Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Matrix-assisted diffusion-ordered NMR spectroscopy with an invisible matrix: a vanishing surfactant†
Robert Evans *a,
Aaron Hernandez-Cid
*a,
Aaron Hernandez-Cid b,
Guilherme Dal Poggetto
b,
Guilherme Dal Poggetto b,
Ashley Vestyb,
Stephan Haiberc,
Gareth A. Morris
b,
Ashley Vestyb,
Stephan Haiberc,
Gareth A. Morris b and
Mathias Nilsson
b and
Mathias Nilsson b
b
aAston Institute of Materials Research, School of Engineering and Applied Science, Aston University, Birmingham, B4 7ET, UK. E-mail: r.evans2@aston.ac.uk
bSchool of Chemistry, University of Manchester, Manchester M13 9PL, UK
cGivaudan, Dept Analyt Res, Huizerstr 28, NL-1411 GP Naarden, The Netherlands
First published on 3rd January 2017
Abstract
The addition of co-solutes to aid the separation of signals from molecules of similar size in DOSY experiments – matrix-assisted DOSY – is a potentially powerful technique for mixture analysis. The additional signals introduced by a co-solute can however greatly complicate analysis. By suitable choice of sample conditions, the NMR peaks of a surfactant matrix can be suppressed, allowing the clear resolution of molecular species according to chemistry and structure, without extraneous interference.
1 Introduction
DOSY (diffusion-ordered spectroscopy) is an effective way of analysing mixtures by NMR. Separation of the signals of mixture components is typically achieved when the components are significantly different in size, and therefore diffuse at measurably different rates. However, many mixtures of interest contain molecules that are very similar in size, e.g. isomers. The possibility of deliberately adding a co-solute to a mixture, changing the matrix within which the different species diffuse, in order to perturb diffusion differentially was quickly recognised as a useful tool in DOSY.1–4 A wide range of methods, under names such as matrix-assisted DOSY (MAD), micelle-assisted DOSY, and chromatographic DOSY, have been used to aid mixture resolution by DOSY. These include adding chromatographic stationary phases,5 polymers,6 cyclodextrins,7 lanthanide shift reagents8 and, most commonly, surfactants.9–15The role of the matrix in these experiments is analogous to that of the stationary phase in chromatography. There is the same potential for using different matrices, with different functionalities and properties, to manipulate systematically the diffusion coefficients of species in a sample. The choice of matrix is limited by some key considerations: it must have a suitable solubility; if it is a surfactant, it must have a Krafft temperature and critical micelle concentration (cmc) that allow a suitable concentration of micelles to form under the conditions required; and its proton spectrum should not overlap with signals of interest in the mixture. This last condition arises from the difficulty of separating individual contributions to the diffusional decays of overlapping signals,16,17 and the fact that the co-solute will often have much stronger signals than the analytes. A matrix with only a few signals, preferably far from the spectral region of interest, is a good choice where possible, but most useful matrices have a significant 1H footprint. One exception is polydimethylsiloxane, with a single peak close to 0 ppm, which has been shown to separate signals in a number of mixtures, including some based on the Suzuki reaction.18 Polyethylene glycol, with a single peak at around 3.5 ppm, has been shown to resolve mixtures of compounds including natural products such as β-estradiol and testosterone.19 Deuterated9,20 and fluorinated14 surfactants are 1H-NMR invisible; both are commercially available and have been used successfully in MAD experiments.
We show here that the same advantage of invisibility can be conferred on a common cationic surfactant, hexadecyltrimethylammonium bromide (also known as cetyltrimethylammonium bromide, CTAB), by using solution conditions that cause it to form very large structures with fast T2 relaxation. Using a DOSY pulse sequence that incorporates significant transverse relaxation weighting then allows the matrix signals to be filtered out. CTAB has long been identified as a potential matrix for MAD experiments3 and its use has recently been revisited.21 In addition to spherical micelles, CTAB can form a range of structures in solution, from rod shapes22 to extended networks of large worm-like micelles.23 Various experimental conditions are known to promote the formation of these extended structures; for example, suitable choice of counter-ions can facilitate the formation of larger species, by screening the head group charge.24 Both hydrotropic species, such as salicylate ions25 and catechol,26 and high ionic strength solutions22,27 have been shown to induce a transition from spherical to rod-like and worm-like micelles in cationic surfactants. Like surfactants, hydrotropes contain hydrophilic and hydrophobic parts, but do not spontaneously form micelles on their own accord. They facilitate the formation of micelles by other surfactants and, in the case of CTAB, the hydroxyl groups screen the cationic head-groups.
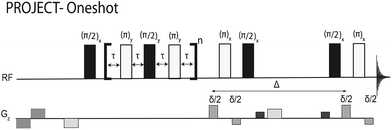
The formation of very large species often causes problems in NMR experiments, because the resultant slow molecular motion results in rapid T2 relaxation and hence broad spectral lines, but here it is used to simplify the acquired spectra. A new pulse sequence, PROJECT-Oneshot, is used to generate diffusion-weighted spectra in which the surfactant signals have been rendered invisible by T2 weighting. The ‘invisible matrix’ approach demonstrated here produces DOSY spectra in which the CTAB co-solute disperses the analyte signals without itself contributing any signals to the spectrum. This greatly expands the range of mixture analysis problems amenable to micellar matrix-assisted DOSY.
2 Experimental
Materials and reagents
All chemicals used were commercially available and used without further purification. The TSP reference solution was contained in a capillary insert to avoid interactions with the micelles. All experiments were carried out with temperature regulation at 28 °C, to ensure that the samples were above the normal Krafft temperature of CTAB.NMR experiments
NMR measurements for the results presented in the paper were carried out, non-spinning, on a 400 MHz Varian INOVA spectrometer using a 5 mm indirect detection probe equipped with a z gradient coil producing a nominal maximum gradient of 30 G cm−1. Additional NMR experiments, reported in the ESI,† were carried out on a 300 MHz Bruker AVANCE spectrometer using a 5 mm indirect detection probe equipped with a z gradient coil producing a nominal maximum gradient of 53 G cm−1.DOSY data were acquired using the Oneshot method28 and the new PROJECT-Oneshot pulse sequence of Fig. 1, in which a PROJECT sequence29 is used to add T2 weighting to Oneshot while avoiding J modulation. Both sequences used a total diffusion-encoding pulse duration, δ, of 2.5 ms, a diffusion delay, Δ, of 0.1 s, and 12 nominal gradient amplitudes ranging from 3.0 to 27.6 G cm−1 in equal steps in gradient squared. The echo time, 4τ, in the PROJECT sequence was 4 ms, and 25 cycles were used to give 100 ms of T2 weighting, sufficient to remove all surfactant signals.
DOSY spectra were constructed in the DOSY Toolbox30 by fitting to a modified Stejskal–Tanner equation, parametrized to take into account the effects of pulsed field gradient non-uniformity.2
3 Results
Fig. 2 compares Oneshot (Fig. 2a–c) and PROJECT-Oneshot (Fig. 2d) DOSY spectra of a 10 mM mixture of 2-methylpropan-1-ol (isobutanol) and butan-2-ol (sec-butanol) in (Fig. 2a) D2O, (Fig. 2b) a 150 mM solution of CTAB in D2O, and (Fig. 2c and d) a 150 mM solution of CTAB in D2O saturated with NaCl. The NaCl causes the CTAB to form long worm-like micelles that produce an entangled, highly viscous gel that restricts the CTAB motion sufficiently to reduce the T2s of the CTAB protons to less than 10 ms. The formation of a gel complicates sample handling, but is very effective at suppressing convection.31,32 It is possible to make short T2 CTAB gels with lower salt concentrations if a hydrotrope such as catechol is included, but some hydrotrope signal survives the T2 filtration so such mixtures are less useful. The effectiveness of the filtration and the use of matrices containing hydrotropes are illustrated in more detail in the ESI.†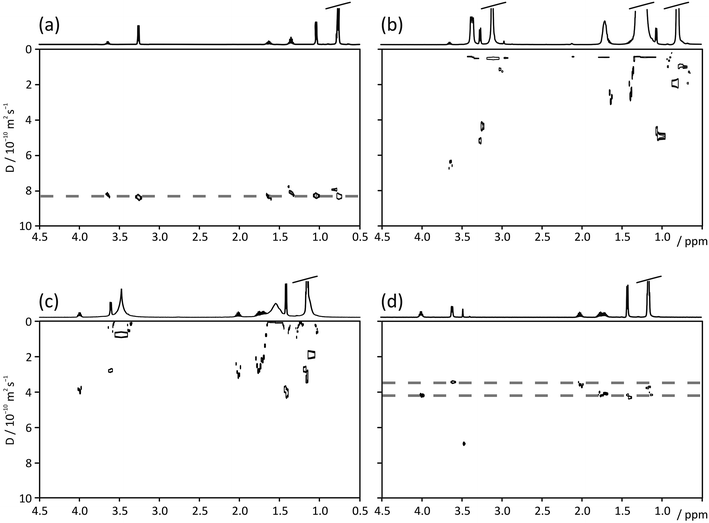 | ||
| Fig. 2 DOSY spectra of a solution of 10 mM each of 2-methylpropan-1-ol (isobutanol) and butan-2-ol (sec-butanol) in (a) D2O, (b) D2O and 150 mM CTAB, (c) the ‘invisible matrix’ solution, containing 150 mM CTAB in a saturated solution of NaCl in D2O, all acquired with Oneshot; and (d) DOSY spectrum of 2-methylpropan-1-ol and butan-2-ol in the ‘invisible matrix’ solution, acquired with the PROJECT-Oneshot sequence of Fig. 1. Dashed lines in (a) and (d) indicate diffusion coefficients of butanol isomers. | ||
In simple D2O solution the butanol signals are, as expected, not resolved in the diffusion dimension of a standard DOSY spectrum (Fig. 2a). In a micellar solution of CTAB in D2O (Fig. 2b), almost all the butanol signals are swamped by the much stronger CTAB signals. The addition of NaCl (Fig. 2c) greatly broadens the CTAB peaks, making it easier to distinguish some of the butanol signals but still not allowing clean diffusion resolution. However, when T2 filtration is added to the Oneshot experiment, to give the pulse sequence of Fig. 1, the resultant experimental data contain no significant CTAB signal and the clean, well-resolved DOSY spectrum of Fig. 2d results. The peak at 3.4 ppm in Fig. 2d is most probably a low MW CTAB impurity.
The separation can be interpreted on the basis of the hydrophobicities of the species in the sample. 2-Methylpropan-1-ol (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 0.8) interacts with the core of the micelles more strongly than butan-2-ol (log
P = 0.8) interacts with the core of the micelles more strongly than butan-2-ol (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 0.68) and the effective diffusion coefficients observed in Fig. 2d reflect this.
P = 0.68) and the effective diffusion coefficients observed in Fig. 2d reflect this.
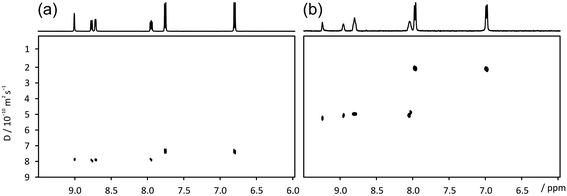
A further demonstration of the technique is shown in Fig. 3, using a sample of 4 mM nicotinic and 3 mM 4-aminobenzoic acid. The ‘invisible matrix’ solution contained 150 mM CTAB in a saturated solution of NaCl in 98% D2O/2% DMSO, with the DMSO added to improve the solubility of the analytes.33 While the two species exhibit reduced diffusion coefficients, both as a result of interaction with the surfactant and due to obstruction effects, the small difference in diffusion coefficient observed in aqueous solution is enhanced almost 5 times by the addition of the ‘invisible matrix’. This behaviour is a reflection of the relative hydrophobicities of the two species; 4-aminobenzoic acid (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 0.68) is the more hydrophobic (log
P = 0.68) is the more hydrophobic (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P of nicotinic acid = −2.43). In this example the use of hydrotropic species such as catechol would have introduced overlapping signals that would only have been partially suppressed by the T2-filter.
P of nicotinic acid = −2.43). In this example the use of hydrotropic species such as catechol would have introduced overlapping signals that would only have been partially suppressed by the T2-filter.
4 Conclusions
This work shows the use of ‘invisible matrices’ – using the surfactant chemistry of CTAB to make large, worm-like micelles and removing their NMR signals by T2 weighting – as an alternative to purchasing expensive or specialist NMR-silent chemicals in matrix-assisted DOSY experiments. Very good suppression of surfactant signals can be obtained by appropriate choice of sample conditions and NMR experimental parameters, as shown in Fig. SI 3C of the ESI.† The ‘invisible matrix’ solution separates both positional and structural isomers, as well as the pair of structurally similar substituted benzoic acids of Fig. 3. This approach will allow a much wider range of mixtures to be analysed, as sample signals across the entire range of proton chemical shifts can be observed.Acknowledgements
This work was supported by the Engineering and Physical Sciences Research Council (grant numbers EP/E05899X/1 and EP/H024336/1) and by Givaudan Flavors Division. Aaron Hernandez-Cid thanks the Mexican Scientific and Technology Council (CONACyT), for financial support. Guilherme Dal Poggetto is supported by Science without Borders – Brazil (CNPq reference number 233163/2014-0).References
- C. S. Johnson, Prog. Nucl. Magn. Reson. Spectrosc., 1999, 34, 203–256 CrossRef CAS.
- P. Hodge, P. Monvisade, G. A. Morris and I. Preece, Chem. Commun., 2001, 239–240 RSC.
- K. F. Morris, P. Stilbs and C. S. Johnson, Anal. Chem., 1994, 66, 211–215 CrossRef CAS.
- R. Evans and I. J. Day, RSC Adv., 2016, 6, 47010–47022 RSC.
- S. Viel, F. Ziarelli and S. Caldarelli, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 9696–9698 CrossRef CAS PubMed.
- J. S. Kavakka, I. Kilpelainen and S. Heikkinen, Org. Lett., 2009, 11, 1349–1352 CrossRef CAS PubMed.
- R. W. Adams, J. A. Aguilar, J. Cassani, G. A. Morris and M. Nilsson, Org. Biomol. Chem., 2011, 9, 7062–7064 CAS.
- A. K. Rogerson, J. A. Aguilar, M. Nilsson and G. A. Morris, Chem. Commun., 2011, 47, 7063–7064 RSC.
- M. E. Zielinski and K. F. Morris, Magn. Reson. Chem., 2009, 47, 53–56 CrossRef CAS PubMed.
- R. Evans, S. Haiber, M. Nilsson and G. A. Morris, Anal. Chem., 2009, 81, 4548–4550 CrossRef CAS PubMed.
- C. F. Tormena, R. Evans, S. Haiber, M. Nilsson and G. A. Morris, Magn. Reson. Chem., 2010, 48, 550–553 CrossRef CAS PubMed.
- C. F. Tormena, R. Evans, S. Haiber, M. Nilsson and G. A. Morris, Magn. Reson. Chem., 2012, 50, 458–465 CrossRef CAS PubMed.
- R. E. Hoffman, H. Arzuan, C. Pemberton, A. Aserin and N. Garti, J. Magn. Reson., 2008, 194, 295–299 CrossRef CAS PubMed.
- C. Pemberton, R. E. Hoffman, A. Aserin and N. Garti, Langmuir, 2011, 27, 4497–4504 CrossRef CAS PubMed.
- J. Cassani, M. Nilsson and G. A. Morris, J. Nat. Prod., 2012, 75, 131–134 CrossRef CAS PubMed.
- M. Nilsson, M. A. Connell, A. L. Davis and G. A. Morris, Anal. Chem., 2006, 78, 3040–3045 CrossRef CAS PubMed.
- A. A. Colbourne, G. A. Morris and M. Nilsson, J. Am. Chem. Soc., 2011, 133, 7640–7643 CrossRef CAS PubMed.
- S. Huang, J. Gao, R. Wu, S. Li and Z. Bai, Angew. Chem., Int. Ed., 2014, 53, 11592–11595 CrossRef CAS PubMed.
- J. S. Kavakka, V. Parviainen, K. Wahala, I. Kilpelainen and S. Heikkinen, Magn. Reson. Chem., 2010, 48, 777–781 CrossRef CAS PubMed.
- R. E. Hoffman, E. Darmon, A. Aserin and N. Garti, Colloids Surf., A, 2016, 507, 218–226 CrossRef CAS.
- N. V. Gramosa, N. M. S. P. Ricardo, R. W. Adams, G. A. Morris and M. Nilsson, Magn. Reson. Chem., 2016, 54, 815–820 CrossRef PubMed.
- T. Imae, R. Kamiya and S. Ikeda, J. Colloid Interface Sci., 1985, 108, 215–225 CrossRef CAS.
- T. Shikata, Y. Sakaiguchi, H. Uragami, A. Tamura and H. Hirata, J. Colloid Interface Sci., 1987, 119, 291–293 CrossRef CAS.
- S. Berr, R. R. M. Jones and J. S. Johnson, J. Phys. Chem., 1992, 96, 5611–5614 CrossRef CAS.
- Z. Lin, J. J. Cai, L. E. Scriven and H. T. Davis, J. Phys. Chem., 1994, 98, 5984–5993 CrossRef CAS.
- J. Meng, Y. Lu, L. Li, H. Wu and X. Zhang, Z. Phys. Chem., 2009, 223, 689 CrossRef CAS.
- W. Zhang, G. Li, J. Mu, Q. Shen, L. Zheng, H. Liang and C. Wu, Chin. Sci. Bull., 2000, 45, 1854–1857 CrossRef CAS.
- M. D. Pelta, G. A. Morris, M. J. Stchedroff and S. J. Hammond, Magn. Reson. Chem., 2002, 40, S147–S152 CrossRef CAS.
- J. A. Aguilar, M. Nilsson, G. Bodenhausen and G. A. Morris, Chem. Commun., 2012, 48, 811–813 RSC.
- M. Nilsson, J. Magn. Reson., 2009, 200, 296–302 CrossRef CAS PubMed.
- I. Swan, M. Reid, P. W. Howe, M. A. Connell, M. Nilsson, M. A. Moore and G. A. Morris, J. Magn. Reson., 2015, 252, 120–129 CrossRef CAS PubMed.
- T. M. Barbosa, R. Rittner, C. F. Tormena, G. A. Morris and M. Nilsson, RSC Adv., 2016, 6, 95173–95176 RSC.
- J. T. Rubino and W. S. Berryhill, J. Pharm. Sci., 1986, 75, 182–186 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra26144b |
| This journal is © The Royal Society of Chemistry 2017 |