samples were evaluated via cyclic voltammetry (CV) and charge–discharge (CD) measurements with a mass loading of ∼2 mg for each symmetric electrode. In the case of VSe2, the suitable working potential window was found to be ∼1 V, (as can be seen in Fig. S9, ESI†), beyond which it tends to show an irreversible oxygen evolution reaction. Fig. S10 (ESI†) shows the detailed supercapacitor performances of pristine VSe2. The curves obtained for VSe2 showed significant deviations from a rectangular shape, clearly suggesting its dominating pseudocapacitive behavior. This is further corroborated by the CD curves which attained asymmetric shapes with multiple slopes. For the VSe2 sample, a specific capacitance of ∼103 F g−1 was obtained at a normalized current of 1 A g−1. Similar measurements were performed for the RGO sample also. The working potential window in the case of RGO was set to 1 V (Fig. S11, ESI†), beyond which it seems to undergo the oxygen evolution reaction. The supercapacitor data for RGO are elucidated in Fig. S12 (ESI†). The CV curves were of almost rectangular shape suggesting the dominant EDLC properties of RGO. Furthermore, the CD curves were highly symmetric and were in good agreement with the CV curves. For the RGO sample, the highest capacitance of ∼126 F g−1 was obtained at a mass normalized current of 1 A g−1. The resultant data plots for the V/G_0.3 hybrid are shown in Fig. 2. The obtained CV curves (Fig. 2a) were of quasi-rectangular shape which can be attributed to the pseudocapacitive effect of VSe2 and internal resistance (due to electrode/electrolyte interaction) of the fabricated device. However, the high resemblance of the I–V characteristic curves to those observed in the case of RGO clearly suggests the dominant effect of EDLC based charge storage properties of the hybrid. Fig. 2b shows the CD curves of the hybrid obtained at different current densities showing the cumulative effect of EDLC and pseudocapacitance based charge storage. Supercapacitor evaluation data for samples V/G_0.15 and V/G_0.75 are elucidated in Fig. S13 and S14 (ESI†), respectively.
For a mass normalized current of 1 A g−1, V/G_0.3 showed a capacitance of ∼680 F g−1 whereas V/G_0.15 and V/G_0.75 respectively showed capacitance values of ∼409 and ∼585 F g−1. Table S1 (see the ESI†) shows a detailed comparison of supercapacitor performances of all the samples. Also, a comparative plot illustrating the variation of capacitance with current densities of all the samples is given in Fig. S15 (ESI†). Hybrid V/G_0.3 shows better supercapacitor performances than the other reported samples due to the enhanced synergistic effect of both pseudocapacitance and EDLC arising out of the excellent coordination between VSe2 and RGO. A long cyclic stability test (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of charge–discharge) for V/G_0.3 was conducted to check the long term usability of the fabricated device. Fig. S16 (ESI†) shows the stability data in terms of the initial (Fig. S16a, ESI†) and final (Fig. S16b, ESI†) five cycles along with the percentage retention calculation. After 10
000 cycles of charge–discharge) for V/G_0.3 was conducted to check the long term usability of the fabricated device. Fig. S16 (ESI†) shows the stability data in terms of the initial (Fig. S16a, ESI†) and final (Fig. S16b, ESI†) five cycles along with the percentage retention calculation. After 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, V/G_0.3 still showed a retention of 81%, revealing the good long term stability of the hybrid. The Ragone plot containing energy density and power density values of all the samples is shown in Fig. S17 (ESI†). Hybrid V/G_0.3 showed a high energy density of the order of ∼212 W h kg−1 (at a power density of ∼0.9 kW kg−1) and a high power density of ∼3.3 kW kg−1 (at an energy density of ∼160 W h kg−1). The value of energy density is very much comparable to those of some of the best performing supercapacitor devices reported in the literature. In our previous report, we obtained an energy density of ∼174 W h kg−1 from a ternary hybrid VS4/CNT/RGO. The ternary hybrid also showed a maximum power density of ∼13.85 kW kg−1.31 In another report, Jana et al. have found a maximum energy density of ∼68.6 W h kg−1 in the case of a RGO/nano-structured cobalt sulfide hybrid.32 In another report, a maximum energy density of ∼25 kW kg−1 and power density of 4.5 kW kg−1 were found in the case of a titanium doped-vanadium oxide-vertically aligned CNT composite.33
000 cycles, V/G_0.3 still showed a retention of 81%, revealing the good long term stability of the hybrid. The Ragone plot containing energy density and power density values of all the samples is shown in Fig. S17 (ESI†). Hybrid V/G_0.3 showed a high energy density of the order of ∼212 W h kg−1 (at a power density of ∼0.9 kW kg−1) and a high power density of ∼3.3 kW kg−1 (at an energy density of ∼160 W h kg−1). The value of energy density is very much comparable to those of some of the best performing supercapacitor devices reported in the literature. In our previous report, we obtained an energy density of ∼174 W h kg−1 from a ternary hybrid VS4/CNT/RGO. The ternary hybrid also showed a maximum power density of ∼13.85 kW kg−1.31 In another report, Jana et al. have found a maximum energy density of ∼68.6 W h kg−1 in the case of a RGO/nano-structured cobalt sulfide hybrid.32 In another report, a maximum energy density of ∼25 kW kg−1 and power density of 4.5 kW kg−1 were found in the case of a titanium doped-vanadium oxide-vertically aligned CNT composite.33
To justify why V/G_0.3 shows much better capacitive properties in contrast to VSe2, RGO, V/G_0.15, and V/G_0.75, detailed electrochemical impedance spectroscopy (EIS) was carried out for the as fabricated supercapacitor devices. The EIS technique gives valuable information regarding the charge-transfer characteristic of a sample under study which is an essential parameter in determining the quality and feasibility of that sample as an electrode material for supercapacitor devices. The Nyquist plot for the samples obtained from the EIS study is shown in Fig. S18 (ESI†). The inset contains both a simplified Randles equivalent circuit and a magnified portion of the plot. The magnified portion demonstrates the resistivity arising out of electrode/electrolyte interaction in the case of the composites. The resistivity in the case of VSe2 was found to be the lowest, whereas in the case of RGO, it was found to be the highest. The lowest resistive property of VSe2 is due primarily to its metallic properties. The resistive properties of hybrid samples were found to be of intermediate values. However, despite its high conductivity owing to the metallic character, VSe2 has very poor charge-transfer characteristic as can be seen from the large semicircle it possesses. The semicircle with minimal curvature was observed in the case of V/G_0.3 indicating its excellent charge-transfer characteristic. Table S2 (see the ESI†) shows the charge transfer resistance values of all the samples calculated by fitting the respective EIS spectra with the help of the Randles equivalent circuit.
X-ray diffraction spectra for the V/G_0.3 hybrid before and after the long cycle test were compared to check a possible intercalation process. As shown in Fig. S19 (ESI†), the two diffraction spectra have marked differences between them. Except for the peak at ∼29.2° corresponding to the (002) phase growth of pure VSe2 in the hybrid, all other peaks were found to be unaltered after the long cycle measurement. Variation in the intensity of the (002) peak with respect to the (011) peak (highest intensity peak for pure VSe2) can be explained in terms of a possible intercalation of ion/atom/molecule into the VSe2 layer structure. The process might have triggered a quasi-exfoliation step relatively increasing the intensity of the (002) peak in contrast to the (011) peak.24 The shift in the 2θ value can be correlated with the doping effect of the intercalated ion/atom/molecule.34 The above analyses and observations infer that the supercapacitor performance of the hybrid is governed by the dominating charge intercalation technique with minor contributions from surface kinetics based charge storage. The additional peaks were found to match with those of K2SO4, the aqueous solution of which was taken as the electrolyte. An optical image of the two supercapacitor devices (in series) powering a light emitting diode is provided in Fig. S20 (ESI†).
In summary, a simple one-step hydrothermal technique has been devised for the synthesis of VSe2/RGO hybrids of highest quality at different weight-percentages of GO. Detailed electrochemical investigations were carried out to check the possible supercapacitive performance of the as-synthesized hybrids by implementing a typical CR2032 coin cell arrangement. An enhanced working potential window of 1.5 V was observed during the device characterization step. The fabricated coin cell type device delivered a specific capacitance of the order of ∼680 F g−1 at a mass normalized discharge current of 1 A g−1, which was found to be ∼6 and ∼5 fold higher than the specific capacitances delivered by pristine VSe2 and pristine RGO, respectively. Furthermore, 81% retention of the initial capacitance even after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of charge–discharge was observed, strongly suggesting the potential application of VSe2/RGO hybrids as efficient and high-performance supercapacitor electrodes.
000 cycles of charge–discharge was observed, strongly suggesting the potential application of VSe2/RGO hybrids as efficient and high-performance supercapacitor electrodes.
This study was supported by the Department of Science and Technology (DST), SERB, Govt. of India, through the award of a research grant (SR/S1/IC-04/2012) to Dr J. N. Behera. Dr C. S. Rout would like to thank DST (Government of India) for the Ramanujan fellowship (Grant no. SR/S2/RJN-21/2012). This work was supported by the DST-SERB Fast-track Young scientist (Grant no. SB/FTP/PS-065/2013), UGC-UKIERI thematic awards (Grant no. UGC-2013-14/005) and BRNS-DAE (Grant no. 37(3)/14/48/2014-BRNS/1502). Also, part of this work was supported by the Indo-US Science and Technology Forum (IUSSTF) through a joint INDO-US centre grant and the Ministry of Human Resources Development (MHRD), India, through a center of excellence grant.
References
- Q. Tang and Z. Zhou, Prog. Mater. Sci., 2013, 58, 1244–1315 CrossRef CAS.
- X. Chia, A. Y. S. Eng, A. Ambrosi, S. M. Tan and M. Pumera, Chem. Rev., 2015, 115, 11941–11966 CrossRef CAS PubMed.
- N. Zibouche, A. Kuc, J. Musfeldt and T. Heine, Ann. Phys., 2014, 526, 395–401 CrossRef CAS.
- A. Carvalho, R. M. Ribeiro and A. H. Castro Neto, Phys. Rev. B: Condens. Matter Mater. Phys., 2013, 88, 115205 CrossRef.
- W. Zhao, R. M. Ribeiro and G. Eda, Acc. Chem. Res., 2015, 48, 91–99 CrossRef CAS PubMed.
- H. Wang, H. Feng and J. Li, Small, 2014, 10, 2165–2181 CrossRef CAS PubMed.
- M. Pumera and A. H. Loo, TrAC, Trends Anal. Chem., 2014, 61, 49–53 CrossRef CAS.
- C. S. Rout, R. Khare, R. V. Kashid, D. S. Joag, M. A. More, N. A. Lanzillo, M. Washington, S. K. Nayak and D. J. Late, Eur. J. Inorg. Chem., 2014, 5331–5336 CrossRef CAS.
- S. Ratha and C. S. Rout, ACS Appl. Mater. Interfaces, 2013, 5, 11427–11433 CAS.
- M. D. Stoller, S. Park, Z. Yanwu, J. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498–3502 CrossRef CAS PubMed.
- A. K. Geim, Science, 2009, 324, 1530–1534 CrossRef CAS PubMed.
- Z. Ni, H. Bu, M. Zou, H. Yi, K. Bi and Y. Chen, Phys. B, 2010, 405, 1301–1306 CrossRef CAS.
- P. Blake, P. D. Brimicombe, R. R. Nair, T. J. Booth, D. Jiang, F. Schedin, L. A. Ponomarenko, S. V Morozov, H. F. Gleeson, E. W. Hill, A. K. Geim and K. S. Novoselov, Nano Lett., 2008, 8, 1704–1708 CrossRef PubMed.
- E. G. Da Silveira Firmiano, A. C. Rabelo, C. J. Dalmaschio, A. N. Pinheiro, E. C. Pereira, W. H. Schreiner and E. R. Leite, Adv. Energy Mater., 2014, 4, 1301380 CrossRef.
- C. S. Rout, P. D. Joshi, R. V Kashid, D. S. Joag, M. A. More, A. J. Simbeck, M. Washington, S. K. Nayak and D. J. Late, Appl. Phys. Lett., 2014, 105, 43109 CrossRef.
- S. Ratha, S. R. Marri, N. A. Lanzillo, S. Moshkalev, S. K. Nayak, J. N. Behera and C. S. Rout, J. Mater. Chem. A, 2015, 3, 18874–18881 CAS.
- F. Li, K. Tu and Z. Chen, J. Phys. Chem. C, 2014, 118, 21264–21274 CAS.
- R. Guzma'n, P. Lavela, J. Morales and J. L. Tirado, J. Appl. Electrochem., 1997, 27, 1207–1211 CrossRef.
- D. W. Murphy and J. N. Carides, J. Electrochem. Soc., 1979, 126, 349–351 CrossRef CAS.
- W. Fang, H. Zhao, Y. Xie, J. Fang, J. Xu and Z. Chen, ACS Appl. Mater. Interfaces, 2015, 7, 13044–13052 CAS.
- Y. Wang, B. Qian, H. Li, L. Liu, L. Chen and H. Jiang, Mater. Lett., 2015, 141, 35–38 CrossRef CAS.
- L. F. Schneemeyer, A. Stacy and M. J. Sienko, Inorg. Chem., 1980, 19, 2659–2662 CrossRef CAS.
- A. Kuc, Chem. Modell., 2014, 11, 1–29 Search PubMed.
- K. Xu, P. Chen, X. Li, C. Wu, Y. Guo, J. Zhao, X. Wu and Y. Xie, Angew. Chem., Int. Ed., 2013, 52, 10477–10481 CrossRef CAS PubMed.
- N. D. Boscher, C. S. Blackman, C. J. Carmalt, I. P. Parkin and A. G. Prieto, Appl. Surf. Sci., 2007, 253, 6041–6046 CrossRef CAS.
- J. Gao, X. Di, W. Li, L. Feng, J. Zhang, L. Wu, B. Li, W. Wang, G. Zeng and J. Yang, Thin Solid Films, 2014, 550, 638–642 CrossRef CAS.
- S. Jeong, D. Yoo, J. T. Jang, M. Kim and J. Cheon, J. Am. Chem. Soc., 2012, 134, 18233–18236 CrossRef CAS PubMed.
- C. Webb and P. M. Williams, Phys. Rev. B: Solid State, 1975, 11, 2082–2086 CrossRef CAS.
- G. Hopfengärtner, D. Borgmann, I. Rademacher, G. Wedler, E. Hums and G. W. Spitznagel, J. Electron Spectrosc. Relat. Phenom., 1993, 63, 91–116 CrossRef.
- M. Shenasa, S. Sainkar and D. Lichtman, J. Electron. Spectrosc. Relat. Phenom., 1986, 40, 329–337 CrossRef CAS.
- S. Ratha, S. R. Marri, J. N. Behera and C. S. Rout, Eur. J. Inorg. Chem., 2016, 259–265 CrossRef CAS.
- M. Jana, S. Saha, P. Samanta, N. C. Murmu, N. H. Kim, T. Kuila and J. H. Lee, Nanotechnology, 2015, 26, 75402 CrossRef PubMed.
- P. H. Jampani, O. Velikokhatnyi, K. Kadakia, D. H. Hong, S. S. Damle, J. A. Poston, A. Manivannan and P. N. Kumta, J. Mater. Chem. A, 2015, 3, 8413–8432 CAS.
- D. Aurbach, J. Electrochem. Soc., 1995, 142, 1746 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cc08035a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2017 |