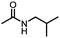
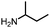
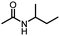
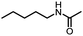
An unexpected copper-catalyzed carbonylative acetylation of amines†
Yahui
Li‡
a,
Changsheng
Wang‡
b,
Fengxiang
Zhu
a,
Zechao
Wang
a,
Jean
François Soulé
b,
Pierre H.
Dixneuf
b and
Xiao-Feng
Wu
*a
aLeibniz-Institut für Katalyse e.V. an der Universität Rostock, Albert-Einstein-Straße 29a, 18059 Rostock, Germany. E-mail: Xiao-Feng.Wu@catalysis.de
bCatalyse et Organométalliques, Institut Sciences Chimiques de Rennes, UMR 6226-CNRS-Université de Rennes, Av. Général Leclerc, 35042 Rennes, France
First published on 1st December 2016
Abstract
A novel copper-catalyzed carbonylative acetylation of amines has been developed. With peroxide as the oxidant as well as the methyl source with a copper catalyst under CO pressure, good yields of N-acetyl amides could be obtained. Notably, this is the first example of carbonylative acetylation.
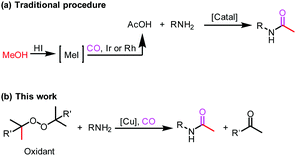
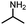
The acetylation of amine is one of the fundamental transformations in organic chemistry.1 Additionally, its importance has also been verified by the numerous applications in pharmaceutical and agricultural industries.2 Traditionally, the acetylation of amines usually took place with acetic acid/acetic anhydride/acetyl chloride under either basic or acidic conditions (Scheme 1, eqn (a)).3 Although acetylation with acetic acid represents the most straightforward procedure, however from the acetic acid synthesis point of view, acetic acid is mainly produced via a carbonylation procedure (Cativa process and Monsanto process).4 With methanol as the starting material under CO pressure and with Ir or Rh catalysts, acetic acid is produced in million of tonnes per year. According to retrosynthetic analysis,5 it should be possible and interesting to realize the application of carbonylation in acetylation. Nevertheless, the choice of the methyl source is a challenge. With methyl iodide (the intermediate in the Cativa process by the reaction of MeOH with HI) as the methyl source, the methylation of amines via the nucleophilic substitution reaction between MeI and amines is much faster; with MeOH as the methyl source, the needed HI is not compatible with amines. Hence, no carbonylative acetylation has been developed to date.
On the other hand, transitional metal-catalyzed carbonylative transformations have already become a powerful toolbox in modern organic synthesis.6 With noble metals as the catalysts, CO can be easily introduced into the parent molecules. Taking into consideration the high price of noble metals and the required phosphine ligands, the exploration of non-noble catalysts for this area is a challenge and will be interesting. By looking at the catalytic behaviours and their advantages, copper catalysts will be a good option.7 Indeed, copper catalysts are rarely applied in carbonylation reactions.8,9 We recently studied the application of copper catalysts in carbonylative transformations of alkanes with amines and amides.9 With copper as the catalyst and DTBP (di-tert-butyl peroxide) as the oxidant, amides or imides can be produced effectively from the corresponding alkanes and amines or amides. Interestingly, in some cases, acetylation of amine was observed during the optimization process. Under thermal conditions or in the presence of a metal catalyst, peroxides could decompose into the corresponding alkoxy radical, which could give a methyl radical through β-scission of the alkoxy radical.10 In the analysis of the reaction conditions, we realized that the methyl group is from DTBP and trapped by CO to give the acetyl group. With the awareness of the importance of acetylation reactions and also the academic meaningfulness of using peroxide as a methyl source in carbonylation, we focused on this transformation (Scheme 1, eqn (b)). In this procedure, peroxide acts both as an oxidant and a methyl source to give the first example of carbonylative acetylation.
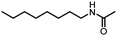
With 1-octanamine as the model substrate, we established this catalytic system. With dicumyl peroxide11 as the oxidant as well as the methyl source, using CuF2 (10 mol%) and 1,10-phenanthroline hydrate (1,10-phen., 10 mol%) as the catalyst system in chlorobenzene under CO pressure at 140 °C, 79% of N-octylacetamide can be isolated (Table 1, entry 1). Only a trace amount of acetamide can be detected in the absence of copper or a ligand (Table 1, entries 2 and 3). A decreased reaction efficiency was observed upon catalyst or ligand variation (Table 1, entries 4–6). And decreasing the reaction temperature or CO pressure resulted in decreased yields of acetamide (Table 1, entries 7–9). DTBP is shown to be a suitable reagent as well, 56% of N-octylacetamide was isolated (Table 1, entry 10); while no product could be detected with TBHP as the reagent (Table 1, entry 11).§
| Entry | Variations from the standard conditions | Yieldb (%) |
|---|---|---|
| a 1-Octanamine (0.5 mmol), catalyst (10 mol%), ligand (10 mol%), 1 mmol DCP, 40 bar CO, PhCl (2 mL), 24 h. b Isolated yields, the yields were calculated based on the amount of amine used. TBHP: t-butyl hydroperoxide solution 70 wt% in H2O. DCP: dicumyl peroxide. DTBP: di-tert-butyl peroxide. 1,10-phen: 1,10-phenanthroline hydrate. | ||
| 1 | — | 79 |
| 2 | Without CuF2 | Trace |
| 3 | Without 1,10-phen | Trace |
| 4 | CuBr(Me2S) instead of CuF2 | 31 |
| 5 | 2,9-Dimethyl-1,10-phenanthroline instead of 1,10-phen | 61 |
| 6 | 4,4′-Di-tert-butyl-2,2′-dipyridyl instead of 1,10-phen | 52 |
| 7 | 100 °C, 20 bar CO | 48 |
| 8 | 120 °C, 20 bar CO | 55 |
| 9 | 20 bar CO | 67 |
| 10 | DTBP instead of DCP | 56 |
| 11 | TBHP instead of DCP | 0 |
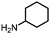
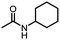
With the best reaction conditions in hand (Table 1, entry 1), we performed substrate testing of this novel procedure (Table 2). Good to excellent yields of acetamide can be produced with the tested alkyl amines (Table 2, entries 1–6).
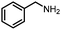
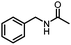
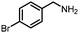
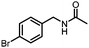
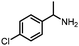
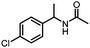
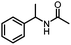
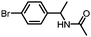
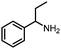
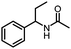
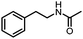
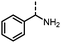
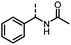
Benzylic and other aromatic substituted amines are proven to be suitable substrates as well; moderate to good yields can be obtained in general (Table 2, entries 7–14). The chirality of the starting amine was retained, and a good yield of the acetylated product can be isolated (Table 2, entry 14). Additionally, aniline was tested as well. Around 30% of N-phenylacetamide was obtained together with aniline-oxidized products.
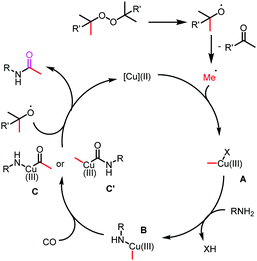
Based on our results, a possible reaction mechanism is proposed (Scheme 2). The reaction starts with a copper(II)-catalyzed or thermal hemolytic cleavage of a peroxide to generate the corresponding alkoxy radical. A methyl radical is generated through β-scission of the alkoxy radical, which reacts with the copper(II) species to give the corresponding Cu(III)-methyl species A. Then complex A reacts with the amine through X ligand exchange to produce the Cu(III) intermediate B. Then the insertion of CO forms the intermediate C or C′, which will give the final carbonylation product after reductive elimination. Meanwhile, the formed Cu(I) intermediate reacted with the alkoxy radical to produce Cu(II) species for the next catalytic cycle.
In conclusion, an interesting copper-catalyzed carbonylative acetylation of amine has been developed. With peroxide as the oxidant as well as the methyl source, good yields of N-acetyl amides were formed from the corresponding amines. Notably, this is the first example of carbonylative acetylation.
The authors thank the Chinese Scholarship Council for financial Support. The analytical support from Dr W. Baumann, Dr C. Fisher, S. Buchholz, and S. Schareina is gratefully acknowledged. We also appreciate the generous support from Professor Matthias Beller in LIKAT.
Notes and references
- (a) R. C. Larock, Comprehensive Organic Transformations, VCH, Weinheim, Germany, 2nd edn, 1989 Search PubMed; (b) T. W. Greene and P. G. M. Wuts, Protective Groups in Organic synthesis, John Wiley & Sons, Inc., New York, 3rd edn, 1999 CrossRef.
- (a) J. M. Humphrey and A. R. Chamberlin, Chem. Rev., 1997, 97, 2243 CrossRef CAS PubMed; (b) A. Scozzafava, T. Owa, A. Mastrolorenzo and C. T. Supuran, Curr. Med. Chem., 2003, 10, 925 CrossRef CAS PubMed; (c) J. S. Carey, D. Laffan, C. Thomson and M. T. Williams, Org. Biomol. Chem., 2006, 4, 2337 RSC; (d) F. Abbate, C. T. Supuran, A. Scozzafava, P. Orioli, M. T. Stubbs and G. Klebe, J. Med. Chem., 2002, 45, 3583 CrossRef CAS PubMed.
- (a) A. Vogel, Practical Organic Chemistry, Langman Scientific & Technical and Wiley, New York, 1989 Search PubMed; (b) J. March, Advanced Organic Chemistry, John Wiley & Sons, New York, 4th edn, 1992 Search PubMed.
- (a) J. H. Jones, Platinum Met. Rev., 2000, 44, 94 CAS; (b) G. J. Sunley and D. J. Watson, Catal. Today, 2000, 58, 293 CrossRef CAS.
- (a) E. J. Corey, Chem. Soc. Rev., 1988, 17, 111 RSC; (b) E. J. Corey, Angew. Chem., Int. Ed., 1991, 30, 455 CrossRef.
- For selected recent reviews on carbonylation reaction, see: (a) X. F. Wu, H. Neumann and M. Beller, Chem. Rev., 2013, 113, 1 CrossRef CAS PubMed; (b) X. F. Wu, H. Neumann and M. Beller, Chem. Soc. Rev., 2011, 40, 4986 RSC; (c) X.-F. Wu and H. Neumann, ChemCatChem, 2012, 4, 447 CrossRef CAS; (d) Q. Liu, H. Zhang and A. Lei, Angew. Chem., Int. Ed., 2011, 50, 10788 CrossRef CAS PubMed; (e) X. F. Wu, H. Neumann and M. Beller, ChemSusChem, 2013, 6, 229 CrossRef CAS PubMed; (f) C. H. Schiesser, U. Wille, H. Matsubara and I. Ryu, Acc. Chem. Res., 2007, 40, 303 CrossRef CAS PubMed; (g) S. Sumino, A. Fusano, T. Fukuyama and I. Ryu, Acc. Chem. Res., 2014, 47, 1563 CrossRef CAS PubMed; (h) B. Gabriele, R. Mancuso and G. Salerno, Eur. J. Org. Chem., 2012, 6825 CrossRef CAS; (i) S. D. Friis, A. T. Lindhardt and T. Skrydstrup, Acc. Chem. Res., 2016, 49, 594 CrossRef CAS PubMed.
- S. E. Allen, R. R. Walvoord, R. Padilla-Salinas and M. C. Kozlowski, Chem. Rev., 2013, 113, 6234 CrossRef CAS PubMed.
- (a) J. M. Liu, R. Z. Zhang, S. F. Wang, W. Sun and C. G. Xia, Org. Lett., 2009, 11, 1321 CrossRef CAS PubMed; (b) P. Tambade, Y. Patil, N. Nandurkar and B. Bhanage, Synlett, 2008, 886 CAS; (c) S.-K. Kang, T. Yamaguchi, T.-H. Kim and P.-S. Ho, J. Org. Chem., 1996, 61, 9082 CrossRef CAS.
- (a) Y. Li, K. Dong, F. Zhu, Z. Wang and X.-F. Wu, Angew. Chem., Int. Ed., 2016, 55, 7227 CrossRef CAS PubMed; (b) Y. Li, F. Zhu, Z. Wang and X.-F. Wu, ACS Catal., 2016, 6, 5561 CrossRef.
- P. Gray and A. Williams, Chem. Rev., 1959, 59, 239 CrossRef CAS.
- T. Kubo and N. Chatani, Org. Lett., 2016, 18, 1698 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: General procedure, analytical data and NMR spectra. See DOI: 10.1039/c6cc08929a |
| ‡ These authors contributed equally to this work. |
§ General procedure: A 4 mL screw-cap vial was charged with CuF2 (5.05 mg, 10 mol%), 1,10-phenanthroline hydrate (9.9 mg, 10 mol%) and an oven-dried stirring bar. The vial was closed by a Teflon septum and a phenolic cap and connected with a needle. After amine (0.5 mmol), DCP (1 mmol) and PhCl (2 mL) were injected using a syringe, the vial was fixed in an alloy plate and put into a Paar 4560 series autoclave (300 mL) under an argon atmosphere. At room temperature, the autoclave was flushed with carbon monoxide thrice and 40 bar of carbon monoxide was charged. The autoclave was placed on a heating plate equipped with a magnetic stirrer and an aluminium block. Then the reaction was heated at 140 °C for 24 hours. Afterwards, the autoclave was cooled to room temperature and the pressure was carefully released. After the removal of the solvent under reduced pressure, the pure product was obtained by column chromatography on silica gel (eluent![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) pentane/ethyl acetate = 1 pentane/ethyl acetate = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). 1). |
| This journal is © The Royal Society of Chemistry 2017 |