Towards improved halogenated BODIPY photosensitizers: clues on structural designs and heavy atom substitution patterns†
Antonio J.
Sánchez-Arroyo
,
Eduardo
Palao
,
Antonia R.
Agarrabeitia
,
María J.
Ortiz
* and
David
García-Fresnadillo
 *
*
Departamento de Química Orgánica I, Facultad de Ciencias Químicas, Universidad Complutense de Madrid, E-28040 Madrid, Spain. E-mail: dgfresna@ucm.es
First published on 30th November 2016
Abstract
The singlet oxygen (1O2) production quantum yield (ΦΔ) of 14 halogenated BODIPY dyes has been determined (0.01 < ΦΔ < 0.99). 1O2 production and photostability have been evaluated considering the BODIPY structure, the substitution pattern, and the number and type of heavy atoms and quenching rate constants of 1O2 by the sensitizer. In view of the experimental results and principal component analysis (PCA), guidelines for an improved design of efficient and photostable halo-BODIPY sensitizers are proposed.
BODIPY dyes have emerged as relevant fluorophores and 1O2 photosensitizers for different applications.1–3 The inclusion of heavy atoms such as Br or I in the BODIPY core is a common strategy to enhance the excited state (S1 → T1) intersystem crossing. This modification induces fluorescence attenuation and efficient energy transfer to the ground state molecular oxygen, leading to singlet oxygen production.4 However, photobleaching of BODIPYs is often attributed to 1O2. Moreover, the influence of heavy atoms on 1O2 production by BODIPY dyes has been barely studied. An increment in ΦΔ values has been reported for polybrominated BODIPYs as the number of Br atoms is increased.5 On the other hand, the inclusion of I atoms in positions 3 and/or 5 of polyiodinated derivatives does not produce a significant increment in ΦΔ.6 Therefore, the structural design of halogenated BODIPYs combining efficient 1O2 production and high photostability is still under debate.
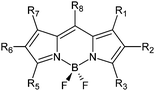
In order to shed light on this topic, a series of polyhalogenated BODIPY dyes ranging from disubstituted to hexasubstituted derivatives has been synthesized (Fig. 1 and Table 1, ESI†) and characterized concerning 1O2 production (ΦΔ) and quenching (kSensqΔ) in acetonitrile. Their photostability has also been evaluated in terms of initial photodegradation rates (r) and photodegradation quantum yields (Φpd), and PCA has been used to find out potential correlations among the parameters. The general structures of the BODIPY dyes studied in this work are polyhalogenated 2-ethyl-4,4-difluoro-1,3-dimethyl-4-bora-3a,4a-diaza-s-indacenes (trialkylside-BODIPYs 1–5) or 4,4- difluoro-8-(4-tolyl)-4-bora-3a,4a-diaza-s-indacenes (8-tolyl-BODIPYs 6–14), which represent two common structural frames often studied in the BODIPY literature. The triplet state lifetimes of these BODIPY structures are usually above 20 μs,2,3b,5,6 and for long-lived triplets and [O2] in the mM range (typical of air-equilibrated organic solvents such as acetonitrile), the excited triplet states are quantitatively quenched by molecular oxygen and ΦΔ generally becomes independent of [O2].7
| R1 | R2 | R3 | R5 | R6 | R7 | R8 | |
|---|---|---|---|---|---|---|---|
| 1 | Br | Br | H | Me | Et | Me | H |
| 2 | H | Br | Br | Me | Et | Me | H |
| 3 | I | I | H | Me | Et | Me | H |
| 4 | H | I | I | Me | Et | Me | H |
| 5 | I | I | I | Me | Et | Me | H |
| 6 | H | Br | H | H | Br | H | 4-Tolyl |
| 7 | H | H | Br | Br | H | H | 4-Tolyl |
| 8 | H | Br | Br | Br | Br | H | 4-Tolyl |
| 9 | Br | Br | Br | Br | Br | Br | 4-Tolyl |
| 10 | Br | Br | CH(CO2Et)2 | CH(CO2Et)2 | Br | Br | 4-Tolyl |
| 11 | H | I | CH(CO2Et)2 | I | I | H | 4-Tolyl |
| 12 | H | I | NH(CH2)2CO2H | I | I | H | 4-Tolyl |
| 13 | H | I | NH-p-(C6H4)OMe | I | I | H | 4-Tolyl |
| 14 | H | I | I | I | I | H | 4-Tolyl |
For ΦΔ determination (Table 2), the 1O2 phosphorescence decay traces at 1270 nm after laser pulse excitation (532 nm) were recorded at different laser powers (ESI†). Rose bengal was used as the reference sensitizer (ΦΔ = 0.71).6 The quenching rate constants of 1O2 deactivation by the BODIPYs (kSensqΔ) were determined by Stern–Volmer analysis from the 1O2 emission lifetimes (τΔ) measured in the photosensitization experiments.
λ
maxabs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a/nm a/nm |
ε /M−1 cm−1 |
Φ
appΔ
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
|
τ Δ /μs | k SensqΔ/108 M−1 s−1 | Φ Δ | r /10−8 M s−1 | Φ pd /10−6 | |
|---|---|---|---|---|---|---|---|---|
| a Absorption maximum in the visible region. Uncertainty ±2 nm from duplicate samples. b Uncertainty ±10%. c Apparent singlet oxygen production quantum yield, uncorrected for 1O2 quenching by the sensitizer. d Singlet oxygen phosphorescence lifetimes measured for BODIPY solutions with an optical density of 0.1 at 532 nm (dye concentrations in the 10−4–10−6 M range). τΔ values reported in the literature for photosensitizers with lower kSensqΔ than the BODIPYs studied in this work are 83.7 μs8 and 81.4 μs9 for 1H-phenalen-1-one and [Ru(dip)3]Cl2, respectively, in acetonitrile. e Singlet oxygen production quantum yield corrected for 1O2 quenching by the photosensitizer. f Uncertainty ±10%. g Uncertainty ±15%. h Φ Δ = 0.87 ± 6%.6 i Not determined. | ||||||||
| 1 | 488 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
0.83 ± 0.03 | 76.9 ± 3.3 | 0.3 ± 0.1 | 0.90 ± 0.04 | 1.6 | 5.8 |
| 2 | 509 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
0.55 ± 0.05 | 76.8 ± 2.3 | 1.0 ± 0.5 | 0.60 ± 0.05 | 2.4 | 8.0 |
| 3 | 503 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
0.90 ± 0.06 | 75.6 ± 2.2 | 1.0 ± 0.4 | 0.99 ± 0.06 | 2.6 | 8.5 |
| 4 | 521 | 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
0.55 ± 0.06 | 76.3 ± 1.1 | 2.6 ± 0.4 | 0.60 ± 0.06 | 1.3 | —i |
| 5 | 525 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
0.56 ± 0.04 | 77.5 ± 2.1 | 2.5 ± 0.5 | 0.60 ± 0.04 | —i | —i |
| 6 | 531 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.54 ± 0.11 | 77.9 ± 1.2 | 4.3 ± 0.9 | 0.57 ± 0.11 | 1.4 | 1.7 |
| 7 | 515 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
0.23 ± 0.02 | 72.7 ± 3.6 | 1.5 ± 0.6 | 0.26 ± 0.02 | 1.5 | 2.0 |
| 8 | 546 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
0.52 ± 0.07 | 74.1 ± 2.1 | 4.4 ± 0.8 | 0.58 ± 0.07 | 4.2 | 5.8 |
| 9 | 544 | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
0.45 ± 0.06 | 80.2 ± 0.9 | 1.7 ± 0.3 | 0.47 ± 0.06 | 1.7 | —i |
| 10 | 534 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.15 ± 0.02 | 79.4 ± 3.4 | 1.3 ± 0.4 | 0.16 ± 0.02 | 9.5 | —i |
| 11 | 559 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
0.72 ± 0.09 | 73.8 ± 1.1 | 2.6 ± 0.5 | 0.81 ± 0.09 | 9.3 | 12.1 |
| 12 | 497 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
0.43 ± 0.04 | 46.8 ± 0.8 | 0.8 ± 0.1 | 0.77 ± 0.05 | 9.5 | —i |
| 13 | 518 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
0.011 ± 0.001 | 66.8 ± 3.4 | 4.7 ± 0.5 | 0.014 ± 0.001 | —i | —i |
| 14 | 573 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
0.85 ± 0.04h | 75.4 ± 1.1 | 1.7 ± 0.5 | 0.94 ± 0.04 | 4.0 | 6.1 |
Since the kSensqΔ values obtained for the BODIPY dyes under study are rather high (107–108 M−1 s−1, compared to ∼104 M−1 s−1 typical of reference 1O2 photosensitizers such as phenalenone or rose bengal),7b the apparent 1O2 production quantum yields (ΦappΔ) calculated from the phosphorescence decay traces were corrected for the 1O2 quenching by the sensitizer itself by using the following equation: ΦappΔ/ΦΔ = kd/(kd + kSensqΔ × [Sens]), where kd is the rate constant of 1O2 deactivation by the solvent (1/τΔ)8,9 and [Sens] is the concentration of the BODIPY sensitizer.10 It has to be noted that this important correction, especially when 1O2 is deactivated by the solvent and by the sensitizer in similar timescales, is often neglected in the calculation of ΦΔ values. In this way, more accurate ΦΔ values could be determined by taking into account kSensqΔ and the dye concentration (Table 2). According to these results, some general trends in 1O2 production depending on the halogen and the substitution pattern could be observed. Firstly, iodinated derivatives tend to show higher ΦΔ values when compared with their brominated counterparts, as it is evidenced by ΦΔ of the 1 and 3 or 8 and 14 pairs of trialkylside-BODIPYs or 8-tolyl-BODIPYs, respectively. The higher spin–orbit coupling of iodine vs. bromine may well account for this effect.11 Secondly, when the trialkylside-BODIPY family is considered, a significant role concerning 1O2 production ability is played by the presence of halo atoms at position 1 (beneficial) vs. 3 (detrimental). In particular, substitution at position 1 tends to increase ΦΔ, while substitution at position 3 promotes a substantial decrease of ΦΔ (cf. ΦΔ of compounds with Br or I atoms at positions 1 and 2, ΦΔ(1) = 0.90 and ΦΔ(3) = 0.99, vs. positions 2 and 3, ΦΔ(2) = 0.60 and ΦΔ(4) = 0.60). The predominant detrimental effect on ΦΔ due to substitution in position 3 can also be observed in the triiodinated compound 5 (ΦΔ(5) = 0.60, despite its potentially advantageous substitution in position 1 as well, which suggests a major effect of substitution in position 3 vs. 1). Regarding the substitution pattern by bromine in the symmetric 8-tolyl-BODIPYs 6–10, although the presence of Br atoms in positions 3 and 5 seems to support the previous statement in the dibromo derivatives (ΦΔ(6) = 0.57 and ΦΔ(7) = 0.26), this trend is not followed in the case of the tetrabromo or hexabromo compounds 8, 9 and 10, which also shows that an increased number of heavy atoms does not necessarily increase ΦΔ (ΦΔ(8) = 0.58, ΦΔ(9) = 0.47 and ΦΔ(10) = 0.16). Concerning the asymmetric triiodo 8-tolyl-BODIPYs 11–13, all of them show lower ΦΔ values than their nonalkylated equivalent (R2, R5, R6 = I, R3 = H, ΦΔ = 0.86)6 or their symmetric tetraiodo counterpart (ΦΔ(14) = 0.94), and suggest that the type of nonhalogenated substituent can induce strong changes in ΦΔ when it is alkyl or (amino)alkyl/aryl (ΦΔ(11) = 0.81 and ΦΔ(12) = 0.77, while ΦΔ(13) = 0.014). Overall, when substitution of halo atoms in position 3 by alkyl or N-alkyl/aryl is considered, lower ΦΔ values are always observed (e.g., ΦΔ(9) > ΦΔ(10); ΦΔ(14) > ΦΔ(11), ΦΔ(12) and ΦΔ(13)). It should be noted that the inclusion of nonhalogenated substituents in the BODIPY structure is important for their functionalization with other subunits or biomolecules, or for their immobilization onto solid supports, in order to develop different photosensitization applications.1–3
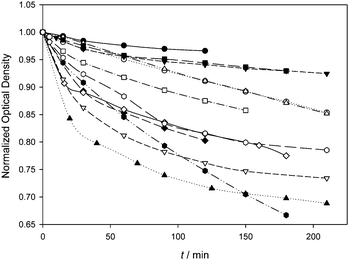
The photodegradation process of a series of BODIPYs studied in this work was monitored by following the optical density changes at λmaxabs with the irradiation time under green light illumination. However, when the different experimental procedures reported in the literature about the photodegradation of BODIPY dyes are examined,4a,12–19 a common methodology for the determination of a specific experimental parameter quantitatively related to dye photostability is not easy to find. Among the different approaches considered, the comparison with other well-known photosensitizers,4a the remaining absorbance,12–19 the determination of kinetic half-degradation times,17 and the calculation of the photodegradation quantum yield18,19 are the most relevant methods. In our case, since the irradiation experiments revealed that most of the studied dyes tend to show an asymptotic behaviour of the remaining optical density after 3 h of illumination, the initial degradation rates (r) and the photodegradation quantum yields (Φpd) were calculated from the evolution of the optical density after 30 minutes of irradiation (Fig. 2 and Table 2, ESI†).‡
Φ Δ should correlate with r and with Φpd if the photodegradation of the studied BODIPYs were a bimolecular photooxidation process, as their relatively high kSensqΔ values suggest,§ however, the trialkylside-BODIPYs, with moderate to high ΦΔ values, seem to suffer much less from photodegradation when compared with the 8-tolyl-BODIPYs (Fig. 2). This result agrees well with the lower r values determined in the case of the trialkylside-BODIPYs 1–4 ((1.3–2.6) × 10−8 M s−1 range, Table 2), while the 8-tolyl-BODIPYs 8, 11, 12 and 14, also with moderate to high ΦΔ values (0.6 < ΦΔ < 1), display higher photodegradation rates ((4.0–9.5) × 10−8 M s−1 range, Table 2). The weak ΦΔ–r correlation is also evidenced by 10 (low ΦΔ and high r) when compared with 8 or 9. From the r values, alkyl/N-alkyl substitution in 10–12 seems to be detrimental concerning photostability if these BODIPYs are compared with 8, 9, or 14. When the series of polybrominated symmetric 8-tolyl-BODIPYs (except compound 10) is considered, their photodegradation rates tend to be low despite their moderate ΦΔ values. Interestingly, dibromo 8-tolyl-BODIPYs 6 and 7, and hexabromo derivative 9 with moderate to low ΦΔ, display similar r values to the dihalogenated trialkylside-BODIPYs that are more efficient singlet oxygen photosensitizers. These facts point out that factors such as the hydrocarbon structural frame of the BODIPY, and not ΦΔ nor the total number and position of the halogens, are influencing the photostability of the BODIPYs. When the amount of photons absorbed by the dye is taken into account and Φpd is discussed, a weak correlation between Φpd and ΦΔ is observed, and the influence of other structural features appears to be of little relevance as well.
Therefore, since different structural and photophysical factors might influence the singlet oxygen production and/or photostability of BODIPY dyes, a principal component analysis (PCA) approach was considered in order to qualitatively elucidate, from a statistical point of view, the strongest possible correlations, or lack of correlations, between all the potential variables. The set of prospective variables or parameters used to perform the different PCAs was composed by ΦΔ, kSensqΔ, Φpd, r, the relative positions substituted by halogen (1, 2 and 3), and the type and number of halogens (details on the PCA methodology are summarized in the ESI†).
Among all the PCAs carried out, some of them supported the existence of certain positive or negative correlations, while other PCAs did not provide significant information. The most relevant results are discussed below. The PCA displayed in Fig. 3, describing 61% of the variance, shows the existence of a strong correlation between ΦΔ and the relative position 1 of the BODIPYs under study (since those more related variables display closer vectors between them). Conversely, an opposite link between ΦΔ and position 3, and ΦΔ and kSensqΔ is detected. Interestingly, the data points of compounds 1 and 3 (belonging to the 1,2-dihalo pattern in trialkylside-BODIPYs) are very close to the ΦΔ vector, supporting the existence of this strong correlation. On the other hand, Fig. 4, accounting for 81% of the variance, shows a lack of correlation between ΦΔ and the number of halogen atoms, and the influence (in favour of iodine) of the type of halogen atom. Finally, regarding the analysis of the factors that could influence the photostability of the BODIPYs under study, Fig. 5, where 85% of the variance is justified, shows the inverse relationship between the quantum yields (ΦΔ mainly, and Φpd) and kSensqΔ, and only a certain connection between ΦΔ and Φpd, while ΦΔ displays a weak link with r. From these results, regarding the photostability of the BODIPYs studied in this work, the bimolecular reaction with the photogenerated singlet oxygen does not seem to be the determining factor concerning the photodegradation of these dyes, and it is likely that unimolecular photoprocesses (e.g., a C–X bond fission)20 could also have a strong influence.
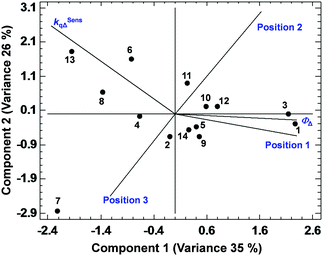 | ||
| Fig. 3 PCA showing the correlations between ΦΔ, kSensqΔ and the relative positions 1, 2 and 3 of halogen atoms in the BODIPY dyes studied in this work. | ||
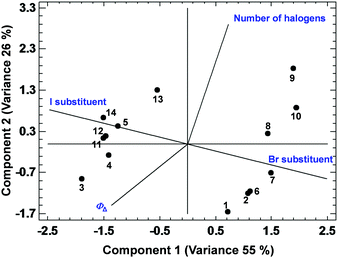 | ||
| Fig. 4 PCA showing the correlations between ΦΔ and the type and number of heavy atoms in the BODIPY dyes studied in this work. | ||
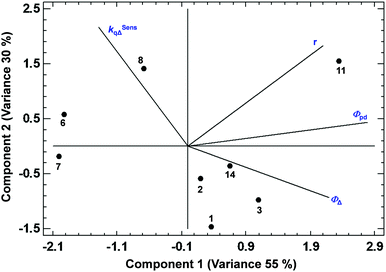 | ||
| Fig. 5 PCA showing the correlations between ΦΔ, Φpd, r, and kSensqΔ in the BODIPY dyes studied in this work. | ||
In summary, for the series of polyhalogenated BODIPYs presented in this work (trialkylside- and 8-tolyl-BODIPYs), a relevant influence of the substitution pattern by halogens on ΦΔ has been observed. In general, the presence of halogens (particularly iodine) in the relative position 1/7 is beneficial for efficient 1O2 production, while halogenation of the relative position 3 is detrimental. Moreover, the presence of (N)-alkyl/aryl substituents in the relative position 3/5 tends to decrease 1O2 production in comparison with their halogenated counterparts. On the other hand, a high number of halogen atoms in the BODIPY structure are not necessarily linked to higher ΦΔ values. Regarding the photostability, trialkylside-BODIPYs are, in general, more photostable than 8-tolyl-BODIPYs, and the photodegradation process seems to be more related to intramolecular pathways rather than to the bimolecular reaction with photogenerated 1O2. With this facts in mind, an improved design of efficient and photostable polyhalogenated BODIPY photosensitizers is possible via the appropriate selection of the BODIPY structural frame and the introduction of the halogens in suitable positions of the BODIPY structure.
Financial support from the MINECO, Spain, is acknowledged (MAT-2014-51937-C3-2-P and MAT-2015-68837-REDT (to M. J. O.) and CTQ2014-52045-R (to D. G.-F.)). The work of A. J. S.-A. was funded by UCM (predoctoral grant ref. CT4/14).
Notes and references
- N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130–1172 RSC.
- A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, Chem. Soc. Rev., 2013, 42, 77–88 RSC.
- (a) T. Kowada, H. Maeda and K. Kikuchi, Chem. Soc. Rev., 2015, 44, 4953–4972 RSC; (b) J. Zhao, K. Xu, W. Yang, Z. Wang and F. Zhong, Chem. Soc. Rev., 2015, 44, 8904–8939 RSC.
- (a) T. Yogo, Y. Urano, Y. Ishitsuka, F. Maniwa and T. Nagano, J. Am. Chem. Soc., 2005, 127, 12162–12163 CrossRef CAS PubMed; (b) Y. C. Lai, S. Y. Su and C. C. Chang, ACS Appl. Mater. Interfaces, 2013, 5, 12935–12943 CrossRef CAS PubMed.
- X. F. Zhand and X. Yang, J. Phys. Chem. B, 2013, 117, 5533–5539 CrossRef PubMed.
- M. J. Ortiz, A. R. Agarrabeitia, G. Duran-Sampedro, J. Bañuelos-Prieto, T. Arbeloa-Lopez, W. A. Massad, H. A. Montejano, N. A. García and I. Lopez-Arbeloa, Tetrahedron, 2012, 68, 1153–1162 CrossRef CAS.
- (a) F. Wilkinson, W. P. Helman and A. B. Ross, J. Phys. Chem. Ref. Data, 1993, 22, 113–263 CrossRef CAS; (b) D. García-Fresnadillo and S. Lacombe, in Singlet Oxygen. Applications in Biosciences and Nanosciences, ed. S. Nonell and C. Flors, Royal Society of Chemistry, Cambridge, 2016, vol. 1, ch. 6, pp. 105–143 Search PubMed.
- O. Shimizu, J. Watanabe, K. Imakubo and S. Naito, Chem. Lett., 1999, 67–68 CrossRef CAS.
- A. J. Sánchez-Arroyo, Z. D. Pardo, F. Moreno-Jiménez, A. Herrera, N. Martín and D. García-Fresnadillo, J. Org. Chem., 2015, 80, 10575–10584 CrossRef PubMed.
- (a) D. Garcia-Fresnadillo, Y. Georgiadou, G. Orellana, A. M. Braun and E. Oliveros, Helv. Chim. Acta, 1996, 79, 1222–1238 CrossRef CAS; (b) I. Gutiérrez, S. G. Bertolotti, M. A. Biasutti, A. T. Soltermann and N. A. García, Can. J. Chem., 1997, 75, 423–428 CrossRef.
- N. Epelde-Elezcano, V. Martinez Martinez, E. Peña-Cabrera, C. F. A. Gómez-Durán, I. Lopez Arbeloa and S. Lacombe, RSC Adv., 2016, 6, 41991–41998 RSC.
- C. Jiao, K. W. Huang and J. Wu, Org. Lett., 2011, 13, 632–635 CrossRef CAS PubMed.
- L. Zeng, C. Jiao, X. Huang, K. W. Huang, W. S. Chin and J. Wu, Org. Lett., 2011, 13, 6026–6029 CrossRef CAS PubMed.
- D. Wang, J. Fan, X. Gao, B. Wang, S. Sun and X. Peng, J. Org. Chem., 2009, 74, 7675–7683 CrossRef CAS PubMed.
- T. Terai, K. Hanaoka, Y. Urano, T. Mineno and T. Nagano, Chem. Commun., 2011, 47, 10055–10057 RSC.
- Y. C. Lai, S. Y. Su and C. C. Chang, ACS Appl. Mater. Interfaces, 2013, 5, 12935–12943 CAS.
- S. Banfi, G. Nasini, S. Zaza and E. Caruso, Tetrahedron, 2013, 69, 4845–4856 CrossRef CAS.
- G. Vives, C. Giansante, R. Bofinger, G. Raffy, A. del Guerzo, B. Kauffmann, P. Batat, G. Jonusauaskas and N. D. McClenaghan, Chem. Commun., 2011, 47, 10425–10427 RSC.
- K. K. Jagtap, N. Shivran, S. Mula, D. B. Naik, S. K. Sarkar, T. Mukherjee, D. K. Maity and A. K. Ray, Chem. – Eur. J., 2013, 19, 702–708 CrossRef CAS PubMed.
- E. Palao, T. Slanina, L. Muchová, T. Šolomek, L. Vítek and P. Klán, J. Am. Chem. Soc., 2016, 138, 126–133 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Synthesis, characterization data and NMR spectra of the BODIPY dyes, UV-VIS absorption spectra, determination of ΦappΔ, kSensqΔ, r and Φpd, chemical actinometry and principal component analyses. See DOI: 10.1039/c6cp06448e |
| ‡ Dye samples (3 mL, air-equilibrated acetonitrile) were irradiated in a quartz cell of 1 cm optical path length using a green LED lamp (λmaxem = 515 nm and FWHM = 33 nm). The incident photon flux, qp = (2.2 ± 0.2) × 10−7 einstein s−1, was determined by Reinecke's salt actinometry. For the calculation of photodegradation quantum yields, since the amount of photons absorbed by the dye during the irradiation process progressively decreased due to photobleaching, the total number of absorbed photons was determined by considering the time-dependent absorbance (ESI†). |
| § It has to be noted that kSensqΔ represents the rate constant of total 1O2 quenching by the sensitizer itself (physical quenching and chemical reaction).10a |
| This journal is © the Owner Societies 2017 |

![[hexagon filled, point down]](https://www.rsc.org/images/entities/char_e124.gif) ) and 14 (
) and 14 (![[hexagon open, flat-side down]](https://www.rsc.org/images/entities/char_e1c0.gif) ).
).