A metal-free fluorescence turn-on molecular probe for detection of nucleoside triphosphates†
Debabrata
Maity
,
Mao
Li
,
Martin
Ehlers
and
Carsten
Schmuck
 *
*
Institute for Organic Chemistry, University of Duisburg-Essen, 45117, Essen, Germany. E-mail: carsten.schmuck@uni-due.de
First published on 25th November 2016
Abstract
We report a fluorescence probe 1, which contains a naphthalimide fluorophore with two symmetric peptidic arms equipped with a tailor-made anion-binding motif, the guanidiniocarbonyl pyrrole moiety, for the detection of nucleoside triphosphates. Upon binding to nucleoside triphosphates, especially ATP, 1 shows significant turn-on fluorescence response. Probe 1 can also be applied for the imaging of ATP in cells.
The development of metal-free host molecules for the recognition of anions in water remains a demanding and ongoing challenge for chemists as a result of the high free energies of hydration of most anions.1 Nucleoside polyphosphates are one of the most targeted anionic species because of their ubiquitous presence in biological systems.2 ATP is the universal energy currency in living cells, an extracellular signalling mediator in many biological processes and also involved in DNA synthesis.3 GTP is involved in RNA synthesis, citric acid cycle and acts as an energy source for protein synthesis.4 UTP serves as a donor for energy transduction in organisms and participates in enzymatic reactions as a control element in metabolic processes.5 TTP is an essential building block in DNA replication and cell division.6 Therefore, plenty of effort has been devoted to the development of luminescent detection systems for these nucleoside polyphosphates.7 Many chemosensors bearing imidazolium or quaternary ammonium groups as the binding motif were developed as imidazolium or quaternary ammonium ions can form ionic bonds with nucleoside triphosphate group.8 Azacrown, amide and urea based chemosensors were reported as they bind to phosphate via hydrogen bonding.9 Artificial receptors for nucleoside polyphosphates detection most often contain Zn2+-complexes as a common binding motif.10 Some other metals (such as Yb3+, Ru2+, Eu3+, Tb3+, Y3+, Mn2+ and Co2+) complexes as the binding motif have also been reported as they possess coordinative unsaturated central metal ions and, therefore, have vacant coordination sites for the anions.11 However, metal-based sensors do have some disadvantages for application in living systems as such metal ions are often toxic or under suspicion to be associated with pathological disorders such as Alzheimer's disease, epilepsy and Parkinson's disease etc. Furthermore, several of the reported chemosensors are not sensitive enough towards ATP in water as sometimes they detect ATP only at high millimolar concentrations. Therefore, metal-free chemosensors which allow detecting ATP with high sensitivity and which can therefore also be used for cellular imaging are still needed.
A statistical evaluation of 3003 X-ray crystal structures of phosphate binding proteins revealed that two thirds of them do not utilize a metal ion for phosphate binding. More than half of the metal-free proteins use either lysine or an arginine moiety; especially those phosphate binding sites which are located on more solvent-accessible surfaces.12 Consequently, ammonium and guanidinium residues seem to be well suited to bind phosphate anions in natural systems even when they are exposed to the competitive influence of surrounding water molecules and other counter ions. Therefore, incorporating guanidinium moieties into artificial systems can be an excellent starting point for nucleotide binding. Till date, Anslyn et al. reported a guanidinium-based peptidic chemosensor for detection of ATP with a binding constant of Ka 3.4 × 104 M−1.13 However, it was not tested for cellular ATP detection. Peptide based chemosensors for detection of nucleotides are still rare in literature. Small cationic peptidic probes can both bind to nucleoside polyphosphates and also sometimes penetrate cells; their use for imaging of nucleotides within cells is thus attractive.
Our group has long-standing expertise in the development of artificial receptors and sensors for anionic biomolecules.14 Our idea is now to use an artificial nucleotide-binding peptide as a probe for imaging of nucleotides in cells. We present here, a naphthalimide fluorophore functionalized probe 1 with two symmetric peptidic arms which are equipped with a modified guanidinium-based anion-binding site and a tryptophan for π–π interaction with nucleobases (Scheme 1). The structure of peptidic probe 1 is very flexible enabling it to bind to ATP in tweezer-like fashion. It consists of two identical peptidic arms, each attached via its C-terminus to a central highly flexible spacer. Each arm includes a head group, a guanidiniocarbonyl pyrrole (GCP) moiety, an anion-binding site developed by our working group.15 It is very effective in binding oxoanions even under physiological conditions by means of a salt bridge strengthened by multiple hydrogen bonds. Additionally, each side chain consists of one lysine for additional charge–charge interactions. These two arms are also functionalised with tryptophan to potentially differentiate nucleoside triphosphates as they might undergo dissimilar π–π interactions with the various nucleobases. The central spacer is further tagged with a green emitting aminonaphthalimide fluorophore as reporter unit which should exhibit significant changes in fluorescence properties upon nucleotide binding. The synthesis of probe 1 was carried out by means of microwave-assisted solid-phase peptide synthesis.
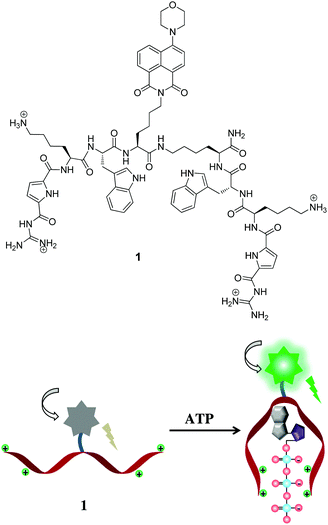 | ||
| Scheme 1 Top: Molecular structure of peptidic probe 1. Bottom: Schematic representation for detection of ATP. | ||
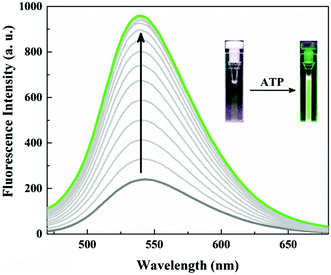
Probe 1 (20.0 μM) unexpectedly showed only a weak fluorescence emission at 540 nm in neutral aqueous conditions (10 mM HEPES, pH 7.4). Obviously the fluorescence of the naphthalimide in probe 1 is somehow quenched. A remarkably large fluorescence enhancement was observed as shown in Fig. 1, when ATP was added to the solution of probe 1. The fluorescence intensity at 540 nm increased by more than 4-fold upon addition of 10 μM ATP, and the plot of the fluorescence intensity change (I/I0) showed typical saturation behaviour. The binding constant was calculated to be 2.2 × 105 M−1 for ATP which is one order of magnitude larger than for the Anslyn peptide. A fluorescence Job plot between 1 and ATP confirmed a binding stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S1, ESI†). The photograph of the turn-on fluorescence of probe 1 upon binding to ATP clearly demonstrated the naked eye detection of ATP using this peptidic probe (Fig. 1). This excellent property of 1 as a fluorescent probe allowed us to sense submicromolar concentration of ATP (Fig. S2, ESI†).
1 (Fig. S1, ESI†). The photograph of the turn-on fluorescence of probe 1 upon binding to ATP clearly demonstrated the naked eye detection of ATP using this peptidic probe (Fig. 1). This excellent property of 1 as a fluorescent probe allowed us to sense submicromolar concentration of ATP (Fig. S2, ESI†).
The fluorescence selectivity of probe 1 towards different type of biologically relevant anions was also evaluated by fluorescence titration experiments. Table 1 summarizes the binding constant and increase in emission intensity ratio (I/I0 at 540 nm) obtained under neutral aqueous conditions. Probe 1 showed strong binding affinity (in the order of 105 M−1) towards all nucleoside triphosphates (GTP, CTP, TTP and UTP) and also pyrophosphates. However, the changes in fluorescence intensity are quite different (vide infra). Nucleoside diphosphates (ADP, UDP) showed binding affinities in the order of 104 M−1. The fluorescence enhancement by these polyphosphates was found to be in the order ATP > GTP > UTP > CTP > TTP > PPi ≫ ADP > UDP. Fig. 2 shows differential fluorescence responses of different nucleotides towards probe 1. In contrast, the fluorescence change was not induced by monophosphorylated species such as HPO42−, c-AMP, AMP, CMP, GMP, UMP, TBAP, MMP or by other anions (SO42−, NO3−, HCO3−, CH3COO−). These results suggest that probe 1 is a useful probe for the fluorescence detection of nucleoside triphosphates and pyrophosphate. The distinguishability of ATP from ADP and AMP is also important, since ATP is made from ADP or AMP, and its use in metabolism converts it back into these precursors. The ratio of ATP to ADP in cells is an important modulator for a variety of cellular events.12
| Anion species | K (M−1) | I/I0 |
|---|---|---|
| ATP | 2.2 × 105 | 4.1 |
| ADP | 3.8 × 104 | 1.3 |
| AMP | Not determined | No change |
| GTP | 2.2 × 105 | 3.2 |
| CTP | 1.5 × 105 | 2.5 |
| TTP | 1.8 × 105 | 2.2 |
| UTP | 2.2 × 105 | 2.7 |
| PPi | 1 × 105 | 2 |
| UDP | 3.6 × 104 | 1.2 |
| HPO42− | Not determined | No change |
| c-AMP | Not determined | No change |
| CMP | Not determined | No change |
| GMP | Not determined | No change |
| UMP | Not determined | No change |
| TBAP | Not determined | No change |
| MMP | Not determined | No change |
| SO42− | Not determined | No change |
| NO3− | Not determined | No change |
| HCO3− | Not determined | No change |
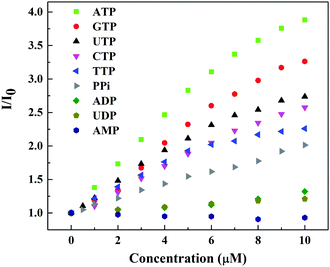 | ||
| Fig. 2 Fluorescence response curve of probe 1 (20.0 μM) (λex = 410 nm) with increasing concentration of different nucleotides in 10 mM HEPES buffer, pH = 7.4. | ||
Addition of ADP to probe 1 displayed only 1.3 fold increase in emission intensity. AMP failed to induce any significant changes in emissions. The ratio for ATP (I/I0) is much larger than that for ADP, which is good enough to discriminate ATP from ADP. Thus, probe 1 can monitor the exact level of ATP free from hindrance of ADP and AMP.
To gain insight into the polyphosphate binding induced fluorescence enhancement phenomena of probe 1, we performed pH dependent fluorescence study (Fig. S10, ESI†). Whereas there was no change in emission intensity of probe 1 at acidic and neutral pH, the fluorescence of a solution of probe 1 was increased in basic solutions above pH 9. We have also checked the fluorescence property of a naphthalimide fluorophore precursor with tryptophan methyl ester and a TREN(GCP)3 compound separately (Fig. S12, ESI†). Tryptophan showed no influence on fluorescence intensity of the fluorophore but addition of TREN(GCP)3 increases it. In general naphthalimide is an environment sensitive fluorophore, which is weakly fluorescent in polar media but becomes highly fluorescent in non-polar hydrophobic media. In agreement with this, cationic probe 1 is weakly fluorescent at acidic and neutral pH. Due to the overall cationic nature most likely probe 1 adopts a rather open and extended conformation exposing the fluorophore to the aqueous medium with subsequent fluorescence quenching. Under basic conditions probe 1 is overall less-polar, adopting most likely a more compact folded conformation with increased fluorescent. Binding of a nucleotide triphosphate to the probe 1 has the same effect. The net charge in the complex is reduced, the conformation becomes more compact (see Scheme 1) and overall the microenvironment around the fluorophore less-polar (similar to the addition of TREN(GCP)3). Thus the fluorescence of the naphthalimide moiety increases.
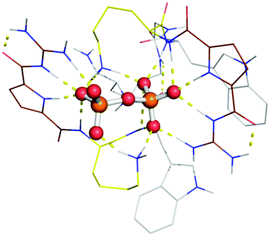
Molecular modelling was carried out for a simplified version of probe 1 (without the naphthalimide group) and pyrophosphate (PPi) as substrate (Fig. 3). Within the energy minimized complex, the peptide binds tightly to the negatively charged oxygen atoms of the PPi by ion pair interactions with both our tailor-made GCP moiety as with ammonium groups of lysine. In addition several hydrogen bonds are formed between probe 1 and PPi. The observed larger fluorescence of triphosphates over di- or monophosphates may be ascribed to the difference in electrostatic interaction with GCP and lysine due to their different anionic charges. The unequal fluorescence response of different nucleoside triphosphates (ATP, GTP, CTP, UTP and TTP) is most likely due to differential interactions of the nucleobases with the tryptophan residues and naphthalimide fluorophore which varies the hydrophobic microenvironment around the fluorophore depending on the hydrophobic nature of the nucleobase (A > G > C, T, U > PPi).
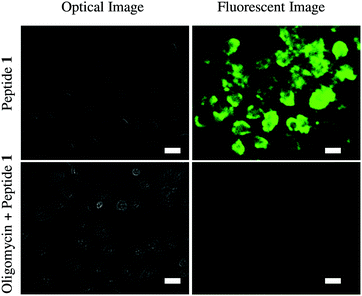
Finally, we have tested the application of probe 1 in cultured HeLa cells using fluorescence microscopy (Fig. 4). Cellular ATP concentration is normally in the millimolar range. Oligomycin generally inhibits ATP synthesis and decreases cellular ATP levels. Therefore, one set of HeLa cells was treated with 10 μM oligomycin for 30 min and the other set was not. Then the cells were exposed to probe 1 (50 μM) for 5 min and then washed. Microscopy showed probe 1 successfully imaged ATP in the cells. Probe 1 clearly displayed green fluorescence in the normal HeLa cells, whereas only a very weak fluorescence was observed in the oligomycin induced cells due to the lower concentration of ATP in those cells.
These results clearly indicate that probe 1 is cell membrane permeable and able to detect intracellular ATP. Cytotoxicity of probe 1 toward HeLa cell was measured using a standard alamar blue assay (Fig. S14, ESI†). At concentrations of 10–50 μM, cell viabilities were found to be high (70%) after incubation for 24 h suggesting low toxicity of probe 1.
In conclusion, we have presented here a metal-free peptidic fluorescent turn-on probe 1 which allows detection of nucleotide triphosphates. The key feature of this probe is a tailor-made anion binding site, the GCP moiety incorporated into the peptide arms of 1. This group serves as an efficient binding site for the triphosphates of the nucleotides allowing ATP binding with submicromolar affinity in water. This binding increases the fluorescence of the naphthalimide fluorophore enabling the use of 1 as a fluorescence turn-on sensor. The utility of 1 as a bioanalytical molecular tool has also been demonstrated by fluorescence imaging of ATP in living cells.
Notes and references
- (a) C. Schmuck and H. Wennemers, Highlights in Bioorganic Chemistry: Methods and Applications, Wiley-VCH, Weinheim, 2002 Search PubMed; (b) D. Maity and C. Schmuck, Synthetic Receptors for Amino Acids and Peptides, Royal Society of Chemistry, 2015, vol. 8, pp. 326–368 Search PubMed.
- D. Voet, J. G. Voet and C. W. Pratt, Fundamentals of Biochemistry, Wiley-VCH, Weinheim, 2002 Search PubMed.
- (a) J. R. Knowles, Annu. Rev. Biochem., 1980, 49, 877–919 CrossRef CAS PubMed; (b) F. M. Ashcroft and F. M. Gribble, Diabetologia, 1999, 42, 903–919 CrossRef CAS PubMed; (c) P. B. Dennis, A. Jaeschke, M. Saitoh, B. Fowler, S. C. Kozma and G. Thomas, Science, 2001, 294, 1102–1105 CrossRef CAS PubMed.
- B. Alberts, A. Johnson, J. Lewis, M. Raff, K. Roberts and P. Walter, Molecular Biology of the Cell, Garland Science, New York, 2002 Search PubMed.
- D. Lecca and S. Ceruti, Biochem. Pharmacol., 2008, 75, 1869–1881 CrossRef CAS PubMed.
- S. Pullarkat, J. Stoehlmacher, V. Ghaderi, Y. P. Xiong, S. A. Ingles, A. Sherrod, R. Warren, D. Tsao-Wei, S. Groshen and H. J. Lenz, Pharmacogenomics J., 2001, 1, 65–70 CrossRef CAS PubMed.
- (a) Y. Zhou, Z. Xu and J. Yoon, Chem. Soc. Rev., 2011, 40, 2222–2235 RSC; (b) A. E. Hargrove, S. Nieto, T. Zhang, J. L. Sessler and E. V. Anslyn, Chem. Rev., 2011, 111, 6603–6782 CrossRef CAS PubMed.
- (a) H. Abe, Y. Mawatari, H. Teraoka, K. Fujimoto and M. Inouye, J. Org. Chem., 2004, 69, 495–504 CrossRef CAS PubMed; (b) Z. Xu, N. J. Singh, J. Lim, J. Pan, H. N. Kim, S. Park, K. S. Kim and J. Yoon, J. Am. Chem. Soc., 2009, 131, 15528–15533 CrossRef CAS PubMed; (c) S. Atilgan and E. U. Akkaya, Tetrahedron Lett., 2004, 45, 9269–9271 CrossRef CAS; (d) P. P. Neelakandan, M. Hariharan and D. Ramaiah, Org. Lett., 2005, 7, 5765–5768 CrossRef CAS PubMed; (e) G. Zong, L. Xian and G. Lu, Tetrahedron Lett., 2007, 48, 3891–3894 CrossRef CAS; (f) Z. Kejík, K. Záruba, D. Michalík, J. Šebek, J. Dian, S. Pataridis, K. Volka and V. Král, Chem. Commun., 2006, 1533–1535 RSC; (g) J. Y. Kwon, N. J. Singh, H. N. Kim, S. K. Kim, K. S. Kim and J. Yoon, J. Am. Chem. Soc., 2004, 126, 8892–8893 CrossRef CAS PubMed; (h) S. K. Kim, B.-S. Moon, J. H. Park, Y. I. Seo, H. S. Koh, Y. J. Yoon, K. D. Lee and J. Yoon, Tetrahedron Lett., 2005, 46, 6617–6620 CrossRef CAS; (i) S. Wang and Y.-T. Chang, J. Am. Chem. Soc., 2006, 128, 10380–10381 CrossRef CAS PubMed; (j) P. P. Neelakandan, M. Hariharan and D. Ramaiah, J. Am. Chem. Soc., 2006, 128, 11334–11335 CrossRef CAS PubMed; (k) H. N. Kim, J. H. Moon, S. K. Kim, J. Y. Kwon, Y. J. Jang, J. Y. Lee and J. Yoon, J. Org. Chem., 2011, 76, 3805–3811 CrossRef CAS PubMed; (l) X. Li, X. Guo, L. Cao, Z. Xun, S. Wang, S. Li, Y. Li and G. Yang, Angew. Chem., Int. Ed., 2014, 126, 7943–7947 CrossRef; (m) C. Li, M. Numata, M. Takeuchi and S. Shinkai, Angew. Chem., Int. Ed., 2005, 44, 6371–6374 CrossRef CAS PubMed.
- (a) C. Bazzicalupi, S. Biagini, A. Bencini, E. Faggi, C. Giorgi, I. Matera and B. Valtancoli, Chem. Commun., 2006, 4087–4089 RSC; (b) C. Bazzicalupi, A. Bencini, S. Biagini, E. Faggi, S. Meini, C. Giorgi, A. Spepi and B. Valtancoli, J. Org. Chem., 2009, 74, 7349–7363 CrossRef CAS PubMed; (c) G. V. Zyryanov, M. A. Palacios and P. Anzenbacher, Jr., Angew. Chem., Int. Ed., 2007, 46, 7849–7852 CrossRef CAS PubMed; (d) H. Wang and W.-H. Chan, Org. Biomol. Chem., 2008, 6, 162–168 RSC.
- (a) A. Ojida, H. Nonaka, Y. Miyahara, S. Tamaru, K. Sada and I. Hamachi, Angew. Chem., Int. Ed., 2006, 45, 5518–5521 CrossRef CAS PubMed; (b) A. Ojida, I. Takashima, T. Kohira, H. Nonaka and I. Hamachi, J. Am. Chem. Soc., 2008, 130, 12095–12101 CrossRef CAS PubMed; (c) T. Sakamoto, A. Ojida and I. Hamachi, Chem. Commun., 2009, 141–152 RSC; (d) H. T. Ngo, X. Liu and K. A. Jolliffe, Chem. Soc. Rev., 2012, 41, 4928–4965 RSC; (e) E. J. O’Neil and B. D. Smith, Coord. Chem. Rev., 2006, 250, 3068–3080 CrossRef; (f) Y. Kurishita, T. Kohira, A. Ojida and I. Hamachi, J. Am. Chem. Soc., 2010, 132, 13290–13299 CrossRef CAS PubMed; (g) A. Ojida, Y. Miyahara, J. Wongkongkatep, S. Tamaru, K. Sada and I. Hamachi, Chem. – Asian J., 2006, 1, 555–563 CrossRef CAS PubMed.
- (a) E. A. Weitz, J. Y. Chang, A. H. Rosenfield and V. C. Pierre, J. Am. Chem. Soc., 2012, 134, 16099–16102 CrossRef CAS PubMed; (b) S. Li, W. Yuan, C. Zhu and J. Xu, Anal. Biochem., 2004, 331, 235–242 CrossRef CAS PubMed; (c) K. M. K. Swamy, S. K. Kwon, H. N. Lee, S. M. S. Kumar, J. S. Kim and J. Yoon, Tetrahedron Lett., 2007, 48, 8683–8686 CrossRef CAS; (d) H. Wu, C. He, Z. Lin, Y. Liu and C. Duan, Inorg. Chem., 2009, 48, 408–410 CrossRef CAS PubMed.
- A. K. H. Hirsch, F. R. Fischer and F. Diederich, Angew. Chem., Int. Ed., 2007, 46, 338–352 CrossRef CAS PubMed.
- S. E. Schneider, S. N. O’Neil and E. V. Anslyn, J. Am. Chem. Soc., 2000, 122, 542–543 CrossRef CAS.
- (a) H. Y. Kuchelmeister and C. Schmuck, Chem. – Eur. J., 2011, 17, 5311–5318 CrossRef CAS PubMed; (b) J. Wu, Y. Zou, C. Li, W. Sicking, I. Piantanida, T. Yi and C. Schmuck, J. Am. Chem. Soc., 2012, 134, 1958–1961 CrossRef CAS PubMed; (c) D. Maity, J. Jiang, M. Ehlers, J. Wu and C. Schmuck, Chem. Commun., 2016, 52, 6134–6137 RSC; (d) D. Maity and C. Schmuck, Chem. – Eur. J., 2016, 22, 13156–13161 CrossRef CAS PubMed; (e) J. Wu, A. Zawistowski, M. Ehrmann, T. Yi and C. Schmuck, J. Am. Chem. Soc., 2011, 133, 9720–9723 CrossRef CAS PubMed.
- (a) C. Schmuck, Chem. – Eur. J., 2000, 6, 709–718 CrossRef CAS PubMed; (b) C. Schmuck, M. Heil, K. Baumann and J. Scheiber, Angew. Chem., Int. Ed., 2005, 44, 7208–7212 CrossRef CAS PubMed; (c) C. Schmuck and L. Geiger, Chem. Commun., 2005, 772–774 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6cc08386b |
| This journal is © The Royal Society of Chemistry 2017 |