An all-solid-state asymmetric device based on a polyaniline hydrogel for a high energy flexible supercapacitor†
Hamid
Heydari
and
Mohammad B.
Gholivand
*
Faculty of Sciences, Razi University, Kermanshah, Iran. E-mail: mbgholivand@razi.ac.ir; Fax: +98 831-4274559; Tel: +98 831-4274557
First published on 5th December 2016
Abstract
The demand for low cost, flexible energy storage devices with enhanced energy/power density has increased with the rapid development of portable, flexible electronics. Herein, we report on an all-solid-state flexible, high energy density asymmetric supercapacitor (SC) based on a polyaniline (PANI) hydrogel made using a two-component mixing strategy. PANI hydrogel consists of polymeric networks with high levels of hydration and three-dimensional (3D) microstructures, which lead to faster electron and mass transport and offer a large accessible surface area. The 3D design is mechanically robust and flexible with large internal surfaces designed to pull water via capillary action. The design features ensure a strong interaction between the electrode and electrolyte solution for effective charge storage reactions, while at the same time giving the electrode sufficient mechanical flexibility to work under a bent condition. The PANI hydrogel electrode exhibits an excellent electrochemical performance with a specific capacitance of 862 F g−1 at 1 A g−1. Utilizing this nanocomposite as the positive electrode in a PANI//AC asymmetric configuration results in devices with a remarkable performance including good capacitance (261 F g−1 at 1 A g−1), great rate capability (64% capacitance retention), excellent cycle life (18% loss after 5000 cycles), a maximum energy density of 92.7 W h kg−1, and a high power density of 16 kW kg−1. Furthermore, the areal capacitance, areal energy density, volumetric capacitance, and volumetric energy density of the fabricated supercapacitor are 522 mF cm−2, 0.185 mW h cm−2, 17.4 F cm−3, and 6.16 mW h cm−3, respectively. Even under continuous bending and unbending, the device still provides a stable electrochemical performance without noticeable changes. We also demonstrate the promise of these SCs for various applications as we light up a green LED. This excellent performance holds great promise for next-generation flexible electronics.
1. Introduction
Consumers increasingly demand flexible electronics, including products ranging from wearable electronics to flexible solar cell arrays and displays.1–4 Because of their bendability, portability, durability, and even rollability, flexible devices seem to lead future electronics. Yet problems exist when developing flexible electronics as thin, light, and flexible energy storage devices (ESDs). In order to combat these problems, the development of various power sources and ESDs has arisen; such power sources and ESDs include flexible, thin lithium-ion batteries and supercapacitors (SCs) with varying shapes, sizes, and mechanical properties.5–8More recently, fiber based SCs have attracted great attention due to their high flexibility, integration of lightweight materials, and tiny volume, favoring portable applications.9–12 Nevertheless, SCs typically suffer from low energy density, which limits their practical applications. To overcome the limitation of using asymmetric supercapacitors AC is an effective approach to increase the energy density and extend the operating voltage window.13,14 For instance, fiber-shaped asymmetric SCs utilizing carbon fiber/graphene and metal fiber/Co3O4 nanowires with a volumetric energy density of 0.62 mW h cm−3 have been reported by Shen et al.11 However, the tendency of metal fibers to corrode during charge/discharge, to oxidize under ambient conditions, and to fail due to fatigue under constant bending make them unfit for use in flexible and wearable electronics.15 Furthermore, Cui et al. reported planar flexible SCs based on conductive textiles fabricated using carbon nanotubes (CNTs) and achieved an area capacitance of 0.48 F cm−2. Nonetheless, a great amount of boundaries due to randomly dispersed CNTs in the CNT fibers can result in lowered conductivity leading to a decrease in efficiencies during charging/discharging. Besides, the relatively low conductivity in comparison with metal current collectors, when CNT fibers are used as both current collectors and active materials will cause an increase in the equivalent series resistance.16,17
One of the most efficient ways to overcome the above-mentioned limitations is by using a conducting polymer18 (CP) such as polyaniline (PANI). PANI is ideal for SC fabrication because of its ease of synthesis, low material cost, high pseudocapacitive energy density, and wide range of applications.19–21 The redox reactions in PANI can easily be reversed; reversals include the doping and dedoping of counterions22,23 in order to exhibit the high power characteristics of the SCs. As a result, electron transfer in PANI is through a conjugated double bond. Because of this, passing an electric current in a coherent wrap is facile and hard between two independent parts.24 Bulk PANI provides superior energy storage, but it is plagued by deficiencies in its mechanical properties and cycling stability. Commercial uses of PANI pseudocapacitors are also limited during the counterions' doping and dedoping processes; due to large variations in volume, the polymer backbone disintegrates during cycling, reducing the capacity.17,25 Nevertheless, PANI's cycling stability may potentially be improved through development of a PANI 3D network, and its hydrogel has a number of ESD uses, including in biofuel cells and SCs.
Hydrogels are polymeric networks containing high levels of hydration and three-dimensional (3D) structures.1,14,26 CP hydrogels (e.g. PANI,27,28 polythiophene,7 and polypyrrole29–31) combine the electrical properties of conducting polymers with the unique properties of hydrogels and offer such features as intrinsic 3D conducting frameworks facilitating the transport of electrons, ions, and molecules.1,32,33
SCs are high-power ESDs with a much greater capacitance than normal capacitors.34–37 Flexible SCs are used in hybrid electric vehicles,38 portable electronic devices,39 and medical devices.40 There are two main categories of SCs: (i) redox SCs and (ii) electric double layer capacitors (EDLCs).1,32 A SC electrode material primarily includes transition-metal oxides,41,42 conducting polymers,7,18 and high-surface carbons.31,39 The materials on the nano-size scale should significantly improve the performance of the electrode due to enhancement in the electrode/electrolyte interface areas, which will minimize the ion diffusion path31,39,43,44 within the active materials. This results in an increased energy storage capacity.
In order to synthesize a CP hydrogel, CPs must be copolymerized with a non-conductive polymer, or a CP monomer must be polymerized in a medium of non-conductive hydrogel. This synthesis must be accomplished with a template, such as Hep-MA/PANI hydrogel,45 which is created by cross-linked heparin methacrylate hydrogel. To synthesize a stretchable conducting hybrid hydrogel, α-CD-containing polyacrylamide (α-CDPAAm) hydrogel46 must be pre-organized. However, insulating the hydrogel template can result in decreased electrical properties, which is an issue of these hydrogels.
In order to create a hierarchical PANI hydrogel nanostructure, we used amino trimethylene phosphoric acid (ATMP) as the dopant and gelator to the PANI network, which formed quickly due to the lack of non-conductive polymers; such a method was suggested by the Xu research group.27 In this process, conductive PANI constructs a 3D interconnected conductive network that enhances both electron transport and flexibility, properties desirable for new generation high-performance SC electrodes. Besides, the suggested direct synthesis of PANI hydrogel on carbon cloth improves the interaction between PANI and carbon clothes and thus improves electron transfer.39 Other notable properties derived from this process are high capacitance, good cycling stability, and rate capability.
The SCs energy density must be improved if it will contribute to advance the energy storage device market. To do so, positive and negative electrodes of various chemical natures should ideally be combined in an asymmetric configuration (AC) by integrating a pseudocapacitive positive electrode (PANI) and an EDLC negative electrode in the same cell. The device's energy density will improve because of this process due to the high overpotential of reversible hydrogen electrosorption on the carbon EDLC electrodes.
2. Experimental
2.1 Materials
An aniline monomer was distilled and stored in a refrigerator. ATMP and ammonium peroxysulfate (APS) were purchased from Sigma-Aldrich and used as received. Carbon cloth (CC) was purchased from Niru Battery Company.2.2 Preparation of PANI hydrogels
We synthesized the PANI hydrogel by the fast mixing of two components.10 Typically, Solution A was prepared by mixing 10 mmol aniline, 5 mmol ATMP (50%, w/w in water), and 4 mL DI water. Also, 2.5 mmol ammonium persulfate (APS) was dissolved in 2 mL DI water (solution B). Carbon cloths (1 cm × 2 cm) were used as the substrate and current collectors. Solution A and B were mixed quickly and immediately dropped onto the carbon cloth (available area of 1 cm × 1 cm) and reacted for 4 h. The prepared electrodes were dried at room temperature and washed by immersing in DI water for one day. In the last step, a vacuum oven was used to dry the electrodes for electrochemical measurements.2.3 Characterization and electrochemical investigations
We used a scanning electron microscope (SEM, Philips and JEOL-JSM-6700) and a transmission electron microscope (TEM, Zeiss model EM10C) to monitor the morphology of the samples. We confirmed the PANI hydrogel's chemical structure via Fourier transform infrared spectroscopy (FTIR). The prepared samples' crystal structures were investigated using powder X-ray diffraction (XRD, Philips X'pert diffractometer) with Cu Kα radiation (λ = 1.5406 Å).Cyclic voltammetry (CV), galvanostatic charge/discharge (CD), and electrochemical impedance spectroscopy (EIS) experiments enabled us to verify the prepared samples' electrochemical performance. Electrochemical measurements were performed in an aqueous H2SO4 solution (1 M) at room temperature. All electrochemical measurements were performed using a biologic VSP-300 potentiostat instrument. The counter electrode was a platinum plate, and an Ag/AgCl electrode served as the reference electrode.
2.4 Preparation of the asymmetric SC
As an asymmetric device was needed, we used PANI as a positive electrode, AC electrodes were used as negative electrodes, and cellulosic paper served as a separator. Polyvinyl alcohol (PVA) is used for the preparation of a gel electrolyte and so an all-solid-state device (Fig. 1). To prepare the gel electrolyte, we dissolved 1 g PVA in 10 mL water and stirred vigorously for 30 minutes, then 0.56 mL stoke of H2SO4 was added to produce a 1 M H2SO4 gel electrolyte. We calculated an optimal mass ratio of 0.33 between the positive and negative electrodes (m+/m−), according to the charge balance theory. This aided us in achieving maximum capacitance and operating potential window. So, two electrodes with 0.5 mg of PANI and 1.5 mg of AC were prepared. The geometric surface area of each electrode was 1 cm−2. Therefore, the total mass loading of the two electrodes was 2 mg cm−2.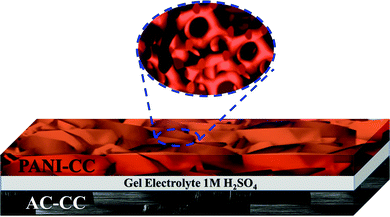 | ||
| Fig. 1 Schematic illustration of the asymmetric device setup including a positive electrode, a negative electrode and a gel electrolyte added to the separator. | ||
The specific capacitances (Csp), energy densities (ED) and power densities (PD) were calculated from the discharge curves using the following equations:
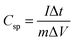 | (1) |
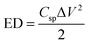 | (2) |
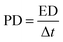 | (3) |
We activated PANI before it was assembled in the asymmetric cell by running 200 charge/discharge cycles in a three-electrode system, thus utilizing PANI's full pseudocapacitance. Next, we subjected the as-fabricated PANI//AC asymmetric SCs to CV, CD, and EIS measurements, and tested the asymmetric SC's cycle life through more than 5000 charge/discharge cycles at a current density of 5 A g−1.
3. Results and discussion
We used the ATMP for PANI hydrogel gelation and microstructure formation. ATMP is used to produce the 3D PANI hydrogel as follows: each ATMP molecule reacts with and protonates some of the nitrogen groups in the PANI chain; the resulting crosslinks form branched 3D network structures. Strongly acidic ATMP phosphorus groups dope the PANI chains, making them highly hydrophilic; increased doping increases the conductivity of the conjugated polymer network. To synthesize the highly doped PANI hydrogel, we combined a solution containing the oxidant APS with a solution of the gelator (ATMP) and monomer (aniline).3.1 Characterization
SEM was used to investigate the structure and morphology of the PANI hydrogel electrodes. Fig. 2a–c show the FE-SEM images of the as-synthesised PANI hydrogel at different magnifications, which clearly illustrate the well-defined 3D interconnected network of the PANI hydrogel with a continuous porous structure containing pores of sub-micrometer to several micrometers in size. Furthermore, as can be seen, PANI nanosheets are constructed of interconnected hierarchical PANI. 3D structured PANI formation makes it easy to achieve a high surface area platform. In addition, several micro-sized pores stick out across those PANI sheets that supply direct pathways for impressive ion transport in thin PANI hydrogel sheets. It is worth mentioning that two kinds of pores are observed in the FE-SEM images marked by the white arrows. The first one, which is the average gap size, is the pores among the branched nanofibers, and the second one is the bigger micron sized pores. Fig. 2d shows that the Brunauer–Emmett–Teller (BET) surface area of the PANI hydrogel is 37.8 m2 g−1, which is analogous to other chemically synthesized PANI hydrogels.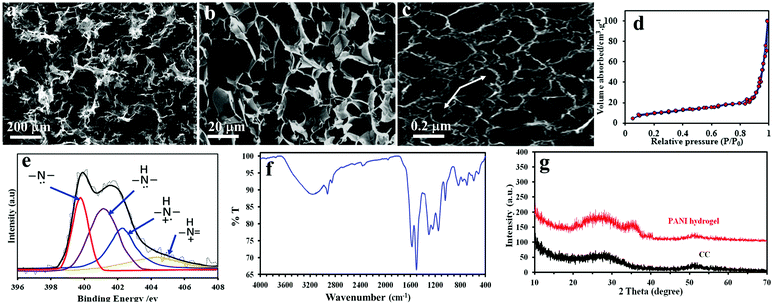 | ||
| Fig. 2 (a)–(c) Fe-SEM images, (d) BET, (e) XPS, (f) FTIR spectrum of the 3D hierarchical nanostructured PANI hydrogel, and (g) the powder XRD patterns of CC and the PANI hydrogel. | ||
We used XPS analysis to investigate the synthesized PANI hydrogel's chemical structure. As seen in Fig. 2e, for the N 1s XPS spectrum, four peaks of N-containing groups of PANI are demonstrated at 399.6 eV, 401.2, 402.4 eV, and 404.1 eV, respectively. These peaks correspond to the nitrilo-2,5-cyclohexadiene-1,4-diylidenenitrilo-1,4-phenylene, imino-1,4 phenylene, radical cation of imino-1,4-phenylene, and protonated imino-1,4-phenylene groups, respectively.19
Further investigation of the PANI hydrogel on CC using FTIR spectroscopy shows the generation of an emeraldine salt form, not solely in the pernigraniline or leucoemeraldine forms (Fig. 2f). This point is proven by the bands at 1576 and 1503 cm−1, which are due to the stretching vibrations of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C in the quinonoid and benzenoid rings. The C–N stretching vibrations of planar bending of C–H in the aromatic moieties peak at 1000 cm−1, and the secondary aromatic amines form a peak of 1301 cm−1. Moreover, the peaks between 798 and 505 cm−1 indicate that the C–H experienced a bending vibration within the aromatic ring. The peak at 1240 cm−1 is attributable to a C
C in the quinonoid and benzenoid rings. The C–N stretching vibrations of planar bending of C–H in the aromatic moieties peak at 1000 cm−1, and the secondary aromatic amines form a peak of 1301 cm−1. Moreover, the peaks between 798 and 505 cm−1 indicate that the C–H experienced a bending vibration within the aromatic ring. The peak at 1240 cm−1 is attributable to a C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond, while the 2925 cm−1 peak indicates C–H vibration. It has to be noticed that higher peaks, reaching 3100 to 3600 cm−1 in all of the FTIR spectra, mark stretching vibrations within the OH group; such vibrations are due to absorbed water molecules.27,47
N bond, while the 2925 cm−1 peak indicates C–H vibration. It has to be noticed that higher peaks, reaching 3100 to 3600 cm−1 in all of the FTIR spectra, mark stretching vibrations within the OH group; such vibrations are due to absorbed water molecules.27,47
X-Ray Diffraction (XRD) analysis was also been utilized to examine the PANI hydrogel samples' structure; the CC's XRD patterns both with and without the PANI hydrogel appear in Fig. 2g. The characteristic of the CC sheet is a broad peak at 20 °C to 35 °C. As can be seen, the PANI hydrogel shows two peaks around 2θ of 25° and 35° that demonstrate the fabrication of PANI hydrogel on the CC piece.27
3.2 Electrochemical evaluation
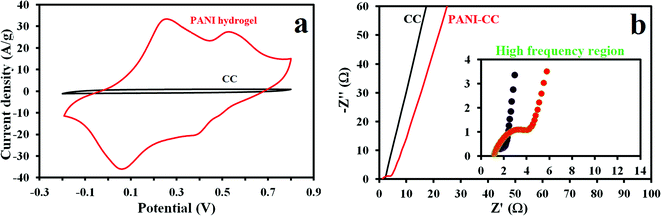
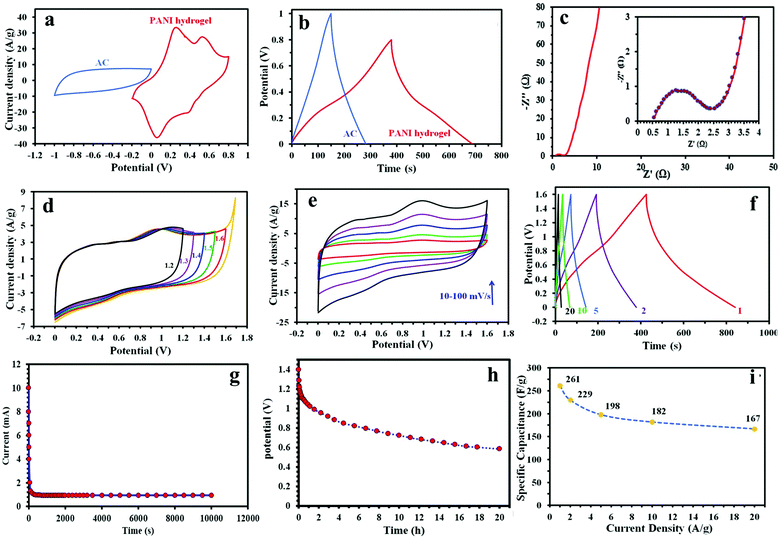
We investigated the electrode's electrochemical performance using a 1 M H2SO4 solution as the electrolyte. The typical CV curves of the PANI hydrogel and CC electrodes at a scan rate of 50 mV s−1 with a potential window of −0.2 to 0.8 versus the Ag/AgCl reference electrode are presented in Fig. 3a. Because of the increase in the CV integrated area of the PANI hydrogel electrode composed of PANI synthesized on CC, we inferred that its current density and specific capacitance improved in comparison with the CC electrode alone. Conversely, the CC's limited integrated area suggests little capacitance. Two pairs of PANI redox peaks can be clearly seen. Evidence of the improved PANI hydrogel electrode's electrochemical performance is seen in the EIS measurements and is highlighted in the PANI hydrogel and CC electrode Nyquist plots (Fig. 3b). Such plots show that the CC electrode possesses similar features to those of an EDLC supercapacitor;48 the almost vertical line observed in the low-frequency region of Fig. 3b visualizes this. Likewise, the PANI hydrogel electrode's behavior is similar, albeit in the high-frequency region. As can be seen in Fig. 3b, the PANI hydrogel electrode forms a tiny semicircle caused by the redox quinonoid/benzenoid couple's charge transfer resistance (Rct = 3.15), and thus high electrode conductivity and high rate of charge transfer. Because the CC electrode consists of carbon materials, it forms no semicircle. Furthermore the calculated capacitance based on EIS data is 331 mF cm−2.We tested the positive (PANI hydrogel) and negative (AC) electrodes separately in a three-electrode setup before fabricating the asymmetric supercapacitor. Our tests determined the capacitance and stable potential of both electrodes. As Fig. 4a shows, AC activates in a potential window of −1 to 0 V and with a specific capacitance as calculated from a CD of approximately 862 for PANI and 280 F g−1 for AC half-cells at a current density of 1 A g−1 (Fig. 4b). During device assembly, charge balance theory and the maximum potential window are achieved through positive and negative electrodes with 0.5 and 1.5 mg cm−2 mass loading, respectively. Based on the EIS spectrum shown in Fig. 4c, the charge transfer resistance and capacitance are 1.7 Ω and 305 mF cm−2, respectively.
The full asymmetric cell achieves a stable potential window of up to 1.6 V in an aqueous electrolyte (Fig. 4d), higher than that of conventional activated carbon symmetric capacitors in a similar medium (0.8–1.0 V).49 The asymmetric device exhibits a rectangular CV curve, indicative of both electric double layer capacitance and pseudo-capacitance in charge storage. Yet this curve retains its shape perfectly and demonstrates minimal shift at scan rates ranging from 10 to 100 mV s−1 (Fig. 4e), characteristic of its superior rate capability and reversibility. The double contribution of EDLC and pseudocapacitance makes it possible to increase the current density from 1 to 20 A g−1 (Fig. 4f).
Our fabricated device has a low leakage current of 0.8 mA and manifests an open circuit voltage of 0.75 V which can be maintained for more than 15 h after being fully charged. CD measurements indicate that a maximum capacitance of 261 F g−1 at 1 A g−1 is achieved for the asymmetric device (Fig. 4i), although a capacitance of 167 F g−1 is certainly achievable at a current density of 20 A g−1 demonstrating the good rate capability of the PANI/AC device (64% capacitance retention). It is worth mentioning that the areal capacitance, areal energy density, volumetric capacitance, and volumetric energy density of the fabricated supercapcitor are 522 mF cm−2, 0.185 mW h cm−2, 17.4 F cm−3 and 6.16 mW h cm−3, respectively.
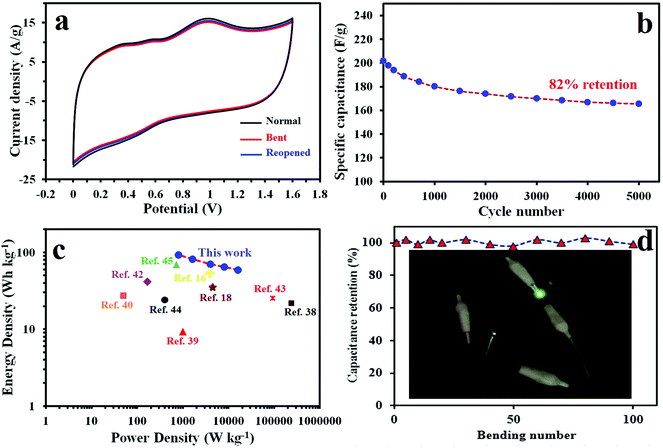
Next generation energy storage devices must be able to function despite undergoing such mechanical stress as bending or twisting. We tested our device's durability and CV behavior under various bending conditions. Fig. 5a reports the results of these tests. Few changes were observed when the device was bent or reopened, meaning it is quite flexible. In order to investigate the long-term cycling stability, we tested the device through 5000 continuous CD cycles at a high current density of 5 A g−1. Interestingly, the device shows excellent cycling stability, with only 18% decay in specific capacitance after 5000 cycles. It should be noted that the decreasing capacitance during charge–discharge cycling may be due to the destruction of PANI caused by the shrinkage and expansion of that during doping and dedoping along the cycling.
We used a Ragone plot to compare the overall performance of the fabricated device with other symmetric and asymmetric supercapacitors previously reported. As illustrated in Fig. 5c, the device exhibits a maximum energy density of 92.7 W h kg−1 and a maximum power density of 15 kW kg−1, which is much higher than symmetric and asymmetric supercapacitors such as P/N/O co-doped carbonaceous material-based SC (21.8 W h kg−1 at 238 kW kg−1),50 PANI/FC//PANI/FC (9.3 W h kg−1 at 1 kW kg−1),51 CMK-5-PANI (27.4 W h kg−1 at 0.05 kW kg−1),52 AC//AC SCs (<10 W h kg−1),53 sGNS/cMWCNT/PANI//aGNS (41.5 W h kg−1 at 0.167 kW kg−1),54 RGO/PANI/eFCC (25.4 W h kg−1 at 92.2 kW kg−1),55 GH-PANI/GP//GH-PANI/GP (24.1 W h kg−1 at 0.4 kW kg−1),56 V2O5-PANI//V2O5-PANI (69.2 W h kg−1 at 0.72 kW kg−1),57 SPAN/MoO (35 W h kg−1 at 4.7 kW kg−1)19 and graphene/polyaniline/MnOx (61.2 W h kg−1 at 4.5 kW kg−1).32 Even though some of the compared studies included additional materials apart from the PANI and used them when normalizing the energy and power density, the value of the energy and power density in this work is comparable with them. In fact this superior performance is due to: (i) the synergistic effects of carbon/PANI which can be operated in a high voltage window of 1.6 V contributing to the high energy density and (ii) the unique 3D porous structure of electrodes facilitating ion transportation and minimizing the diffusion distance to the interior surfaces by effectively absorbing the gelled electrolyte and acting as an electrolyte reservoir.
We also show the reliable performance of the fabricated device after bending 100 times (Fig. 5d). To demonstrate the practical application of our device, we connected two devices in series and after charging up to 3 V, they were able to light up green, round light-emitting diodes (LEDs), 2.7 V (Fig. 5d inset and supporting media, ESI†).
4. Conclusion
We developed a PANI hydrogel on a carbon cloth substrate as a flexible, binder-free electrode containing a 3D interconnected network, nanoscale pores, and high surface area. The electrodes' 3D porous structure combined with the synergistic effects of carbon/polymer to enhance the electric double layer capacitor's efficient contribution. Combined, these contribute to the high flexibility, excellent rate capability and cycle stability, and high energy density of the electrode. The unique 3D porous structure of the electrodes, along with the synergistic effects of carbon/polymer, improved the efficient contribution of the electric double layer capacitor and pseudocapacitance, as well as realizing the high flexibility, long cycle stability and excellent rate capability. By lighting up a green LED, our device shows great promise for energy storage applications. Furthermore, utilizing this gel electrolyte provides us with the chance of having an all-solid-state portable device with easy usage. This approach opens the door to further improvements by utilizing other polymer morphologies with a higher specific capacitance, or electrolytes with a larger operating voltage, etc. We believe that this simple and efficient strategy for the low-cost preparation of flexible electrodes establishes a step forward towards hybrid electrodes for numerous applications such as flexible supercapacitors, lithium ion batteries, fuel cells, catalysis, biosensors, gas sensors and other electronic devices.Acknowledgements
This work was made possible through the Razi University Research Council.References
- P.-Y. Chen, N.-M. D. Courchesne, M. N. Hyder, J. Qi, A. M. Belcher and P. T. Hammond, RSC Adv., 2015, 5, 37970–37977 RSC.
- F. Guo, Q. Liu and H. Mi, Mater. Lett., 2016, 163, 115–117 CrossRef CAS.
- S. Xi, Y. Kang, S. Qu and S. Han, Mater. Lett., 2016, 175, 126–130 CrossRef CAS.
- K. Wang, X. Zhang, C. Li, H. Zhang, X. Sun, N. Xu and Y. Ma, J. Mater. Chem. A, 2014, 2, 19726–19732 CAS.
- S. Zeng, H. Chen, F. Cai, Y. Kang, M. Chen and Q. Li, J. Mater. Chem. A, 2015, 3, 23864–23870 CAS.
- H. Wu, G. Yu, L. Pan, N. Liu, M. T. McDowell, Z. Bao and Y. Cui, Nat. Commun., 2013, 4, 1943 Search PubMed.
- L. Pan, G. Yu, D. Zhai, H. R. Lee, W. Zhao, N. Liu, H. Wang, B. C.-K. Tee, Y. Shi and Y. Cui, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 9287–9292 CrossRef CAS PubMed.
- Z. Zhang, S. Liu, J. Xiao and S. Wang, J. Mater. Chem. A, 2016, 4, 9691–9699 CAS.
- Z. Yang, J. Deng, X. Chen, J. Ren and H. Peng, Angew. Chem., Int. Ed., 2013, 52, 13453–13457 CrossRef CAS PubMed.
- L. Kou, T. Huang, B. Zheng, Y. Han, X. Zhao, K. Gopalsamy, H. Sun and C. Gao, Nat. Commun., 2014, 5, 3754 CAS.
- X. Wang, B. Liu, R. Liu, Q. Wang, X. Hou, D. Chen, R. Wang and G. Shen, Angew. Chem., Int. Ed., 2014, 53, 1849–1853 CrossRef CAS PubMed.
- Z. Zhang, F. Xiao, J. Xiao and S. Wang, J. Mater. Chem. A, 2015, 3, 11817–11823 CAS.
- Z. Zhang, F. Xiao, L. Qian, J. Xiao, S. Wang and Y. Liu, Adv. Energy Mater., 2014, 4, 1400064 CrossRef.
- Z. Zhang, F. Xiao and S. Wang, J. Mater. Chem. A, 2015, 3, 11215–11223 CAS.
- V. T. Le, H. Kim, A. Ghosh, J. Kim, J. Chang, Q. A. Vu, D. T. Pham, J.-H. Lee, S.-W. Kim and Y. H. Lee, ACS Nano, 2013, 7, 5940–5947 CrossRef CAS PubMed.
- G. Sun, J. Zhou, F. Yu, Y. Zhang, J. H. L. Pang and L. Zheng, J. Solid State Electrochem., 2012, 16, 1775–1780 CrossRef CAS.
- L. Li, Z. Wu, S. Yuan and X.-B. Zhang, Energy Environ. Sci., 2014, 7, 2101–2122 CAS.
- Z. S. Wu, K. Parvez, S. Li, S. Yang, Z. Liu, S. Liu, X. Feng and K. Müllen, Adv. Mater., 2015, 27, 4054–4061 CrossRef CAS PubMed.
- L.-J. Bian, H.-L. He and X.-X. Liu, RSC Adv., 2015, 5, 75374–75379 RSC.
- X. Du, Y. Xu, L. Xiong, Y. Bai, J. Zhu and S. Mao, J. Appl. Polym. Sci., 2014, 131, 40827 Search PubMed.
- F. Ke, J. Tang, S. Guang and H. Xu, RSC Adv., 2016, 6, 14712–14719 RSC.
- A. Khosrozadeh, M. Xing and Q. Wang, Appl. Energy, 2015, 153, 87–93 CrossRef CAS.
- Q. Tang, J. Wu, H. Sun, J. Lin, S. Fan and D. Hu, Carbohydr. Polym., 2008, 74, 215–219 CrossRef CAS.
- Y. Shi and G. Yu, Chem. Mater., 2016, 28, 2466–2477 CrossRef CAS.
- L. Lizarraga, E. M. a. Andrade and F. V. Molina, J. Electroanal. Chem., 2004, 561, 127–135 CrossRef CAS.
- P. Du, H. C. Liu, C. Yi, K. Wang and X. Gong, ACS Appl. Mater. Interfaces, 2015, 7, 23932–23940 CAS.
- P. Dou, Z. Liu, Z. Cao, J. Zheng, C. Wang and X. Xu, J. Mater. Sci., 2016, 51, 4274–4282 CrossRef CAS.
- D. Zhai, B. Liu, Y. Shi, L. Pan, Y. Wang, W. Li, R. Zhang and G. Yu, ACS Nano, 2013, 7, 3540–3546 CrossRef CAS PubMed.
- S. Li, J. Liu, X. Zhang, L. Li, X. Yu and Z. Huang, Polym. Bull., 2015, 72, 2891–2902 CrossRef CAS.
- Y. Shi, L. Pan, B. Liu, Y. Wang, Y. Cui, Z. Bao and G. Yu, J. Mater. Chem. A, 2014, 2, 6086–6091 CAS.
- Z. Zhang, K. Chi, F. Xiao and S. Wang, J. Mater. Chem. A, 2015, 3, 12828–12835 CAS.
- A. Jayakumar, Y.-J. Yoon, R. Wang and J.-M. Lee, RSC Adv., 2015, 5, 94388–94396 RSC.
- Z. Tai, X. Yan and Q. Xue, J. Electrochem. Soc., 2012, 159, A1702–A1709 CrossRef CAS.
- S. E. Moosavifard, J. Shamsi, S. Fani and S. Kadkhodazade, RSC Adv., 2014, 4, 52555–52561 RSC.
- S. E. Moosavifard, S. Fani and M. Rahmanian, Chem. Commun., 2016, 52, 4517–4520 RSC.
- P. Simon, Y. Gogotsi and B. Dunn, Science, 2014, 343, 1210–1211 CrossRef CAS PubMed.
- P. Huang, C. Lethien, S. Pinaud, K. Brousse, R. Laloo, V. Turq, M. Respaud, A. Demorti”re, B. Daffos and P. Taberna, Science, 2016, 351, 691–695 CrossRef CAS PubMed.
- Y. A. Ismail, J. Chang, S. R. Shin, R. S. Mane, S.-H. Han and S. J. Kim, J. Electrochem. Soc., 2009, 156, A313–A317 CrossRef CAS.
- X. Xiang, W. Zhang, Z. Yang, Y. Zhang, H. Zhang, H. Zhang, H. Guo, X. Zhang and Q. Li, RSC Adv., 2016, 6, 24946–24951 RSC.
- J. Hur, K. Im, S. W. Kim, U. J. Kim, J. Lee, S. Hwang, J. Song, S. Kim, S. Hwang and N. Park, J. Mater. Chem. A, 2013, 1, 14460–14466 CAS.
- B. C. Kim, M. Rajesh, H. S. Jang, K. H. Yu, S.-J. Kim, S. Y. Park and C. J. Raj, J. Alloys Compd., 2016, 674, 376–383 CrossRef CAS.
- Q. X. Xia, K. San Hui, K. N. Hui, S. D. Kim, J. H. Lim, S. Y. Choi, L. J. Zhang, R. S. Mane, J. M. Yun and K. H. Kim, J. Mater. Chem. A, 2015, 3, 22102–22117 CAS.
- M. M. Pérez-Madrigal, F. Estrany, E. Armelin, D. D. Díaz and C. Alemán, J. Mater. Chem. A, 2016, 4, 1792–1805 Search PubMed.
- S. E. Moosavifard, J. Shamsi, S. Fani and S. Kadkhodazade, Ceram. Int., 2014, 40, 15973–15979 CrossRef CAS.
- H. Ding, M. Zhong, Y. J. Kim, P. Pholpabu, A. Balasubramanian, C. M. Hui, H. He, H. Yang, K. Matyjaszewski and C. J. Bettinger, ACS Nano, 2014, 8, 4348–4357 CrossRef CAS PubMed.
- G.-P. Hao, F. Hippauf, M. Oschatz, F. M. Wisser, A. Leifert, W. Nickel, N. Mohamed-Noriega, Z. Zheng and S. Kaskel, ACS Nano, 2014, 8, 7138–7146 CrossRef CAS PubMed.
- A. Y. Arasi, J. J. L. Jeyakumari, B. Sundaresan, V. Dhanalakshmi and R. Anbarasan, Spectrochim. Acta, Part A, 2009, 74, 1229–1234 CrossRef PubMed.
- J. Ji, L. L. Zhang, H. Ji, Y. Li, X. Zhao, X. Bai, X. Fan, F. Zhang and R. S. Ruoff, ACS Nano, 2013, 7, 6237–6243 CrossRef CAS PubMed.
- C. Zhou, Y. Zhang, Y. Li and J. Liu, Nano Lett., 2013, 13, 2078–2085 CrossRef CAS PubMed.
- X. Qing, Y. Cao, J. Wang, J. Chen and Y. Lu, RSC Adv., 2014, 4, 55971–55979 RSC.
- L.-J. Bian, F. Luan, S.-S. Liu and X.-X. Liu, Electrochim. Acta, 2012, 64, 17–22 CrossRef CAS.
- Z. Lei, X. Sun, H. Wang, Z. Liu and X. Zhao, ACS Appl. Mater. Interfaces, 2013, 5, 7501–7508 CAS.
- Q. Qu, Y. Zhu, X. Gao and Y. Wu, Adv. Energy Mater., 2012, 2, 950–955 CrossRef CAS.
- J. Shen, C. Yang, X. Li and G. Wang, ACS Appl. Mater. Interfaces, 2013, 5, 8467–8476 CAS.
- P. Yu, Y. Li, X. Zhao, L. Wu and Q. Zhang, Langmuir, 2014, 30, 5306–5313 CrossRef CAS PubMed.
- K. Chi, Z. Zhang, J. Xi, Y. Huang, F. Xiao, S. Wang and Y. Liu, ACS Appl. Mater. Interfaces, 2014, 6, 16312–16319 CAS.
- M.-H. Bai, T.-Y. Liu, F. Luan, Y. Li and X.-X. Liu, J. Mater. Chem. A, 2014, 2, 10882–10888 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6nj02266a |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 |