Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Using endogenous ligands for direct superparamagnetic nanoparticle cluster-based body fluid exosome separation†
Hongzhao Qia,
Huanhuan Jiab,
Jimeng Sanga,
Yu Ren*b,
Jin Zhao*a,
Xin Houa and
Xubo Yuana
aTianjin Key Laboratory of Composite and Functional Materials, School of Materials Science and Engineering, Tianjin University, Tianjin 300072, China. E-mail: zhaojin@tju.edu.cn
bTianjin Research Center of Basic Medical Science, Tianjin Medical University, Tianjin 300070, China. E-mail: renyu0425@yahoo.com
First published on 12th January 2017
Abstract
The separation and purification of exosomes is essential for the application of exosomes. Previously we have developed a new strategy based on superparamagnetic nanoparticle clusters for the separation of exosomes. Here, to optimize this method for the direct separation of body fluid exosomes using endogenous ligands, we unveiled the influence of free ligands on the formation of clusters and the efficiency of exosome separation. Transferrin was chosen as a model endogenous ligand and the concentration of free serum transferrins was adjusted by different times of serum dialysis. To directly separate most of the exosomes from 1 mL untreated serum, at least 360 μg of labelled ligands needed to be used. However, the required amount of labelled ligands reduced to 10 μg when serum was pre-dialyzed for 24 hours. The results demonstrate that the free ligands can compete with ligands labelled on superparamagnetic nanoparticles to affect the formation of clusters. And to separate exosomes from body fluids sufficiently and directly, the amount of labelled ligands must reach a minimum value (i.e., equivalent to the amount of free ligands). In addition, eliminating free ligands by the mild pre-treatment of body fluids facilitates the separation and purification of body fluid exosomes. This study can improve the universality of current immunoaffinity magnetic particle-based methods for exosome separation and facilitate the in vivo clinical translation of exosomes.
Introduction
Exosomes have attracted increasing attention in recent years due to their crucial roles in intercellular communication, immunity regulation and disease progression.1–3 They have been used as tumor vaccines and disease diagnosis markers on account of their special contents, such as lipids, proteins, mRNAs, and miRNAs.4,5 Furthermore, exosomes can be excellent drug delivery vehicles because of their appropriate size, protein–lipid bilayer structure and incomparable biocompatibility.6–8 No matter to which field exosomes are applied, the precise separation and purification of exosomes is the first and most important step. However to date, scientists have yet to standardize desirable methods for separating and purifying exosomes, especially for the separation and purification of body fluid exosomes.Ultracentrifugation seems to be the “gold standard” for the separation of exosomes at the present stage, but this method is crude and non-specific.9 The separated exosomes are frequently contaminated with other proteins and particulates derived from the medium and cell debris.10 And the exosomes pellet often cannot be re-suspended completely, resulting in the difficulty of its in vivo application.11 Even being used, the aggregate proteins could arouse strong immune responses.12 Various technologies have been developed to optimize the ultracentrifugation method. By combining the method with density gradient, the purity of separated exosomes is remarkably increased.13 But exosomes and HIV-1 particles cannot be efficiently separated by this method because of the similarities in their size and density.14 High performance liquid chromatography on a gel exclusion column (HPLC-GEC) can be used to obtain high-purify exosomes. However, this method is also non-specific because of the size dependence of column packing materials. Furthermore, the elution buffer can damage the structure and function of exosomes.15 Compared with density gradient centrifugation and HPLC-GEC, precipitation based methods (such as ExoQuick Precipitation Solution) are more simple as they save time and don't require specialized expensive equipments, but they may co-precipitate other microparticles and proteins.16 And our researches demonstrate that these precipitations can denature the surface proteins of exosomes. Moreover, all methods mentioned above seem to concentrate rather than separate exosomes.
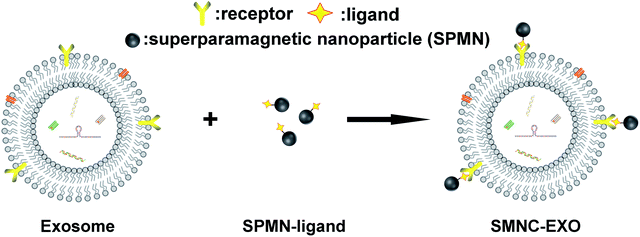
The precise separation and purification of exosomes needs to specifically recognize them. Immunoaffinity magnetic particle-based method is an appropriate choice. Exosomes have been successfully purified by anti-EpCAM antibody-labelled magnetic particles through the interaction of antibody and antigen.17 However, these particles are too large (several hundred nanometers or a few micrometers) to be used for in vivo application, as they are easily trapped in the liver.18 Furthermore, these particles are ferromagnetism and cannot be re-dispersed after magnetic separation resulting in the failure of intravenous administration. To address these limitations, we previously developed a new separation method for exosomes based on superparamagnetic nanoparticles cluster (denoted as SMNC).19 The basic principle of the method was shown in Scheme 1. This method can be used to separate and purify exosomes from any kinds of sources, especially from body fluids, as long as choosing the appropriate ligand and surface receptor of exosomes. And more importantly, the resulting SMNC-EXOs can be directly used in almost all exosomes-related research fields, such as immunoregulation, liquid biopsy and drug delivery because superparamagnetic nanoparticles (denoted as SPMNs) have little influence on exosomes. In other words, SMNC-based separation method can fulfil the integration of exosomes separation and in vivo application.
In this method, the key to the formation of exosomes-based superparamagnetic nanoparticles clusters (denoted as SMNC-EXOs) is the interaction of ligands labelled on SPMNs and receptors expressed on the surface of exosomes. Theoretically, many ligands can be used to form SMNC-EXOs in identical separation medium because of the abundance of receptors on the surface of exosomes. However, the choice of ligands actually needs to be determined by the application of exosomes. Specific exogenous ligands can ensure the purity of exosomes, which will facilitate to optimize the in vitro application of exosomes, such as improvement of the sensitivity of exosomes-based liquid biopsy. Conversely, endogenous ligands are more suitable to form SMNC-EXOs for in vivo application since the endogeneity of ligands can avoid the potential immune response of exogenous ligands and endow SMNC-EXOs with high biological safety.20 But compared with the exogenous ligands, endogenous ligands complicate the separation process because the corresponding free ligands in the separation medium affects the combination of SPMNs and exosomes. These free ligands may bind with the receptors of exosomes resulting in the failure of binding of exosomes receptors with ligands labelled on SPMNs. Furthermore, even if ligands labelled on SPMNs have binded with exosomes receptors, free ligands may compete with them rendering the dissociation of SMNC-EXOs. Although the elimination of free ligands could avoid such potential influence, the pre-treatment of body fluids may damage the structure and function of exosomes. Thus, understanding the influence of free ligands on the formation of SMNC-EXOs and using endogenous ligands to separate exosomes from body fluids directly are both necessary for their in vivo application.
In this study, we chose transferrin as an endogenous ligand to separate exosomes from fresh rat serum by SMNC-based method directly. As control, a group of serum was pre-dialyzed (for 24 hours, unless mentioned otherwise) to eliminate free serum transferrins. According to our previous research, SPMN–transferrin complexes (denoted as M-Tfs) could successfully and easily separate exosomes from pre-dialyzed serum. Through analysis of the differences in the quantities of separated exosomes, the influence of free ligands on SMNC-EXOs formation could be revealed, realizing the direct separation of exosomes from body fluids by endogenous ligands and optimizing the current immunoaffinity magnetic particle-based method for exosomes separation.
Experimental
Materials and reagents
Carboxyl-group functionalized superparamagnetic Fe3O4 nanoparticles were purchased from Nanjing Nanoeast Biotech Co., Ltd. Transferrins were purchased from Sigma-Aldrich. BCA protein assay kit and enzyme-linked immune-sorbent (ELISA) assay kit were purchased from Thermo Scientific.Synthesis of M-Tfs
Carboxyl-group functionalized superparamagnetic Fe3O4 nanoparticles solution (200 μL, 2.5 mg mL−1) was mixed with N1-((ethylimino)methylene)-N3,N3-dimethylpropane-1,3-diamine (EDC) and N-hydroxysuccinimide (NHS) at a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
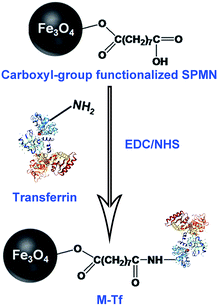
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (pH 5.5). This reaction mixture was incubated at room temperature for 1 h. Then, 1 μL of 2-mercaptoethanol was added to terminate the reaction. The activated superparamagnetic Fe3O4 nanoparticles were purified via magnetic separation and were re-suspended in 200 μL Borate buffer (20 mM, pH 8.5). Then, 50 μg of holo-transferrins were added, and the mixture was incubated for 12 h at 4 °C under nitrogen. Finally, M-Tfs were purified by magnetic separation and washed three times with PBS. The resulting solution (200 μL) was stored at 4 °C until it was used for the separation of exosomes.
3 (pH 5.5). This reaction mixture was incubated at room temperature for 1 h. Then, 1 μL of 2-mercaptoethanol was added to terminate the reaction. The activated superparamagnetic Fe3O4 nanoparticles were purified via magnetic separation and were re-suspended in 200 μL Borate buffer (20 mM, pH 8.5). Then, 50 μg of holo-transferrins were added, and the mixture was incubated for 12 h at 4 °C under nitrogen. Finally, M-Tfs were purified by magnetic separation and washed three times with PBS. The resulting solution (200 μL) was stored at 4 °C until it was used for the separation of exosomes.
The influence of pre-dialysis on the magnetic separation of exosomes
1 mL serum from Kunming mice was first dialyzed against acetate buffer (pH = 5.0) for 8 h and then dialyzed against PBS (pH = 7.2) overnight. All animal experimental protocols were conducted within Tianjin Medical University's guidelines for animal research and were approved by the Institutional Animal Care and Use Committee. The molecular weight cut off of dialysis tube is 300 kD. The resulting serum solution was mixed with 200 μL M-Tfs solution and blended homogeneously using a vortex shaker. This mixture was incubated for 4 h at 4 °C and then submitted to a magnetic field (1 T) for separation. After 2 and 24 hours of magnetic separation, the supernatant was removed and the separated sample was collected respectively. Samples were washed three times with PBS. Finally, they were re-dispersed in 50 μL PBS and the amounts of obtained exosomes were determined by the measurement of protein concentration via BCA protein assay, employing the samples separated from M-Tfs solution as control.In comparison, M-Tfs were mixed with untreated Kunming mice serum. In accordance with the method mentioned above, the samples were separated from the untreated serum and the protein concentration of exosomes was also tested by BCA protein assay.
The size as well as size distributions of the samples after 24 hours of separation from M-Tfs solution, untreated and pre-dialyzed serum were respectively measured by dynamic light scattering (BI-90Plus, Brookhaven Instruments Ltd., USA), and their morphologies were visualized using a high-resolution transmission electron microscope (TEM, JEM-2100F, JEOL Ltd., Japan).
The influence of the amount of M-Tfs on exosomes separation
To study the influence of the amount of M-Tfs on exosomes separation, we have respectively added different amounts of M-Tfs (0.1, 0.3, 0.5, 0.7, 0.9, 1.2, 1.5, 1.8, 2.1, 2.4, 2.7, 3, 3.3, 3.6, 3.9, 4.2 mg) to 1 mL untreated serum. After incubation and 2 hours of magnetic separation, the protein concentrations of separated products were tested using BCA protein assay.Verification of exosomes
3.6 mg of M-Tfs were added to untreated serum to incubate for 4 h. After 2 hours of magnetic separation, the resulting products were analyzed by Western blot analysis as described previously with slight modifications.21 Briefly, products for Western blots were prepared by lysing exosomes in a radioimmunoprecipitation assay (RIPA) buffer containing 1 mM EDTA. Lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were rinsed with PBS for several minutes and blocked with Odyssey blocking buffer for 1 h at 22 °C. The membranes were incubated with primary antibodies against CD9, CD63 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilution; Zhongshan Bio Corp, Beijing, China), followed by incubation with fluorescent secondary antibodies (1
1000 dilution; Zhongshan Bio Corp, Beijing, China), followed by incubation with fluorescent secondary antibodies (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 dilution; Zhongshan Bio Corp, Beijing, China). Images were acquired with an Odyssey infrared imaging system and analyzed using software specified by the Odyssey systems.
1000 dilution; Zhongshan Bio Corp, Beijing, China). Images were acquired with an Odyssey infrared imaging system and analyzed using software specified by the Odyssey systems.
The measurement of transferrins concentration
The concentrations of transferrins in untreated serum and pre-dialyzed (for 24 hours) serum were measured using ELISA assay. PBS solution was used as control.The influence of serum pre-dialysis on exosomes separation
To investigate the influence of serum pre-dialysis on exosomes separation, we have respectively dialyzed serum for different time (0, 2, 4, 6, 8, 10, 12, 14 and 24 h). After pre-dialysis, the concentrations of transferrins in the serum were tested using ELISA assay. At the same time, same amounts of M-Tfs were respectively added to the rest of serum to incubate for 4 h. After 2 hours of magnetic separation, the protein concentrations of products were tested using BCA protein assay.Exosomes separation from pre-dialyzed serum by M-Tfs containing equivalent holo-Tfs
Serum was respectively dialyzed for different time (0, 2, 4, 6, 8, 10, 12, 14 and 24 h) and the concentrations of transferrins of these serum were tested using ELISA assay. Then M-Tfs containing equivalent holo-Tfs were added to the pre-dialyzed serum respectively. After 4 hours of incubation and 2 hours of magnetic separation, the protein concentrations of separated products were tested using BCA protein assay.Results
The separation of exosomes from untreated and pre-dialyzed serum
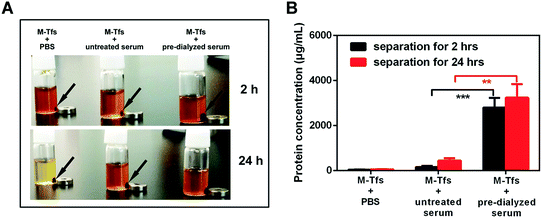
M-Tfs were first synthesized by conjugation of holo-transferrins (iron-loaded transferrin, holo-Tfs) to ∼10 nm Fe3O4 SPMNs. The reaction mechanism was shown in Fig. 1. Holo-Tfs were chosen because only holo-Tfs can bind with transferrin receptors. Then SPMNs can be bound to exosomes through the transferrin–transferrin receptor interaction. The obtained SMNC-EXOs can be magnetically separated to realize the separation and purification of exosomes.To separate exosomes from untreated serum, 0.5 mg of M-Tfs (containing ∼50 μg of holo-Tfs) were mixed with 1 mL untreated serum and incubated for 4 h at 4 °C. After magnetic separation, the situation of separation was photographed (Fig. 2A). The amounts of products separated from PBS and untreated serum were increased when the separation time increased from 2 hours to 24 hours. However, the amount of products separated from pre-dialyzed serum had no significant change. This result implies the separation situation of exosomes from untreated serum and pre-dialyzed serum is different. The variation of protein concentration of separated products (protein concentration can be used to indicate the amount of exosomes) proved the speculation.6 The protein concentration of products (re-dispersed in 50 μL PBS) separated from untreated serum could increase from ∼155.67 μg mL−1 to ∼438.69 μg mL−1 with the extension of separation time, but it was far below that of products (re-dispersed in 50 μL PBS) separated from pre-dialyzed serum (∼3 mg mL−1) (Fig. 2B). As we have previously proved products magnetically separated from pre-dialyzed serum were mainly SMNC-EXOs and the highest protein concentration of exosomes in 1 mL serum was ∼3 mg mL−1. Thus, these results indicate the formation situation of SMNC-EXOs is different when M-Tfs were added to untreated and pre-dialyzed serum.
The characterization of separated products
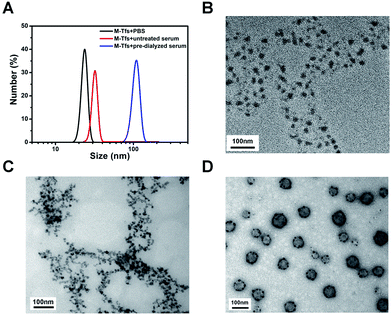
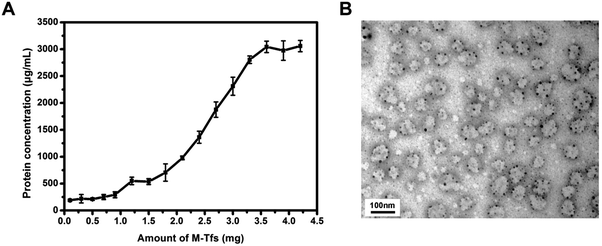
To further verify our conclusion, we characterized the size and morphology of the products separated for 24 hours. The mean size of products separated from untreated serum was ∼32 nm, similar to that of M-Tfs. While the mean size of products separated from pre-dialyzed serum, which were SMNC-EXOs, was ∼105 nm (Fig. 3A). In consideration of the size of exosomes (30–100 nm), we deemed M-Tfs cannot be bound to exosomes to form SMNC-EXOs in untreated serum. The transmission electron microscopy (TEM) results further proved this conclusion. Compared with M-Tfs, the morphology of products separated from untreated serum didn't change much (Fig. 3B and C). But the morphology of products separated from pre-dialyzed serum was round vesicles surrounded by black dots (Fig. 3D). And we have proved round vesicles were exosomes and black dots were SPMNs. Thus, the vast majority of M-Tfs added to untreated serum were not bound to exosomes.But it should be noted not all M-Tfs can't be bound to exosomes in untreated serum. As it can be observed from TEM image (Fig. S1†) that a few of SMNC-EXOs could be found in products separated from untreated serum, though the exosomes were relatively small and the number of SPMNs bound to exosomes was relatively few.
The influence of M-Tfs amount on exosomes separation
To prove whether increasing the amount of M-Tfs could promote the formation of SMNC-EXOs in untreated serum, different amounts of M-Tfs were respectively added to 1 mL untreated serum and magnetically separated for 2 hours after 4 hours of incubation. The protein concentration of separated products had no significant changes when the amount of M-Tfs was less than 1 mg, but further improving the amount of M-Tfs, the protein concentration gradually increased (Fig. 4A). When the amount of M-Tfs increased to 3.6 mg (the amount of holo-Tfs was ∼360 μg), the protein concentration of products separated from untreated serum reached to ∼3 mg mL−1 and further increasing the amount of M-Tfs didn't enhance the protein concentration of products. These products were proved to be SMNC-EXOs as it can be clearly seen from their TEM images that vesicles were surrounded by SPMNs (Fig. 4B) and exosomes marker proteins were also detected (Fig. S2†). As we have mentioned above the highest protein concentration of exosomes in 1 mL serum was ∼3 mg mL−1. So these results demonstrate when the amount of added M-Tfs reached to 3.6 mg, nearly all exosomes have formed SMNC-EXOs and been separated from 1 mL untreated serum.It should be noticed that products separated from untreated serum by 3.6 mg of M-Tfs were impure. A lot of free M-Tfs could be observed in the TEM image (Fig. 4B). And there were more free M-Tfs when separation time was extended to 24 hours (Fig. S3†). These results demonstrate even if excess amount of M-Tfs are added to serum, only part of M-Tfs can be bound to exosomes. Although the magnetic separation time of M-Tfs and SMNC-EXOs are different, the high amount of free M-Tfs still results in the impurity of SMNC-EXOs. Since using endogenous ligand to form SMNC-EXOs is beneficial to the in vivo application of exosomes, the possible side effects of free M-Tfs should be noted.
The influence of free serum transferrins on exosomes separation
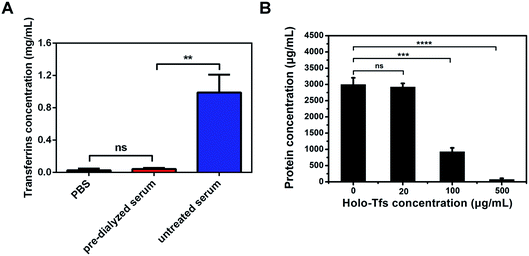
The results of exosomes separation from untreated and pre-dialyzed serum by endogenous ligands were different. But whether these differences were the results of the existence of free ligands was confused. To verify it, the influence of free serum transferrins on exosomes separation was tested. The concentration of free serum transferrins was firstly measured using ELISA assay. Different from the high concentration of free serum transferrins (∼1 mg mL−1) in untreated serum, the concentration of free serum transferrins in serum pre-dialyzed for 24 hours is ∼50 μg mL−1 (Fig. 5A). Compared with pre-dialyzed serum, the high concentration of free serum transferrins in untreated serum may be the chief culprit for the failure of the formation of SMNC-EXOs.It needs special attention that the concentration of free serum transferrins is ∼1 mg mL−1, but the concentration of serum hlolo-Tfs is accounted for ∼30% of total concentration of serum transferrins. So, in 1 mL serum, ∼300 μg serum holo-Tfs rather than all serum transferrins are capable to bind with transferrin receptors.
Pre-dialysis of serum reduced not only the concentration of free serum transferrins, but also that of other serum proteins. To verify the successful formation of SMNC-EXOs in pre-dialyzed serum was due to the elimination of free serum transferrins rather than other proteins, different amounts of holo-Tfs were respectively added to pre-dialyzed serum and protein concentrations of products magnetically separated from serum by 0.5 mg of M-Tfs were tested. The protein concentration reduced as the increase of concentration of holo-Tfs (Fig. 5B). When concentration of holo-Tfs reached to 500 μg mL−1, nearly no exosomes could be separated. But there was little influence on exosomes separation at low concentration of holo-Tfs (20 μg mL−1). The results demonstrate free holo-Tfs affect the formation of SMNC-EXOs and this influence is related to their concentration.
The direct separation of exosomes from untreated serum using M-Tfs
To certify the influence of relations between concentration of free serum transferrins and amount of M-Tfs on exosomes separation, we used same amount of M-Tfs to separate exosomes from serums dialyzed for different time respectively and compared the differences of exosomes separation. We tested the concentration of serum transferrins in serum with different dialysis time (Fig. 6). The concentration of serum transferrins was gradually reduced and reached to ∼40 μg mL−1 (holo-Tfs concentration was ∼12 μg mL−1) after 24 hours of dialysis. Then we respectively added 0.1 mg of M-Tfs (containing ∼10 μg holo-Tfs) to serum dialyzed for different time. After incubation and 2 hours of magnetic separation, the protein concentrations of separated products were tested. The amount of separated exosomes was gradually increased with the extension of dialysis time. And the majority of exosomes could be separated from serum pre-dialyzed for 24 hours by 0.1 mg of M-Tfs. But when the addition amount of M-Tfs increased to 0.5 mg, serum only needed to be pre-dialyzed for 14 hours to realize sufficient separation of exosomes.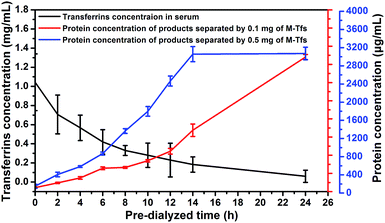 | ||
| Fig. 6 The correlation between residual concentration of transferrins in pre-dialyzed serum and the amount of separated exosomes (expressed using the protein concentration). | ||
Through the analysis, we can make a conclusion that in order to use endogenous ligands to sufficiently separate exosomes from body fluids, the minimum amount of added ligands should be equivalent to the amount of free ligands. To separate adequate exosomes from serum by 0.1 mg of M-Tfs containing ∼10 μg holo-Tfs, serum should be pre-dialyzed for at least 24 hours to reduce the amount of free holo-Tfs to ∼12 μg. But serum just needed to be pre-dialyzed for 14 hours when sufficient exosomes were separated by 0.5 mg of M-Tfs containing ∼50 μg holo-Tfs because the amount of free holo-Tfs in serum pre-dialyzed for 14 hours was ∼54 μg.
To further confirm the conclusion, M-Tfs containing equivalent holo-Tfs were respectively added to serums which have been pre-dialyzed for different time and the protein concentrations of separated products were tested (Table 1). It could be obviously observed that as long as the addition amount of holo-Tfs was equivalent to the amount of free holo-Tfs, exosomes could be adequately separated.
| Pre-dialyzed time (h) | 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 24 |
| Amount of serum transferrins (mg) | 1.04 ± 0.29 | 0.71 ± 0.20 | 0.57 ± 0.13 | 0.42 ± 0.13 | 0.33 ± 0.05 | 0.28 ± 0.13 | 0.23 ± 0.18 | 0.18 ± 0.08 | 0.06 ± 0.06 |
| Addition amount of M-Tfs (mg) | 3.60 | 2.40 | 1.80 | 1.50 | 1.20 | 0.90 | 0.60 | 0.30 | 0.10 |
| Amount of equivalent holo-Tfs (mg) | 0.36 | 0.24 | 0.18 | 0.15 | 0.12 | 0.09 | 0.06 | 0.03 | 0.01 |
| Protein concentration of separated products (mg mL−1) | 3.04 ± 0.11 | 2.98 ± 0.25 | 3.15 ± 0.21 | 2.77 ± 0.30 | 3.09 ± 0.07 | 3.33 ± 0.51 | 2.90 ± 0.23 | 2.65 ± 0.47 | 2.96 ± 0.06 |
Discussion
Exosomes have been widely researched in many fields, such as drug delivery, tumor immunotherapy and cancer liquid biopsy.22–24 But present methods for exosomes separation are barely satisfactory. These methods concentrate exosomes, but do not separate them. The impurity of exosomes results in misleading conclusion about exosomes researches.25 Immunoaffinity magnetic particle-based method can realize the precise separation of exosomes. But the large size of magnetic particles hinders the feasibility of in vivo applications of exosomes. In our SMNC-based method, superparamagnetic nanoparticles are adopted. These nanoparticles have little influence on exosomes and can be well re-dispersed. The resulting SMNC-EXOs can be used in both in vitro and in vivo studies.In addition, current immunoaffinity magnetic particle-based methods use antibodies, such as anti-EpCAM antibody and anti-CD63 antibody, to separate exosomes through the interaction of antibody and antigen.26,27 But in our research, we chose transferrin as ligand. Besides the abundance of blood exosomes expressing transferrin receptors, the high biological safety of transferrins is another important reason for the choice. Using antibody to form SMNC-EXOs, the in vivo application of SMNC-EXOs is compromised due to the potential immune response of exogenous antibody.28,29 Conversely, transferrin being a kind of endogenous protein has a low immunogenicity, thereby facilitating to decrease immune response of SMNC-EXOs.30 So, using endogenous ligands to separate exosomes from body fluids for in vivo application is a better choice.
However, using endogenous ligands to directly separate adequate exosomes from body fluids by a small number of SPMN–ligand complexes is infeasible. Only when the amount of SPMN–ligand complexes increases to a minimum value, can exosomes be separated sufficiently. This is mainly because the free ligands in body fluids could compete with the ligands labelled on SPMNs. When the amount of free ligands in body fluids is much higher than that of labelled ligands, the chance of receptor binding for ligands labelled on SPMNs is small resulting in the failure of formation of SMNC-EXOs. Even if SMNC-EXOs have been formed, the competition of free ligands for labelled ligands may result in the dissociation of SMNC-EXOs. But when the addition amount of labelled ligands is equivalent to the amount of free ligands, the competition ability of labelled ligands is enough for the formation of SMNC-EXOs. Hence, if the concentration of free ligands in body fluids is low, a small quantity of SPMN–ligand complexes can be used to form SMNC-EXOs. In contrary, a mass of SPMN–ligand complexes have to be employed. This could explain why using a small amount of magnetic particle–antibodies complexes to directly separate exosomes from medium is feasible.
According to concentration of free ligands, we can easily adopt SPMN–ligand complexes to separate exosomes from body fluids, improving the universality of SMNC-based method for exosomes separation. And not only that, the influence of free ligands on the addition amount of labelled ligands can be used as reference to current immunoaffinity magnetic separation method for exosomes. In previous studies, large size magnetic particles labelled with antibodies are often used to separate exosomes for their contents detection.31,32 But till now, due to the influence of free ligands on the binding of labelled ligands and receptors could reduce the efficiency of exosomes separation, there have been no reports on adopting endogenous ligands to bind to magnetic particles for exosomes separation. Through our research, the application range of immunoaffinity magnetic separation method can be widely extended because the number of alternative ligands is greatly increased and the efficiency of separation can be raised.
In addition, using endogenous ligands to directly separate exosomes from body fluids greatly reduce the in vitro residence time of exosomes, avoiding the potential damage of the structure and function of exosomes. It can well meet the requirements of the in vivo clinical translation of exosomes: simple protocols and the intact structure and function of exosomes.
However, the impurity of SMNC-EXOs caused by using large quantity of SPMN–ligand complexes should be noticed. The original intention of using endogenous ligands is to facilitate the in vivo application of exosomes. The doped SPMN–ligand complexes in SMNC-EXOs certainly will affect the biodistribution and biosecurity of SMNC-EXOs prejudicing the in vivo application of exosomes. Choosing appropriate endogenous ligands (i.e., the concentration of corresponding free ligands is low) is valid. And reducing the concentration of free ligands in body fluids is also beneficial to keeping the purity of SMNC-EXOs. But how to pre-treat body fluids without damaging exosomes needs to be further researched.
Conclusions
Using transferrin as a model endogenous ligand, we reveal the influence of free ligands on SMNC-EXOs formation and the efficiency of exosomes separation. In accordance with the concentration of free ligands, using equivalent endogenous ligands can directly and sufficiently separate exosomes from body fluids by SMNC-based method. The result improves the universality of immunoaffinity magnetic particle-based method for exosomes separation and facilitates the in vivo clinical translation of exosomes.Acknowledgements
This work was supported by grants from the National Nature Science Foundation of China (Grant No. 51303125, 51673146 and 51473119).References
- M. Simons and G. Raposo, Curr. Opin. Cell Biol., 2009, 21, 575–581 CrossRef CAS PubMed.
- P. D. Robbins and A. E. Morelli, Nat. Rev. Immunol., 2014, 14, 195–208 CrossRef CAS PubMed.
- P. Filipazzi, M. Bürdek, A. Villa, L. Rivoltini and V. Huber, Semin. Cancer Biol., 2012, 22, 342–349 CrossRef CAS PubMed.
- D. H. Hsu, P. Paz, G. Villaflor, A. Rivas, A. Mehta-Damani, E. Angevin, L. Zitvogel and J. B. Le Pecq, J. Immunother., 2003, 26, 440–450 CrossRef CAS PubMed.
- D. D. Taylor and C. Gercel-Taylor, Gynecol. Oncol., 2008, 110, 13–21 CrossRef CAS PubMed.
- L. Alvarez-Erviti, Y. Seow, H. Yin, C. Betts, S. Lakhal and M. J. Wood, Nat. Biotechnol., 2011, 29, 341–345 CrossRef CAS PubMed.
- Y. Tian, S. Li, J. Song, T. Ji, M. Zhu, G. J. Anderson, J. Wei and G. Nie, Biomaterials, 2014, 35, 2383–2390 CrossRef CAS PubMed.
- D. Sun, X. Zhuang, X. Xiang, Y. Liu, S. Zhang, C. Liu, S. Barnes, W. Grizzle, D. Miller and H. G. Zhang, Mol. Ther., 2010, 18, 1606–1614 CrossRef CAS PubMed.
- D. D. Taylor and S. Shah, Methods, 2015, 87, 3–10 CrossRef CAS PubMed.
- H. G. Lamparski, A. Metha-Damani, J. Y. Yao, S. Patel, D. H. Hsu, C. Ruegg and J. B. Le Pecq, J. Immunol. Methods, 2002, 270, 211–226 CrossRef CAS PubMed.
- R. C. Lai, R. W. Y. Yeo, K. H. Tan and S. K. Lim, Biotechnol. Adv., 2013, 31, 543–551 CrossRef CAS PubMed.
- B. J. Tauro, D. W. Greening, R. A. Mathias, H. Ji, S. Mathivanan, A. M. Scott and R. J. Simpson, Methods, 2012, 56, 293–304 CrossRef CAS PubMed.
- C. Théry, S. Amigorena, G. Raposo and A. Clayton, Curr. Protoc. Cell Biol., 2006, 3–22 Search PubMed.
- R. Cantin, J. Diou, D. Bélanger, A. M. Tremblay and C. Gilbert, J. Immunol. Methods, 2008, 338, 21–30 CrossRef CAS PubMed.
- K. E. Petersen, E. Manangon, J. L. Hood, S. A. Wickline, D. P. Fernandez, W. P. Johnson and B. K. Gale, Anal. Bioanal. Chem., 2014, 406, 7855–7866 CrossRef CAS PubMed.
- K. Rekker, M. Saare, A. M. Roost, A. L. Kubo, N. Zarovni, A. Chiesi, A. Salumets and M. Peters, Clin. Biochem., 2014, 47, 135–138 CrossRef CAS PubMed.
- D. D. Taylor and C. Gercel-Taylor, Gynecol. Oncol., 2008, 110, 13–21 CrossRef CAS PubMed.
- J. Dobson, Drug Dev. Res., 2006, 67, 55–60 CrossRef CAS.
- H. Qi, C. Liu, L. Long, Y. Ren, S. Zhang, X. Chang, X. Qian, H. Jia, J. Zhao, J. Sun, X. Hou, X. Yuan and C. Kang, ACS Nano, 2016, 10, 3323–3333 CrossRef CAS PubMed.
- S. P. Vyas and V. Sihorkar, Adv. Drug Delivery Rev., 2000, 43, 101–164 CrossRef CAS PubMed.
- Y. M. S. Tay, W. L. Tam, Y. S. Ang, P. M. Gaughwin, H. Yang, W. Wang, R. Liu, J. George, H. H. Ng, R. J. Perera, T. Lufkin, I. Rigoutsos, A. M. Thomson and B. Lim, Stem Cells, 2008, 26, 17–29 CrossRef CAS PubMed.
- X. Zhuang, X. Xiang, W. Grizzle, D. Sun, S. Zhang, R. C. Axtell, S. Ju, J. Mu, L. Zhang, L. Steinman, D. Miller and H. G. Zhang, Mol. Ther., 2011, 19, 1769–1779 CrossRef CAS PubMed.
- N. Chaput, J. Taïeb, N. E. Schartz, F. Andre, E. Angevin and L. Zitvogel, Cancer Immunol. Immunother., 2004, 53, 234–239 CrossRef CAS PubMed.
- D. R. Santiago-Dieppa, J. Steinberg, D. Gonda, V. J. Cheung, B. S. Carter and C. C. Chen, Expert Rev. Mol. Diagn., 2014, 14, 819–825 CrossRef CAS PubMed.
- A. Clayton, J. Court, H. Navabi, M. Adams, M. D. Mason, J. A. Hobot, G. R. Newman and B. Jasani, J. Immunol. Methods, 2001, 247, 163–174 CrossRef CAS PubMed.
- M. P. Caby, D. Lankar, C. Vincendeau-Scherrer, G. Raposo and C. Bonnerot, Int. Immunol., 2005, 17, 879–887 CrossRef CAS PubMed.
- N. Kosaka, H. Izumi, K. Sekine and T. Ochiya, Silence, 2010, 1, 1 CrossRef PubMed.
- H. Schellekens, Clin. Ther., 2002, 24, 1720–1740 CrossRef CAS PubMed.
- A. S. De Groot and D. W. Scott, Trends Immunol., 2007, 28, 482–490 CrossRef CAS PubMed.
- A. J. Chirino, M. L. Ary and S. A. Marshall, Drug Discovery Today, 2004, 9, 82–90 CrossRef CAS PubMed.
- R. Wubbolts, R. S. Leckie, P. T. Veenhuizen, G. Schwarzmann, W. Möbius, J. Hoernschemeyer, J. W. Slot, H. J. Geuze and W. Stoorvogel, J. Biol. Chem., 2003, 278, 10963–10972 CrossRef CAS PubMed.
- M. Guescini, S. Genedani, V. Stocchi and L. F. Agnati, J. Neural Transm., 2010, 117, 1–4 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra24937j |
| This journal is © The Royal Society of Chemistry 2017 |