Multiphase transition of supramolecular metallogels triggered by temperature†
Bo
Jiang
*,
Li-Jun
Chen
,
Guang-Qiang
Yin
,
Yu-Xuan
Wang
,
Wei
Zheng
,
Lin
Xu
and
Hai-Bo
Yang
*
Shanghai Key Laboratory of Green Chemistry and Chemical Processes, School of Chemistry and Molecular Engineering, East China Normal University, 3663 N. Zhongshan Road, Shanghai, China. E-mail: hbyang@chem.ecnu.edu.cn; 51113300008@ecnu.cn
First published on 25th November 2016
Abstract
We present a new family of supramolecular metallogels undergoing reversible multiphase transition triggered by temperature from amphiphilic alkynylplatinum(II) complexes. Further investigation revealed that intermolecular Pt⋯Pt and π–π stacking interactions facilitate such phase transitions upon increasing the temperature.
Multiphase transition is one of the most fascinating phenomena in nature.1 The most familiar example in daily life is the multiphase transition of water between water vapor, liquid water, and ice. In this case, temperature serves as the main control parameter to determine the phase of the system by altering the strength of intermolecular hydrogen bonds.1a Moreover, in biological systems, proteins can also undergo multiphase transition in response to the stimulus of the external environment, which has proven to play an important role during many important biological processes.1c Notably, mimicking multiphase transition behavior in nature not only promotes a deeper understanding of the self-assembly process in biological systems but also provides a promising methodology for the construction of intelligent materials with broad applications in the field of self-healing materials, multiphase catalysis, and regenerative medicine.2 Thus the investigation of artificial multiphase species has received extensive attention in the past few years.2
Supramolecular gels that exhibit stimuli-responsive behavior have proven to be promising candidates to be multiphase transition systems because of the dynamic nature of non-covalent interactions. However, although a great number of supramolecular gels have been explored, only two phase transitions between a solution and a gel was achieved in most known systems.3–5 Recently, Schneider and co-workers have successfully realized a multiphase transitioning peptide hydrogel.6 However, this system is still limited to multiple stimuli to achieve such a switching process. To the best of our knowledge, supramolecular gels that display multiphase transition triggered by a single stimulus have not been reported. The development of supramolecular gels with multiphase transition behavior induced by a single stimulus is still challenging because it is very difficult to fine tune the change in strength of non-covalent interactions through a single stimulus.
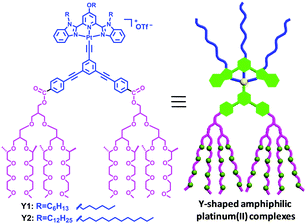
Amphiphilic transition metal complexes have attracted much attention due to their ability to generate highly ordered nanostructures and their tendency to form metal–metal interaction.7,8 Herein, we report a new class of Y-shaped amphiphilic alkynylplatinum(II) bzimpy complexes (Scheme 1), from which supramolecular metallogels with multiphase transition behavior were obtained at room temperature without a heating–cooling process. The resultant metallogels could undergo solution-to-metallogel-to-solid transitions for Y1 or metallogel(I)-to-metallogel(II)-to-solid transitions for Y2 triggered by a change in temperature. Moreover, such phase transition processes for both Y1 and Y2 were completely reversible. Further investigation revealed that intermolecular Pt⋯Pt and π–π stacking interactions play a pivotal role in the self-assembly of amphiphilic platinum(II) complexes, which facilitate the phase transitions upon increasing the temperature. Finally, such a metallogel was successfully utilized as a carrier matrix for the controlled release of a hydrophilic fluorescent molecule at physiological temperature.
Y-shaped amphiphilic platinum(II) complexes Y1 and Y2 were successfully synthesized according to the procedure shown in the ESI† (Schemes S2 and S3). The gelation property was firstly investigated using the “stable-to-inversion-of-test-tube” method due to the presence of multiple non-covalent interactions of complexes Y1 and Y2. Interestingly, it was found that both Y1 and Y2 were able to transform into stable, light red metallogels in a mixed solvent of acetone and water at room temperature without the heating–cooling process. Their critical gelation concentrations (CGCs) were determined to be 20 mg mL−1 for Y1 in the mixed solvent of acetone and water (v/v, 1/30) and 5.5 mg mL−1 for Y2 in the mixed solvent of acetone and water (v/v, 1/4). Moreover, the generation of both metallogels Y1 and Y2 was further confirmed by rheological investigation at 25 °C. As shown in Fig. S15 (ESI†), it was found that the storage modulus (G′) was higher than the loss modulus (G′′) across the whole range of frequencies studied, which revealed a typical solid-like gel nature. Further investigation revealed that both metallogels Y1 and Y2 exhibited multiphase transition behavior triggered by the change in temperature (Fig. 1).
 | ||
| Fig. 1 Photographs of the multiphase transition of metallogels (a) Y1 and (b) Y2 induced by a change in temperature. | ||
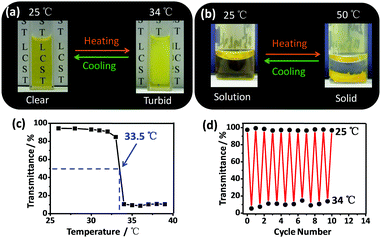
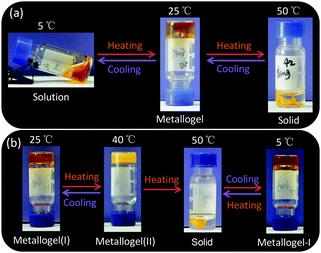
Fig. 1a depicts the temperature-controlled phase transition process of metallogel Y1. At low temperature (5 °C), Y1 (6.4 mM) was dissolved in the mixed solvent of acetone and water (v/v, 1/30) to give a free-flowing red translucent solution. Then, heating the solution to 25 °C resulted in the formation of a free-standing metallogel. Unexpectedly, further heating the metallogel to 50 °C led to the remarkable shrinkage of the metallogel into a small-volume collapsed solid and the solvents encapsulated in the metallogel were expelled from the original gels. Interestingly, the solid structure was re-swelled by cooling the system to 25 °C and the free-standing metallogel was re-formed again after 36 h. The solution–metallogel–solid transitions were fully reversible and could be repeated several times. Notably, the temperature-controlled phase transitions between three states presented in this study were significantly different from those in traditional gels that usually show a typical gel-to-sol transition upon increasing the temperature.3–5
Metallogel Y2 exhibited somewhat different phase transition behavior compared with metallogel Y1. For example, metallogel Y2 was stable at both low temperature and room temperature (referred to as metallogel(I)), which might be attributed to the stronger gelation ability due to the existence of longer alkyl chains. However, upon heating metallogel Y2 to 40 °C, the light red translucent metallogel became turbid, which is referred to as metallogel(II) (Fig. 1b). Further increasing the temperature up to 50 °C led to the remarkable shrinkage of the metallogel into a small-volume collapsed solid and the solvents were expelled from complex Y2. Similarly, the metallogel(I)-to-metallogel(II)-to-solid transitions were reversible and could be repeated at least three times.
Such a unique temperature-controlled multiphase transition process was further studied by using SEM techniques. The SEM images of xerogels displayed a tenuous fibrillar structure for Y1 and intertwined three-dimensional networks of the fibrillar structure for Y2 at room temperature (Fig. S20 and S24, ESI†), which again proved that complex Y2 features stronger gelation ability with higher stability than Y1 because of the higher cross-linking degree. After being heated to 50 °C, the solid samples were taken out and dried on a SiO2/Si substrate under vacuum. Notably, the SEM images of the solid samples for both Y1 and Y2 disclosed dense aggregation morphology (Fig. S21 and S25, ESI†), which might have resulted from the fusion of fibrillar structures due to the enhancement of intermolecular Pt⋯Pt and π–π stacking interactions as well as the hydrophobic effect at high temperature.
In order to further illustrate such reversible multiphase transition behavior, the thermo-responsive behaviors of complexes Y1 and Y2 were investigated in aqueous solution. Interestingly, both Y1 and Y2 exhibited LCST behavior in aqueous media. As shown in Fig. 2a, the clear aqueous solution of Y1 became turbid at 34 °C, and then rapidly became clear again when the solution was cooled to room temperature. Moreover, such a thermo-responsive process could be repeated more than ten times (Fig. 2d). The LCST behavior of Y1 was attributed to the dehydration of oligoether dendritic chains at temperatures above the clouding point (Tcloud) because of the breakup of hydrogen bonding between the ethylene oxide chains and water molecules.9Tcloud was determined through the change in transmittance of Y1 at 551 nm on a temperature-controlled UV-vis spectrometer. As depicted in Fig. 2c, Tcloud of Y1 (3.2 × 10−4 M) was found to be 33.5 °C in aqueous solution. Similar LCST behavior was also observed for complex Y2 in water with Tcloud being determined to be 37 °C (Fig. S12, ESI†).
More interestingly, Y1 exhibited temperature-induced macroscopic aggregation behavior in aqueous solution when the concentration was increased to 1.3 mM (Fig. 2b). The clear yellow solution was able to transform into a small-volume yellow solid at the bottom of the vial when the temperature was increased up to 50 °C. At the same time, the color of solution changed from yellow to colorless, indicating that almost no complex Y1 was dissolved in water. Subsequently, the solid was re-swelled and became the clear yellow solution again after standing at room temperature for 2 h. Such a reversible solution–solid phase transition could be repeated many times.
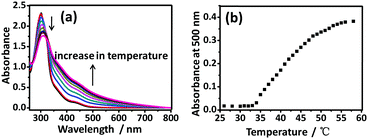
In order to investigate the driving forces for such a thermo-responsive multiphase transition, temperature-dependent UV-vis absorption studies for both Y1 and Y2 were performed in aqueous solution. As shown in Fig. 3a, the UV-vis absorption spectra of Y1 (3.2 × 10−4 M) in aqueous solution exhibited a decrease of absorption band at 300 nm with a concomitant growth of the absorption tail at λ > 500 nm. This change was assignable to the metal–metal-to-ligand charge transfer (MMLCT) [dσ*(Pt) → π*(bzimpy)] transition that was attributed to the intermolecular Pt⋯Pt and π–π stacking interactions.7 The plot of absorbance against temperature showed that the absorbance at 500 nm remained unchanged from 26 °C to 33 °C while a drastic increase was observed when the temperature was increased from 34 °C to 55 °C, which was in good agreement with Tcloud (Fig. 3b). Further increasing the temperature did not give rise to a significant change in the absorbance. It was worthwhile to note that the enhancement of the MMLCT absorption band with the increase of temperature was completely opposite to the commonly observed finding that Pt⋯Pt and π–π stacking interactions are usually disrupted at the elevated temperature.8 Such abnormal thermo-responsive behavior suggested that the Pt centers of Y1 were packed more closely at high temperature, which was in good agreement with the temperature-induced shrinkage behavior in aqueous solution. Similar spectroscopic changes were also observed for complex Y2 in aqueous solution (Fig. S7, ESI†). Additional experiments of PXRD and concentration-dependent 1H NMR were carried out to support the above-mentioned conclusion (Fig. S13 and S14, ESI†). Furthermore, the SEM images of Y1 and Y2 in aqueous solution exhibited irregular blocky structures at room temperature (Fig. S18 and S22, ESI†). Upon increasing the temperature up to 50 °C, ordered cross-linked nanostructures were observed for both Y1 and Y2 (Fig. S19 and S23, ESI†). The formation of such ordered cross-linked aggregation morphology suggested that stronger intermolecular interactions (e.g. Pt⋯Pt and π–π stacking interactions) existed in aqueous solution at high temperature, which was consistent with the results obtained from the spectroscopic studies.
Based on these experimental results, the possible process for such a temperature-controlled multiphase transition was proposed as indicated in Scheme 2. At room temperature, the metallogels were formed through the cross-linking of flexible nanofibers driven by the hydrophobic effect and weak intermolecular Pt⋯Pt and π–π stacking interactions. Above the LCST, the hydrophilic oligoether dendritic chains were dehydrated and self-assembled into molecular globules due to the collapse of hydrogen bonding between the ethylene oxide chains and water molecules. The shrinkage of hydrophilic dendritic chains resulted in the decrease of steric repulsion between the oligoether dendritic chains and the enhanced hydrophobic environment. At the same time, the hydrophobic alkynylplatinum(II) bzimpy moieties might be exposed to the water. To reduce this unfavorable interaction, the alkynylplatinum(II) bzimpy moieties would be packed more closely, thus leading to enhanced aggregation through Pt⋯Pt and π–π stacking interactions. Therefore, the metallogels were shrunk and transformed into the small-volume solid. At low temperature again, the three-dimensional networks of the fibrillar structure were re-formed due to the recovery of hydrogen bonding between ethylene oxide chains and water molecules.
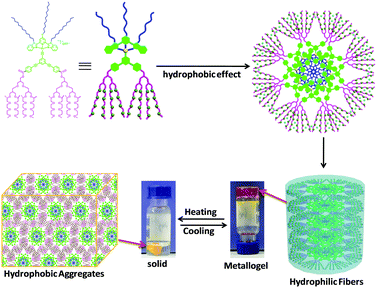 | ||
| Scheme 2 Proposed process for the thermally induced reversible shrinkage/swelling behavior of amphiphilic platinum(II) complex Y2. | ||
By taking advantage of such a phase transition, the temperature controlled release of a fluorescent dye molecule was investigated. Metallogel Y2 was selected as the carrier matrix because of the stronger gelation ability. Water-soluble fluorescein sodium was firstly entrapped in the metallogel during the gel preparation process. SEM images showed that the three-dimensional networks of fibrous structures were maintained even after the addition of guest molecules (Fig. S26, ESI†). Moreover, the metallogel exhibited green fluorescence under a 365 nm UV lamp, implying that fluorescein sodium was successfully encapsulated in the metallogel at room temperature (Fig. S11, ESI†). Interestingly, the water-soluble fluorescein sodium was released concurrently with the shrinkage of the metallogel at 50 °C. The color of the solution became green at the same time. More importantly, fluorescein sodium could be entrapped again along with the re-formation of the metallogel after 48 h at 5 °C. The temperature controlled release of fluorescein sodium was quantitatively determined by using UV-vis spectroscopy. As shown in Fig. 4, there was almost no release of dye within 1.0 h at 20 °C that was lower than the LCST. However, the fast release of fluorescein sodium up to around 70% within 5 min was observed at 37 °C due to the shrinkage of the metallogel. Further extension of time up to 1.0 h did not give rise to any significant change in the absorbance. This result clearly indicated that the present metallogel can be used as a carrier matrix for the controlled release of a fluorescent molecule at physiological temperature.
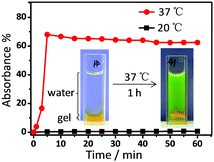 | ||
| Fig. 4 Time courses of the release of the fluorescein sodium substrate from metallogel Y2 at 20 °C and 37 °C. | ||
In summary, we have demonstrated that the rational design of a molecular structure could realize artificial multiphase transition systems induced by temperature. By taking advantage of temperature-controlled Pt⋯Pt and π–π interactions, a new class of self-assembled alkynylplatinum(II) metallogels, which displayed reversible temperature-controlled multiphase transition behavior, were successfully obtained at room temperature without the heating–cooling process. Notably, the multiphase transition metallogels presented in this study were significantly different from the traditional gels that usually show a typical gel-to-sol transition upon increasing the temperature. Moreover, by taking advantage of the phase transition behavior of the metallogel, controlled release of fluorescein sodium at physiological temperature was successfully realized. This study not only represents one of the very few successful examples of supramolecular metallogels undergoing multiphase transition behavior triggered by temperature but also provides new insight into the design of intelligent functional materials.
This work was financially supported by the NSFC/China (No. 21322206) and SSTC (No. 16XD1401000).
Notes and references
- (a) S. C. Weber and C. P. Brangwynne, Cell, 2012, 149, 1188 CrossRef CAS PubMed; (b) L. L. Goff and T. Lecuit, Science, 2009, 324, 1654 CrossRef PubMed; (c) P. G. Vekilov, Soft Matter, 2010, 6, 5254 RSC.
- (a) D. Mozhdehi, S. Ayala, O. R. Cromwell and Z. Guan, J. Am. Chem. Soc., 2014, 136, 16128 CrossRef CAS PubMed; (b) J. Huang, F. Cheng, B. P. Binks and H. Yang, J. Am. Chem. Soc., 2015, 137, 15015 CrossRef CAS PubMed; (c) P. Koria, H. Yagi, Y. Kitagawa, Z. Megeed, Y. Nahmias, R. Sheridan and M. L. Yarmush, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 1034 CrossRef CAS PubMed.
- (a) D. K. Kumar and J. W. Steed, Chem. Soc. Rev., 2014, 43, 2080 RSC; (b) A. Y.-Y. Tam and V. W.-W. Yam, Chem. Soc. Rev., 2013, 42, 1540 RSC; (c) X. Yu, L. Chen, M. Zhang and T. Yi, Chem. Soc. Rev., 2014, 43, 5346 RSC; (d) X. Du, J. Zhou, J. Shi and B. Xu, Chem. Rev., 2015, 115, 13165 CrossRef CAS PubMed.
- (a) Z.-Y. Li, Y. Zhang, C.-W. Zhang, L.-J. Chen, C. Wang, H. Tan, Y. Yu, X. Li and H.-B. Yang, J. Am. Chem. Soc., 2014, 136, 8577 CrossRef CAS PubMed; (b) G.-Z. Zhao, L.-J. Chen, W. Wang, J. Zhang, G. Yang, D.-X. Wang, Y. Yu and H.-B. Yang, Chem. – Eur. J., 2013, 19, 10094 CrossRef CAS PubMed; (c) W. Zheng, L.-J. Chen, G. Yang, B. Sun, X. Wang, B. Jiang, G.-Q. Yin, L. Zhang, X. Li, M. Liu, G. Chen and H.-B. Yang, J. Am. Chem. Soc., 2016, 138, 4927 CrossRef CAS PubMed; (d) N.-W. Wu, L.-J. Chen, C. Wang, Y.-Y. Ren, X. Li, L. Xu and H.-B. Yang, Chem. Commun., 2014, 50, 4231 RSC.
- (a) L. Meazza, J. A. Foster, K. Fucke, P. Metrangolo, G. Resnati and J. W. Steed, Nat. Chem., 2013, 5, 42 CrossRef CAS PubMed; (b) G. O. Lloyd and J. W. Steed, Nat. Chem., 2009, 1, 437 CrossRef CAS PubMed; (c) G. M. Peters, L. P. Skala and J. T. Davis, J. Am. Chem. Soc., 2016, 138, 134 CrossRef CAS PubMed; (d) G. M. Peters, L. P. Skala, T. N. Plank, H. Oh, G. N. M. Reddy, A. Marsh, S. P. Brown, S. R. Raghavan and J. T. Davis, J. Am. Chem. Soc., 2015, 137, 5819 CrossRef CAS PubMed; (e) T. Cardolaccia, Y. Li and K. S. Schanze, J. Am. Chem. Soc., 2008, 130, 2535 CrossRef CAS PubMed; (f) W. Fang, C. Liu, J. Chen, Z. Lu, Z.-M. Li, X. Bao and T. Tu, Chem. Commun., 2015, 51, 4267 RSC; (g) W. Fang, C. Liu, Z. Liu, Z. Sun and T. Tu, Chem. Commun., 2014, 50, 10118 RSC; (h) Z. Shen, Y. Jiang, T. Wang and M. Liu, J. Am. Chem. Soc., 2015, 137, 16109 CrossRef CAS PubMed; (i) Z. Shen, T. Wang and M. Liu, Angew. Chem., Int. Ed., 2014, 53, 13424 CrossRef CAS PubMed; (j) H. Yamaguchi, Y. Kobayashi, R. Kobayashi, Y. Takashima, A. Hashidzume and A. Harada, Nat. Commun., 2012, 3, 603 CrossRef PubMed; (k) A. R. Hirst, B. Escuder, J. F. Miravet and D. K. Smith, Angew. Chem., Int. Ed., 2008, 47, 8002 CrossRef CAS PubMed; (l) Q. Lin, T.-T. Lu, X. Zhu, T.-B. Wei, H. Li and Y.-M. Zhang, Chem. Sci., 2016, 7, 5341 RSC; (m) Q. Lin, T.-T. Lu, X. Zhu, B. Sun, Q.-P. Yang, T.-B. Wei and Y.-M. Zhang, Chem. Commun., 2015, 51, 1635 RSC.
- D. J. Smith, G. A. Brat, S. H. Medina, D. Tong, Y. Huang, J. Grahammer, G. J. Furtmüller, B. C. Oh, K. J. Nagy-Smith, P. Walczak, G. Brandacher and J. P. Schneider, Nat. Nanotechnol., 2015, 11, 95 CrossRef PubMed.
- (a) C. Po, A. Y.-Y. Tam, K. M.-C. Wong and V. W.-W. Yam, J. Am. Chem. Soc., 2011, 133, 12136 CrossRef CAS PubMed; (b) C. Po, A. Y.-Y. Tam and V. W.-W. Yam, Chem. Sci., 2014, 5, 2688 RSC; (c) C. Po and V. W.-W. Yam, Chem. Sci., 2014, 5, 4868 RSC.
- (a) A. Aliprandi, M. Mauro and L. D. Cola, Nat. Chem., 2016, 8, 10 CrossRef CAS PubMed; (b) N. K. Allampally, M. Bredol, C. A. Strassert, C. A. Strassert and L. D. Cola, Chem. – Eur. J., 2014, 20, 16863 CrossRef CAS PubMed; (c) X.-S. Xiao, W. Lu and C.-M. Che, Chem. Sci., 2014, 5, 2482 RSC; (d) L.-B. Xing, S. Yu, X.-J. Wang, G.-X. Wang, B. Chen, L.-P. Zhang, C.-H. Tung and L.-Z. Wu, Chem. Commun., 2012, 48, 10886 RSC.
- (a) Z. Huang, S.-K. Kang, M. Banno, T. Yamaguchi, D. Lee, C. Seok, E. Yashima and M. Lee, Science, 2012, 337, 1521 CrossRef CAS PubMed; (b) K.-S. Moon, H.-J. Kim, E. Lee and M. Lee, Angew. Chem., Int. Ed., 2007, 46, 6807 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, measurements, synthesis and characterization data, UV-vis spectra and emission spectra. See DOI: 10.1039/c6cc08382j |
| This journal is © The Royal Society of Chemistry 2017 |