Co-delivery of all-trans-retinoic acid enhances the anti-metastasis effect of albumin-bound paclitaxel nanoparticles†
Hai
Huang
a,
Hongdong
Shi
a,
Jing
Liu
b,
Yuanzeng
Min
c,
Yucai
Wang
b,
Andrew Z.
Wang
c,
Jun
Wang
b and
Yangzhong
Liu
 *a
*a
aCAS Key Laboratory of Soft Matter Chemistry, CAS High Magnetic Field Laboratory, Department of Chemistry, University of Science and Technology of China, Hefei, Anhui 230026, China. E-mail: liuyz@ustc.edu.cn
bSchool of Life Sciences, University of Science and Technology of China, China
cDepartment of Radiation Oncology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina 27599, USA
First published on 5th December 2016
Abstract
Co-delivery of all-trans-retinoic acid and paclitaxel using albumin-bound nanoparticles demonstrated a significantly improved anti-metastatic effect to breast cancer both in vitro and in vivo. Notably, the co-delivery nanoparticles exhibited more pronounced therapeutic effects than the combination of two free drugs or two HSA loaded single drugs.
Cancer metastasis is responsible for >90% of cancer related patient deaths,1 therefore, the treatment of metastatic cancer is highly desirable in clinics. Although targeted therapeutics, such as Her-2 targeting, are highly effective for the treatment of metastatic breast cancer, chemotherapeutics, including paclitaxel (PTX), are also widely used in clinics.2 Recently, nanoparticle albumin-bound paclitaxel (nab-paclitaxel, or Abraxane®) has demonstrated improved therapeutic effects and reduced toxicity when compared to paclitaxel.2,3 Nab-paclitaxel shows nearly double the response rate of standard paclitaxel (33% vs. 19%),4 however, this value is still far from optimal. Therefore, there is a strong need for the development of more effective systemic agents for metastatic breast cancer. Importantly, systemic agents should also target cancer stem cells (CSCs), which are responsible for the development of new metastatic diseases.5
Although paclitaxel is a standard treatment for metastatic breast cancer, it has been reported that paclitaxel can even increase the motility and invasion of cancer cells.5,6 On the other hand, treatment with paclitaxel also increases the proportion of CSCs whilst reducing the number of overall cancer cells; this consequence is often associated with cancer recurrence and metastasis.7 These observations could be correlated with the insufficient anti-metastatic effect of paclitaxel. Hence, reducing the drug induced cell motility and the CSCs can be a promising strategy for combating metastasis while curing cancers.
All-trans-retinoic acid (ATRA) is a cell differentiation agent with limited toxicity. ATRA has been shown to be effective in a number of different cancers, such as acute promyelocytic leukemia.8 ATRA functions through induction of differentiation pathways in CSCs, which then renders these cells susceptible to other cytotoxic therapies.9 We theorized that the combination of ATRA with PTX could inhibit metastasis in the PTX-based treatment. Indeed, ATRA was found to modulate the plasticity and inhibit the motility of breast cancer cells,10 implying its anti-metastatic properties.11 The co-delivery system could offer optimal therapeutic effects since two drugs may have different pharmacokinetics.
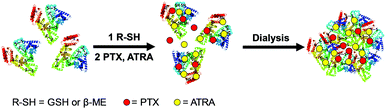
In this work, we designed a co-delivery system for delivering PTX and ATRA using human serum albumin (HSA) nanoparticles as a drug carrier. HSA nanoparticles were prepared by self-assembly of the protein using a self-cross-link strategy modified from the literature method.12 The intramolecular disulfide bonds of HSA were reduced by glutathione (GSH) or β-mercaptoethanol (β-ME) and then two hydrophobic drugs, PTX and ATRA, were loaded into the hydrophobic cavity of HSA.13 The HSA nanoparticles were generated while the formation of intermolecular disulfide bonds occurred during dialysis (Scheme 1). This method allows the formation of HSA nanoparticles without using any toxic chemicals and no external chemical modification was applied to the protein.
HSA nanoparticles were prepared in different encapsulation ratios of PTX and ATRA (Table S1, ESI†). The drug loading efficiency (DLE) was analyzed using HPLC by measuring the amounts of PTX and ATRA in the particles and in solution. The encapsulation of individual drugs showed high DLEs for both PTX (72.5%) and ATRA (95%), giving drug loading contents (DLC) of 22.5% and 27.5%, respectively. In the co-delivery system, the DLE was about 50% for PTX and 90% for ATRA, resulting in a 2–5% loading of PTX and a 19–23% loading of ATRA in the HSA nanoparticles. A higher ATRA ratio was used in this work in order to achieve the optimal therapeutic effect.
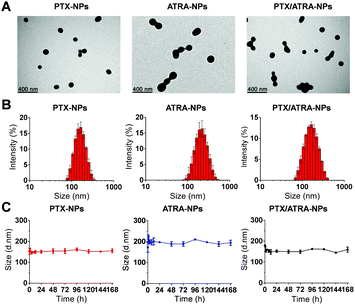
The morphology of the drug loaded HSA nanoparticles was analyzed using transmission electron microscopy (TEM). The results showed that the protein particles were nearly spherical in size and about 100–200 nm (Fig. 1A). Dynamic light scattering (DLS) measurements showed that the average hydrodynamic diameters of the nanoparticles were 164.8 ± 3.5 nm for PTX-NPs and 222.3 ± 9.7 nm for ATRA-NPs, while the co-delivery nanoparticles (PTX/ATRA-NPs) had diameters in the range of 170–185 nm (Fig. 1B). The polydispersion index (PDI, 0.08–0.22) indicated narrow distributions of the particle sizes. Time dependent DLS measurements indicated that the particle sizes did not change over one week (Fig. 1C), suggesting the high stability of HSA nanoparticles in the medium. Drug release from the hydrophobic cavity did not alter the size of the HSA NPs, and sustained drug release was observed over 72 hours (Fig. S1, ESI†).
The in vitro cytotoxicity was evaluated using an MTT assay on highly metastatic 4T1 mouse breast cancer cells. HSA encapsulation slightly enhanced the inhibitory effect of PTX and ATRA by lowering the IC50 values from 1.4 ± 0.6 μg mL−1 to 1.1 ± 0.4 μg mL−1 for PTX and from 5.6 ± 1.8 μg mL−1 to 4.0 ± 1.5 μg mL−1 for ATRA (Fig. S2A, ESI†). The mixture of two single delivery nanoparticles (PTX-NPs and ATRA-NPs) showed no statistical difference in comparison to the mixture of two free drugs, whereas the co-delivery system PTX/ATRA-NPs demonstrated a moderately higher inhibitory effect (Fig. S2B, ESI†). This observation suggests that the synergistic effects of PTX and ATRA are more pronounced in the co-delivery system than the simple mixture of two drugs.
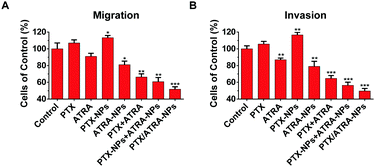
Tumor metastasis is the cumulative result of multiple mechanisms, in which the migration and invasion of tumor cells play important roles.14 To verify the inhibition of the co-delivery nanoparticles on tumor metastasis potentiality, the migration and invasion ability of 4T1 cells was analyzed using the transwell assay. A large number of cells passed through the membrane pore and reached the lower chamber in the control group, showing the high metastatic activity of 4T1 cells without treatment (Fig. S3, ESI†). Similar numbers of cells were observed in the treatment with PTX or PTX-NPs, suggesting that PTX alone or in HSA nanoparticles can hardly prevent the migration and invasion of cancer cells. The quantitative data were obtained by measuring UV absorbance at 550 nm on the migration or invasion cells. Data suggest that the treatment with PTX or PTX-NPs even slightly increased the migration and invasion ability (∼10%) of the cells (Fig. 2). This observation is in agreement with the literature which finds that paclitaxel treatment stimulated the motility of cancer cells.15 On the contrary, treatment with ATRA or ATRA-NPs lowered the migration and invasion ability by about ∼10%. Interestingly, the combination of PTX and ATRA showed a higher inhibitory effect than ATRA alone, although PTX alone can promote the migration and invasion. A more significant result was observed with the co-delivery nanoparticles, which resulted in ∼50% inhibition of migration and invasion. These data clearly indicate that the co-delivery of PTX and ATRA using HSA nanoparticles significantly reduced the ability for migration and invasion of cancer cells.
The in vivo anti-tumor and anti-metastasis effects of drug-loaded nanoparticles were assessed using the 4T1 breast tumor mice model. The drug-loaded HSA nanoparticles were administered through intravenous injection every-other-day 11 times.
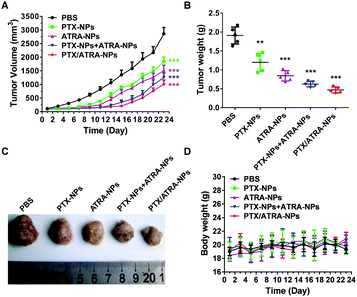
The drug efficacy and toxicity effects were evaluated by monitoring the average tumor size and body weight during the treatment. The results showed that the co-delivery nanoparticles PTX/ATRA-NPs demonstrated superior anti-tumor efficacy to all other treatments (Fig. 3A). It is worth noting that the co-delivery system inhibited the tumor growth more effectively than the mixture of two single delivery particles (PTX-NPs and ATRA-NPs) in equivalent dosages, probably due to the change of the physical properties and pharmacokinetics of two drugs in the co-delivery nanoparticles. The tumor weights and images confirmed this result (Fig. 3B and C). The body weights of the mice were very little affected in all treatments, suggesting the low toxicity of the HSA encapsulated drugs at the dosage used in this measurement (Fig. 3D).
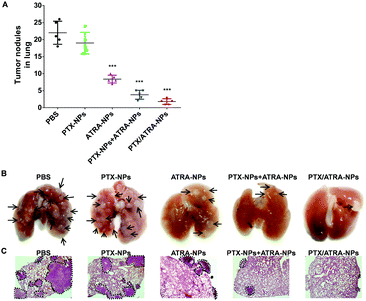
After assessing the anti-tumor efficacy of HSA encapsulated drugs, the tumor metastasis was analyzed by examining the metastatic lesions in the lungs. A number of metastatic tumor nodules were observed on the surface of the lungs in the control group, indicating the strong pulmonary metastasis of 4T1 cancer cells (Fig. 4). Treatment with PTX-NPs almost did not reduce the number of tumor nodules (Fig. 4A), which is in agreement with the literature which found that HSA encapsulated PTX has only a limited anti-metastasis effect. Treatment with the ATRA-loaded nanoparticles clearly reduced the number of metastatic tumor nodules on the lung surface, which is consistent with the in vitro transwell assay. The mixture of PTX-NPs and ATRA-NPs resulted in fewer metastatic tumor nodules, indicating the synergistic effect of PTX and ATRA in the anti-metastasis effect. Interestingly, treatment with the co-delivery nanoparticles PTX/ATRA-NPs further reduced the tumor metastasis and only very few tumor nodules can be observed on the lung. In addition to the metastatic tumor nodules observed on the lung surface, the tumor burden inside the lung tissue was analyzed with H&E staining. There were several large tumor areas in the lung in the case of treatment with PTX-NPs, whereas only a few small tumors were observed with the PTX-NPs and ATRA-NPs treatments, and nearly no tumor was observed inside the lung after treatment with the co-delivery nanoparticles (Fig. 4C). These results indicated that the co-delivery of PTX and ATRA significantly inhibited cancer metastasis in vivo.
The activity of matrix metalloproteinases (MMPs) was measured in order to understand the anti-metastasis effect of HSA encapsulated drugs. MMP is a family of zinc-dependent endopeptidases capable of degrading the extracellular matrix, which plays important roles in tumor invasion and metastasis.16 Inhibition of MMP activity is an effective method for anti-metastasis treatment.17 The gelatin zymography assay showed two bands on the gel in the control group, indicating the activation of MMP-2 (72 kD) and MMP-9 (92 kD) (Fig. S4, ESI†). Similar bands were observed on the samples treated with PTX or PTX-NPs. The brightness of the bands was marginally reduced on the ATRA and ATRA-NPs groups. The co-delivery system PTX/ATRA-NPs clearly reduced the activity of MMP-2 and MMP-9, resulting in much weaker bands relative to the treatment with single drugs or the mixture of two drugs. This observation is consistent with the results of the transwell assay and the in vivo metastasis measurements. These data highlight the inhibition of MMP activity in the prevention of cancer metastasis by the co-delivery PTX/ATRA-NPs.
In addition to the MMP activity throughout the cancer extra-cellular matrix, CSCs have recently been found to be associated with the increased incidence of cancer metastasis.7b Human breast cancer cell lines with expression of CSC markers demonstrated increased growth, adhesion, migration and invasion both in vitro and in vivo.18 Nevertheless, PTX chemotherapy often increases the ratio of CSCs while reducing the whole number of cancer cells.7a Therefore, suppressing the drug induced stemness of cancer cells is crucial for the inhibition of metastasis.
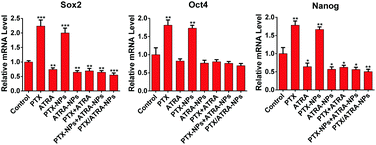
The effect of the co-delivery PTX/ATRA-NPs nanoparticles on the stemness of cancer cells was assessed by measuring the CSC-associated genes, including Sox2, Oct4 and Nanog, which were found highly expressed in breast CSCs.19 The qRT-PCR measurement showed that the expression of these genes clearly increased after treatment with PTX or PTX-NPs (Fig. 5). Although treatment with ATRA had very little influence on gene expression, the incorporation of ATRA prevented the increase of the gene expression induced by PTX. This result suggests that, unlike treatment with single PTX, the co-delivery of PTX/ATRA does not increase the stemness while killing cancer cells. This observation can be associated with the low metastasis effect of PTX/ATRA-NPs observed in the transwell assay. In addition to the in vitro outcome, the nano-delivery system could demonstrate more obvious effects in vivo due to tumor targeting, such as the EPR effect.20
In summary, we have prepared HSA-based nanoparticles for the co-delivery of a cell differentiation agent ATRA and an anticancer drug PTX, aiming to combat cancer metastasis. The co-delivery nanoparticles were formed by self-assembly of the HSA protein together with ATRA and PTX. The results show that the co-delivery of ATRA enhances the drug efficacy of PTX-HSA nanoparticles both in vitro and in vivo. More importantly, the co-delivery nanoparticles significantly inhibit the migration and invasion of cancer cells, and effectively prevent cancer metastasis in the mice model. Further investigations revealed that the activity of cancer cell MMPs, which promote cancer metastasis, is largely reduced by the co-delivery system. In addition, the co-delivery ATRA suppresses the PTX enhanced stemness of cancer cells, which could be correlated with the anti-metastatic effect of this co-delivery system. This work indicates that the co-delivery of the cell differentiation agent ATRA could effectively suppress metastasis while curing cancer. In addition, the biocompatibility of HSA nanoparticles and their facile preparation indicate the pharmaceutical potentiality of this co-delivery system.
This work was supported by the National Basic Research Program of China (973 Program, 2012CB932502), the National Science Foundation of China (U1332210, 21573213, 51503194) and the Collaborative Innovation Center of Suzhou Nano Science and Technology.
Notes and references
- (a) P. S. Steeg, Nat. Rev. Cancer, 2016, 16, 201–218 CrossRef CAS PubMed; (b) S. Valastyan and R. A. Weinberg, Cell, 2011, 147, 275–292 CrossRef CAS PubMed.
- W. J. Gradishar, Expert Opin. Pharmacother., 2006, 7, 1041–1053 CrossRef CAS PubMed.
- (a) N. Desai, V. Trieu and Z. Yao, Clin. Cancer Res., 2006, 12, 3869 CrossRef CAS PubMed; (b) E. Miele, G. P. Spinelli, E. Miele, F. Tomao and S. Tomao, Int. J. Nanomed., 2009, 4, 99–105 CrossRef CAS.
- T. Ogihara, Biol. Pharm. Bull., 2013, 36, 691 CAS.
- H. Kajiyama, K. Shibata, M. Terauchi, M. Yamashita, K. Ino, A. Nawa and F. Kikkawa, Int. J. Oncol., 2007, 31, 277–283 CAS.
- L. Volk-Draper, K. Hall, C. Griggs, S. Rajput, P. Kohio, D. DeNardo and S. Ran, Cancer Res., 2014, 74, 5421–5434 CrossRef CAS PubMed.
- (a) P. Gupta, T. Onder, G. Jiang, K. Tao, C. Kuperwasser, R. Weinberg and E. Lander, Cell, 2009, 138, 645–659 CrossRef CAS PubMed; (b) K. Sampieri and R. Fodde, Semin. Cancer Biol., 2012, 22, 187–193 CrossRef CAS PubMed.
- L. Degos, H. Dombret, C. Chomienne, M. T. Daniel, J. M. Miclea, C. Chastang, S. Castaigne and P. Fenaux, Blood, 1995, 85, 2643–2653 CAS.
- X. Tang and L. Gudas, Annu. Rev. Pathol.: Mech. Dis., 2011, 6, 345–364 CrossRef CAS PubMed.
- A. Zanetti, R. Affatato, F. Centritto, M. Fratelli, M. Kurosaki, M. M. Barzago, M. Bolis, M. Terao, E. Garattini and G. Paroni, J. Biol. Chem., 2015, 290, 17690–17709 CrossRef CAS PubMed.
- (a) M. Ryuto, S. Jimi, M. Ono, S. Naito, Y. Nakayama, Y. Yamada, S. Komiyama and M. Kuwano, Jpn. J. Cancer Res., 1997, 88, 982–991 CrossRef CAS PubMed; (b) T. Palomares, I. Garcia-Alonso, R. San Isidro, J. Mendez and A. Alonso-Varona, J. Surg. Res., 2014, 188, 143–151 CrossRef CAS PubMed.
- W. T. Wang, Y. B. Huang, S. F. Zhao, T. Shao and Y. Cheng, Chem. Commun., 2013, 49, 2234–2236 RSC.
- A. O. Elzoghby, W. M. Samy and N. A. Elgindy, J. Controlled Release, 2012, 157, 168–182 CrossRef CAS PubMed.
- J. Shen, H. Sun, P. Xu, Q. Yin, Z. Zhang, S. Wang, H. Yu and Y. Li, Biomaterials, 2013, 34, 1581–1590 CrossRef CAS PubMed.
- (a) G. Hong, Y. Jeong, S. Lee, E. Lee, J. Oh and H. Lee, Arch. Pharmacal Res., 2011, 34, 407–417 CrossRef CAS PubMed; (b) Y. Ren, X. Zhou, J. Yang, X. Liu, X. Zhao, Q. Wang, L. Han, X. Song, Z. Zhu, W. Tian, L. Zhang, M. Mei and C. Kang, Cancer Lett., 2015, 362, 174–182 CrossRef CAS PubMed.
- C. Gialeli, A. D. Theocharis and N. K. Karamanos, FEBS J., 2011, 278, 16–27 CrossRef CAS PubMed.
- Q. He, S. Guo, Z. Qian and X. Chen, Chem. Soc. Rev., 2015, 44, 6258–6286 RSC.
- A. K. Croker, D. Goodale, J. Chu, C. Postenka, B. D. Hedley, D. A. Hess and A. L. Allan, J. Cell. Mol. Med., 2009, 13, 2236–2252 CrossRef PubMed.
- (a) O. Leis, A. Eguiara, E. Lopez-Arribillaga, M. Alberdi, S. Hernandez-Garcia, K. Elorriaga, A. Pandiella, R. Rezola and A. Martin, Oncogene, 2012, 31, 1354–1365 CrossRef CAS PubMed; (b) C. Liu, Y. Lu, B. Wang, Y. Zhang, R. Zhang, Y. Lu, B. Chen, H. Xu, F. Jin and P. Lu, Ann. Surg., 2011, 253, 1165–1171 CrossRef PubMed; (c) K. Noh, B. Kim, K. Song, H. Cho, Y. Lee, J. Kim, J. Chung, J. Kim, S. Hewitt, S. Seong, C. Mao, T. Wu and T. Kim, J. Clin. Invest., 2012, 122, 4077–4093 CAS.
- (a) W. Wu, J. Wang, Z. Lin, X. Li and J. Li, Macromol. Rapid Commun., 2014, 35, 1679–1684 CrossRef CAS PubMed; (b) Q. Cheng and Y. Liu, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2016 DOI:10.1002/wnan.1410; (c) W. Wu, Q. Zhang, J. Wang, M. Chen, S. Li, Z. Lin and J. Li, Polym. Chem., 2014, 5, 5668–5679 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, drug release kinetics, cytotoxicity assay and measurement of MMPs activity. See DOI: 10.1039/c6cc08146k |
| This journal is © The Royal Society of Chemistry 2017 |