A simple method for preparation of a novel hydrophobic ionic liquid with a per-fluoro-tert-butoxide anion†
Kiki A.
Kurnia
a,
Tânia E.
Sintra
b,
Yann
Danten
c,
Maria Isabel
Cabaço
de,
Marcel
Besnard
c and
João A. P.
Coutinho
 *b
*b
aDepartment of Chemical Engineering, Universiti Teknologi PETRONAS, Bandar Seri Iskandar, Perak 32610, Malaysia
bCICECO – Aveiro Institute of Materials, Department of Chemistry, University of Aveiro, 3810-193, Aveiro, Portugal. E-mail: jcoutinho@ua.pt; Fax: +351 234 370084; Tel: +351 234 370200
cInstitut de Sciences Moléculaires, CNRS (UMR 5255), Université Bordeaux, 351 Cours de a Libération 33405, Talence Cedex, France
dCentro de Física Atómica da UL, Av. Prof. Gama Pinto 2, 1694-003 Lisboa, Portugal
eDepartamento de Física, Instituto Superior Técnico, UTL, Av. Rovisco Pais 1049-001, Lisboa, Portugal
First published on 21st November 2016
Abstract
In this work, we demonstrate a simple and atom-economic method for preparation of a novel hydrophobic ionic liquid (IL) from hydrophilic ILs and its characterization data are disclosed. The simple preparation route also provides opportunities for removal/recovery of the IL during cellulose dissolution.
Ionic liquids (ILs) are organic salts with a melting point below 100 °C that have widespread application in organic synthesis, catalysis, biomass dissolution, and liquid–liquid extraction, among many others.1,2 Their unique feature is essentially determined by their structural characteristics, which originated from, at least, cations, anions, alkyl chain length, and functional groups. It is evaluated that there are about one million possible pure ILs and 1018 ternary liquid mixtures.3 It should, however, be highlighted that hydrophobic ILs are far outnumbered by hydrophilic ILs, even if that class of ILs has been shown to be promising media for the extraction of (bio)molecules and (bio)fuels from aqueous solutions.4–6 With the ever-growing demands for novel ILs with desired structures and functions, the designed synthesis of such compounds has aroused considerable interest. In the absence of predictive computational methods to direct their design, the discovery-based development of novel ILs will remain vital to the field. This is especially the case vis-á-vis heretofore unknown and unused classes of ions when such entities are easily prepared and provide access to potentially unique structural or electronic attributes. In light of these considerations, we report here, for the first time, the synthesis and characterization of the novel IL 1-butyl-3-methylimidazolium per-fluoro-tert-butoxide, [C4C1im][Pftb], made from 1-butyl-3-methylimidazolium acetate, [C4C1im][OAc], or from an ever simpler and cheaper starting material, 1-butyl-3-methylimidazolium chloride, [C4C1im]Cl, both commercially available and simple to synthesize. This new anion offers the potential to further tailor the physical properties, such as density, viscosity, and surface tension, and, most importantly, to create a new family of hydrophobic and water immiscible ionic liquids.
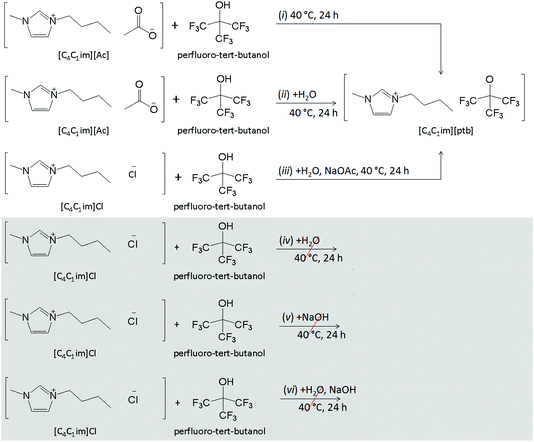
We have explored six different routes for the preparation of [C4C1im][Pftb] as shown in Fig. S1.† The first approach involved the direct mixing of [C4C1im][OAc] and a slightly excess molar ratio of per-fluoro-tert-butanol under solvent free conditions, followed by the removal of the unreacted material and by-product (acetic acid) under vacuum, to give a transparent liquid in 98% yield. This one-step procedure is a typical atom-economic reaction without poisonous by-product. The novel IL was characterized by Fourier transform infra-red (FTIR) spectroscopy; 1H, 13C, and 19F nuclear magnetic resonance (NMR) spectroscopies; and thermogravimetric analysis (TGA). Four changes in the FTIR spectra (Fig. 2) reveal the structural information about the product. (1) The FTIR spectrum of [C4C1im][OAc] displays four bands detected at 630, 1174, 1382, and 1582 cm−1. It is clear that the last two bands (the symmetric and asymmetric stretches of COO−, respectively) disappear for the synthesized novel hydrophobic IL, whereas the first two are observed (bending and vibrations of the imidazolium ring cation), although the second one is found in the large and composed region at around 1205 cm−1. (2) A decrease in CH vibrations is observed at around 2917 cm−1 (symmetry vibration of the methyl acetate) for the synthesized IL. (3) The characteristic bands of the CF stretching of the per-fluoro-tert-butanol spectrum are observed at about 1135 and 1243 cm−1, which overlap with the [C4C1im][Pftb]. Two other bands at 727 (CF3 asymmetry deformation) and 955–972 cm−1 (skeletal stretching) are yet to be detected for the novel IL. (4) The OH stretching and bending at around 3610 and 1380 cm−1, respectively, observed in the per-fluoro-tert-butanol spectrum were not observed in the spectrum of [C4C1im][Pftb]. Thus, it is clear from (1) and (2) behavioural changes that the acetate anion is not present in the final product, and from the third and fourth that the anion [Pftb]− (produced from the per-fluoro-tert-butanol that lost its acidic proton) is detected. Moreover, the formed acetic acid (by-product) is not detected in the NMR spectra of the final product, indicating the high purity of the synthesized novel hydrophobic IL.
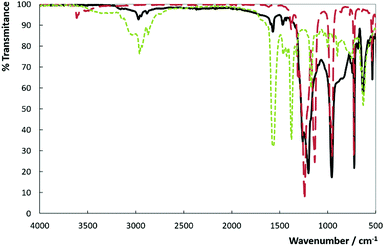 | ||
| Fig. 2 FTIR spectra of the novel synthesized IL and the starting material. [C4C1im][Pftb], solid line; per-fluoro-tert-butanol, dashed-line; [C4C1im][OAc], dotted-line. | ||
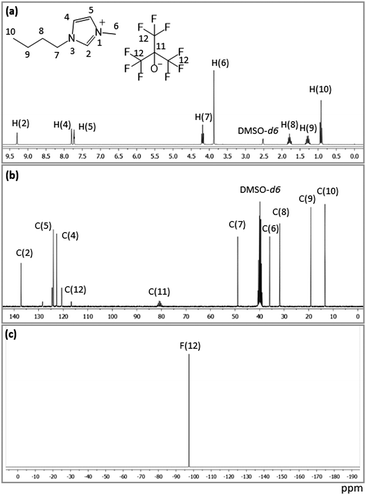
The absence of both acetate anion (starting material) and acetic acid (by-product) in the final product is also confirmed by NMR spectra (cf.Fig. 3). The proton NMR spectrum of the synthesized IL recorded in DMSO-d6 showed eight resonances, as shown in Fig. 3a, which are assigned to the hydrogen atoms of the cationic part of the IL. The absence of extra peaks, particularly for acetate (from the starting material) and acetic acid (produced as the by-product), indicates the high purity of the synthesized IL. The 13C NMR spectrum showed 10 resonances corresponding to the 8 carbons of the cationic part of the IL, while 2 resonances originated from the anionic part. The 13C NMR spectrum of [C4C1im][Pftb] without proton decoupling is presented in Fig. S1 (ESI†). One single resonance is observed at −97.61 ppm in the 19F spectrum of the synthesized IL, assigned to the fluorine atom on the anionic counterpart. No additional peak in both 13C and 19F spectra was observed, further confirming the high purity of the synthesized novel IL, [C4C1im][Pftb]. The assignment of the observed resonances is presented in Table S1 (ESI†).
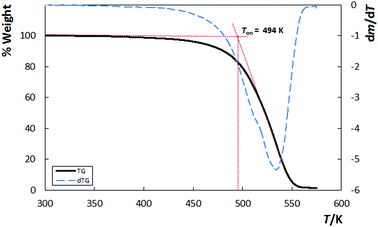
The thermogravimetric curve of the IL showed decomposition onset temperature at 494 K and the thermogravimetric curves of the derivatives showed only one peak at 533 K (Fig. 4). This novel IL appears to have a similar thermal stability to the corresponding starting material, [C4C1im][OAc], whose decomposition temperature is 493 K (ESI†).
The second preparation method attempted (cf.Fig. 1) involved addition of water to a mixture of [C4C1im][OAc] and per-fluoro-tert-butanol. The obtained IL was also characterized by the same techniques used in the first preparation, and showed spectroscopic and thermogravimetric data identical to those of the IL sample prepared using the first method. It is worth mentioning that we also attempted the reaction between [C4C1im][OAc] and tert-butanol, yet no reaction occurred. The reaction between [C4C1im][OAc] and per-fluoro-tert-butanol is an acid–base reaction, in which the latter compound act as the acid (pKa = 5.6),7 providing a hydrogen atom to combine with the acetate anion of the IL, and thus producing acetic acid as the by-product and [C4C1im][Pftb] as the main product. The much higher value of the pKa of tert-butanol (pKa = 16.58)7 accounts for its inability to provide a hydrogen atom in this reaction as it is too weak acid to react with. The replacement of the hydrogen atom by fluorine in tert-butanol to give per-fluoro-tert-butanol significantly increases the acidity. This is due to the fact that the electron-withdrawing character of the fluorine atom causes the hydrogen atom of the hydroxyl group in per-fluoro-tert-butanol (δ+ of H = 0.417) to have a higher partial positive charge (ESI†) when compared to tert-butanol (δ+ of H = 0.383); thus the former has higher acidity that allows the reaction with [C4C1im][OAc].
[C4C1im][Pftb] can also be prepared from other hydrophilic ILs, namely [C4C1im]Cl, and per-fluoro-tert-butanol in the presence of an aqueous solution of sodium acetate, NaOAc (method (iii) in Fig. 1). The synthesized IL, which appears as a new phase, also presents identical spectra and thermograms to the IL prepared using method (i). However, method (iii) gives a somewhat lower yield, 85%, that can be due to the loss of the IL into the NaCl/NaOAc aqueous phase. Direct mixing of [C4C1im]Cl and per-fluoro-tert-butanol, however, did not lead to any chemical reaction. As previously mentioned, the formation of [C4C1im][Pftb] can be simply considered as an acid–base reaction, in which the per-fluoro-tert-butanol serves as the acid source, while the IL anion acts as the base. Thus, the inability of [C4C1im]Cl to directly react with per-fluoro-tert-butanol can be addressed due to the weak basicity of Cl− (solvatochromic β parameter = 0.87) when compared with [OAc]− (β = 1.09).8 In addition, we also made an effort to prepare [C4C1im][Pftb] by mixing [C4C1im]Cl with NaOH in the presence and absence of H2O. Nevertheless, these two methods did not produce the desired product.
The physical properties and toxicity of the novel IL at 298 K were also characterized and the results are presented in Table 1. From these data, it can be concluded that this novel hydrophobic IL displays physical properties and toxicity similar to the well-known fluorine-containing IL [C4C1im][NTf2].9,10 As expected this synthesis can be straightforwardly applied to produce hydrophobic ionic liquids.
| Properties | Value |
|---|---|
| Density (ρ/kg m−3) | 1513.90 |
| Viscosity (η/mPa s) | 215.95 |
| Refractive index (nD) | 1.372878 |
| Surface tension (γ/mN m−1) | 26.82 |
| Water solubility (xH2O) | 0.3632 |
| Solubility in water (xIL) | 6.02 × 10−4 |
| Toxicity (EC50/mg L−1) | 84.41 (5); 84.92 (15); 85.62 (30) |
In addition, it is interesting to observe that this novel IL is immiscible with water, while the starting material, [C4C1im][OAc], is extremely soluble. Here we show that the miscibility of the IL can be easily tuned by changing its counteranion. This unique feature – water miscibility changes – which can be straightforwardly achieved by simply adding the per-fluoro-tert-butanol to the hydrophilic IL, [C4C1im][OAc], to make the hydrophobic IL, [C4C1im][Pftb], has a number of practical implications for designing processes with ILs. For example, both [C4C1im]Cl and [C4C1im][OAc] are reported to be highly efficient solvents for the dissolution and regeneration of cellulose.11–13 The regenerated cellulose could be precipitated from the IL solutions by adding water.11–13 In this separation process, large volumes of dilute aqueous solutions of ILs are produced. The high values of ILs also call for efficient recycling of them from these aqueous solutions. In light of this consideration, we then turned our attention to apply this simple synthesis method to recover the IL from aqueous solution after it was used for the dissolution and regeneration of cellulose. Using this method, we could recover 82% of the IL. Furthermore, the spectroscopic and thermogravimetric data of the recovered IL also are identical to those of the IL prepared using method (i), indicating there is no cellulose in the IL phase. A detailed discussion on this matter will be presented elsewhere.
To summarize, we have shown that a novel hydrophobic IL can be prepared by three simple alternative routes in excellent yields, and as far as we know this is the first report on this type of IL. The simple preparation involved encourages the commercialization of processes based on this type of IL. We anticipate that this novel hydrophobic IL will find application in many areas of interest, not the least in liquid–liquid extraction from aqueous solution.
The authors thank national funding from the Fundação para a Ciência e a Tecnologia (FCT, Portugal), European Union, QREN, FEDER and COMPETE by the projects PEST-C/CTM/LA0011/2013, PTDC/ACC-AMB/119172/2010 and PTDC/QUI-QUI/121520/2010. T. E. Sintra also thanks FCT for the doctoral grant SFRH/BPD/85871/2012.
References
- T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef CAS.
- N. V. Plechkova and K. R. Seddon, Chem. Soc. Rev., 2008, 37, 123–150 RSC.
- R. D. Rogers, Nature, 2007, 447, 917–918 CrossRef CAS PubMed.
- A. Chapeaux, L. D. Simoni, T. S. Ronan, M. A. Stadtherr and J. F. Brennecke, Green Chem., 2008, 10, 1301–1306 RSC.
- D. Rabari and T. Banerjee, Fluid Phase Equilib., 2013, 355, 26–33 CrossRef CAS.
- J. Wang, Y. Pei, Y. Zhao and Z. Hu, Green Chem., 2005, 7, 196–202 RSC.
- W. Reeve, C. M. Erikson and P. F. Aluotto, Can. J. Chem., 1979, 57, 2747–2754 CrossRef CAS.
- M. A. Ab Rani, A. Brant, L. Crowhurst, A. Dolan, M. Lui, N. H. Hassan, J. P. Hallett, P. A. Hunt, H. Niedermeyer, J. M. Perez-Arlandis, M. Schrems, T. Welton and R. Wilding, Phys. Chem. Chem. Phys., 2011, 13, 16831–16840 RSC.
- M. A. A. Rocha, C. M. S. S. Neves, M. G. Freire, O. Russina, A. Triolo, J. A. P. Coutinho and L. M. N. B. F. Santos, J. Phys. Chem. B, 2013, 117, 10889–10897 CrossRef CAS PubMed.
- K. A. Kurnia, T. E. Sintra, C. M. S. S. Neves, K. Shimizu, J. N. Canongia Lopes, F. Goncalves, S. P. M. Ventura, M. G. Freire, L. M. N. B. F. Santos and J. A. P. Coutinho, Phys. Chem. Chem. Phys., 2014, 16, 19952–19963 RSC.
- A. Pinkert, K. N. Marsh, S. Pang and M. P. Staiger, Chem. Rev., 2009, 109, 6712–6728 CrossRef CAS PubMed.
- H. Ohno and Y. Fukaya, Chem. Lett., 2009, 38, 2–7 CrossRef CAS.
- S. Zhu, Y. Wu, Q. Chen, Z. Yu, C. Wang, S. Jin, Y. Ding and G. Wu, Green Chem., 2006, 8, 325–327 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, CHelpG atomic coordinates and charge distribution. See DOI: 10.1039/c6nj02575g |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2017 |