Open Access Article
Open Access ArticleA simple route to prepare a Cu2O–CuO–GN nanohybrid for high-performance electrode materials†
Yanyun Liu *a,
Ling Maa,
Dong Zhangb,
Gaoyi Hancd and
Yunzhen Changcd
*a,
Ling Maa,
Dong Zhangb,
Gaoyi Hancd and
Yunzhen Changcd
aCollege of Chemistry and Chemical Engineering, Jinzhong University, Jinzhong 030619, PR China. E-mail: 312217642@qq.com
bSchool of Material Science and Engineering, Tongji University, Shanghai 200092, PR China
cInstitute of Molecular Science, Innovation Center of Chemistry and Molecular Science, Shanxi University, Taiyuan 030006, PR China
dKey Laboratory of Materials for Energy Conversion and Storage of Shanxi Province, Taiyuan 030006, PR China
First published on 20th February 2017
Abstract
Cu2O–CuO–GN nanohybrid is prepared by a simple electrochemical method by applying a positive and negative pulse electric signal on a dispersion of a mixture of GO and Cu(NO3)2. During the process, reduction of graphene oxide and deposition of Cu2O–CuO on GN occur simultaneously. The nanohybrid is systematically characterized by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Cu2O–CuO nanoparticles with diameters around 100 nm are uniformly distributed on the GN. In addition, the electrochemical properties of the Cu2O–CuO–GN nanohybrid are investigated by cyclic voltammetry (CV), galvanostatic charge/discharge (DC) and electrochemical impedance spectrometry measurements (EIS). The Cu2O–CuO–GN nanohybrid shows a higher specific capacitance (222 F g−1 at 1 A g−1) than that of pure GN (143 F g−1 at 1 A g−1) prepared under the same conditions. This approach opens up the possibility for fabrication of high-performance GN-based electrode materials.
1. Introduction
Graphene (GN), a two-dimensional one-atom thick carbon, has attracted increasing attention in the past several years, mainly due to its outstanding electronic, thermal, and mechanical properties.1–4 Such superior performances endow it with exceptional application potential in many fields including catalysis,5 sensors,6,7 adsorption,8 energy storage,9,10 and so on. However, the exceptional inherent properties of GN are often reduced by GN aggregation and stacking, which is driven by the strong π–π interactions between individual GN sheets.11 In addition, the quality of GN is hardly controllable due to abundant chemical defects formed in the synthetic process. Therefore, the prevention of restacking and the reduction of chemical defects from GN has been a significant issue in developing GN-based electrochemical materials.The addition of extra additives during the GN preparation process has been reported, and is demonstrated to overcome the above mentioned issues. Thus, various GN based composites have been widely reported in the electrochemical application, such as transition metal oxide nanoparticles/GN,12 carbon nanomaterials/GN,13–15 conducting polymer/GN,16 etc. Actually, the extra additive deposited on the surface of GN as a nanospacer can restrain the restacking of GN during the reduction of graphene oxide (GO). Moreover, GN not only offers a 2D conductive backbone for the rapid electron transport, but also allows the decoration by uniform additives to facilitate the utilization of electrode materials.
Among the transition metal oxides, Cu2O, CuO have the unique optical and good electrochemical properties,17–22 which makes it a promising candidate in many applications, such as supercapacitors,23 solar-energy conversion devices,24 and lithium-ion batteries,25 etc. Up to now, Cu2O/GN and CuO/GN nanohybrid have been synthesized by various methods. For example, Wang et al. synthesized Cu2O/CuO/GN nanohybrid through a hydrothermal-assisted reaction.26 Zhu et al. synthesized CuO/GN composite through in situ chemical synthesis approach.27 However, these mentioned methods still have the unsolved problems for regarding Cu2O/CuO decoration of GN: (1) the adopted chemical ways are usually complicated using numerous reducing, precipitating, and stabilizing agents.28–30 (2) It is still a challenge to achieve the convenient production of Cu2O/GN and CuO/GN composites. To overcome these shortcomings, we used a one-pot electrochemical method to prepare Cu2O–CuO–GN nanohybrid. The electrochemical method is a well developed and economical method that has been successfully applied for the deposition of carbon nanotubes and GO particles.31,32
In this paper, Cu2O–CuO–GN nanohybrid is prepared by applying positive and negative pulse electric signal on GO and Cu(NO3)2 mixture dispersion. During the process, reduction of graphene oxide and deposition of nano-Cu2O/nano-CuO on GN occur simultaneously. Our green synthesis approach avoids using harsh, toxic and explosive chemicals such as hydrazine hydrate and sodium borohydride for reducing GO. The electrochemical behavior of as-prepared Cu2O–CuO–GN nanohybrid is examined by cyclic voltammetry (CV) and galvanostatic charge/discharge (DC) and electrochemical impedance spectrometry measurements (EIS). The results show that this method is simple to provide Cu2O–CuO–GN nanohybrid for high-performance electrode materials.
2. Experimental
2.1 Materials and instrument
The graphite oxide was prepared according to the method developed by Hummers' and Offemann,33 and all other chemicals used in this study were of analytical grade. Signal generator (DG1022) and Oscilloscope (DS1052E) were purchased from Beijing Puyuan Technologies Co., Power amplifier (HVP-300A) was bought from Nanjing FonanCo.2.2 Preparation of GN and Cu2O–CuO–GN
The suspension of graphene oxide (GO, 1 mg mL−1) was prepared by dispersing the graphite oxide powder into water with the help of ultrasonication for 2 h. A 1 mL Cu(NO3)2 solution with a concentration of 0.1 mol L−1 was introduced into the as-prepared GO suspension under ultrasonication. Then the mixture suspension was loaded in a glass container. Copper electrodes were used in the experiment. The electrode separation was 10 millimeters. Positive and negative pulse signal whose frequency and peak-to-peak voltage (Vpp) were 5 Hz and 20 V respectively were applied on the electrodes in the mixture dispersion. This signal was produced by the signal generator and amplified by the power amplifier. Electrode which adsorbed thick film was taken out after 2 h and dried in the air. Then flexible Cu2O–CuO–GN thin film was obtained by peeling off from the electrodes and cut by a razor blade into rectangular strips for testing without further modification. For comparison, GN was prepared using the same procedure without adding Cu(NO3)2 solution.2.3 Characterization
The X-ray diffraction (XRD) patterns of samples were obtained by using a D8 Cu Kα radiation (D/max 2550VB3+/PC, Rigaku, Japan). The microstructures and morphology of samples were observed with a field emission scanning electron microscopy (SEM, Quanta 200FEG, FEI) and transmission electron microscope (TEM, JEM-2100F). X-ray photoelectron spectroscopy (XPS) analysis were performed with an ESCALAB 250Xispectrometer (Thermo Electron) using a monochromic Al Kα source at 1486.6 eV.2.4 Electrochemical measurements
The fabrication of the supercapacitors is described as follows: two nearly identical Cu2O–CuO–GN (GN) samples were separated by a filter paper soaked with 1.0 mol L−1 KCl aqueous solution. Before the electrochemical measurements, the slices of samples were also immersed in KCl aqueous solution under vacuum in order to exchange their interior water with electrolyte. Two foam nickels were used as the flexible current collectors. All the components were assembled into a sandwiched structure between the two substrates. Electrochemical performances of the supercapacitors were tested by CV, DC, EIS measurements on a CHI660E electrochemistry workstation (Chenhua, Shanghai). Potential windows for the CV measurements and DC tests ranged from −0.5 to 0.5 V. EIS tests were carried out in the frequency range of 105 to 0.1 Hz at the amplitude of 5 mV, referring to open circuit potential. The specific capacitance (Csc) of the electrodes was calculated based on DC curves:| Csc = 2IΔt/ΔVm |
3. Results and discussion
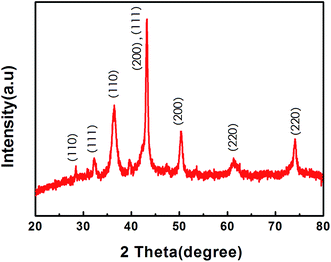
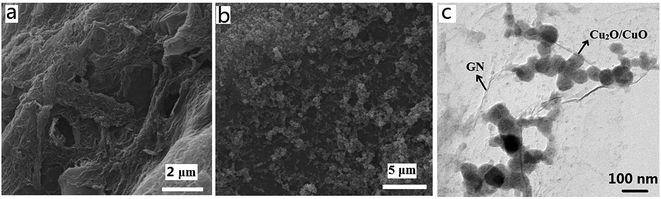
X-ray diffraction (XRD) pattern of as-synthesized Cu2O–CuO–GN indicates the mixed phase of Cu2O and CuO (Fig. 1). For Cu2O–CuO, diffraction peaks centered at 2θ = 28°, 33°, 43° and 62° correspond to the (110), (111), (200) and (220) crystal planes of Cu2O, respectively. Moreover, the peaks located at 37°, 43°, 51°, and 74° can be assigned to the (110), (111), (200), and (220) planes of CuO, respectively. Both of these results suggest the presence of both Cu2O and CuO in the sample.SEM represents the morphology of the sample surface. SEM images of GN and Cu2O–CuO–GN nanocomposite are presented in Fig. 2(a) and (b). It is clearly seen that the as-prepared GN exhibits typical wrinkled structures which prevent the sheets from stacking into dense structure. The wrinkled structures of GN could provide suitable sites for deposition of Cu2O–CuO. Cu2O–CuO–GN shows the formation of flakes composed of the small nanoparticles. It is seen that Cu2O–CuO nanoparticles are uniformly dispersed on the surface of GN. Fig. 2c shows TEM image of Cu2O–CuO–GN nanocomposite. The image indicates that the diameters of nanoparticles are around 100 nm.
The XPS is one of best methods to investigate the chemical composition and electronic structure of the Cu2O–CuO–GN. Fig. 3 shows the wide scan and deconvoluted XPS spectra of Cu2O–CuO–GN and GO (a–d). The elements of Cu, C, and O were clearly observed in Fig. 3(a). Fig. 3(b) and (c) show both the C 1s XPS spectra of GO and Cu2O–CuO–GN. Four different peaks centered at 284.0 eV (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 284.5 eV (C–C), 286.3 eV (C–O) and 288.1 eV (O
C), 284.5 eV (C–C), 286.3 eV (C–O) and 288.1 eV (O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) were detected in GO sample (Fig. 2b). After reaction, the intensities of all C 1s peaks of the carbons binding to oxygen decreased dramatically, revealing that the deoxygenation process accompanied the reduction of GO in the electrochemical reaction. The oxidation state of Cu has also been investigated using XPS (Fig. 3d) and shows the presence of a mixture of oxidation states such as Cu(I) and Cu(II). The result suggests the presence of both Cu2O and CuO in the sample. In this experiment, GO platelets migrated toward the working electrode as soon as a positive pulse was applied. Then these deposited platelets were reduced, meanwhile, Cu2O–CuO nanoparticles deposited on the surface of GN when a negative pulse was applied. The possible reactions are as follow:
C) were detected in GO sample (Fig. 2b). After reaction, the intensities of all C 1s peaks of the carbons binding to oxygen decreased dramatically, revealing that the deoxygenation process accompanied the reduction of GO in the electrochemical reaction. The oxidation state of Cu has also been investigated using XPS (Fig. 3d) and shows the presence of a mixture of oxidation states such as Cu(I) and Cu(II). The result suggests the presence of both Cu2O and CuO in the sample. In this experiment, GO platelets migrated toward the working electrode as soon as a positive pulse was applied. Then these deposited platelets were reduced, meanwhile, Cu2O–CuO nanoparticles deposited on the surface of GN when a negative pulse was applied. The possible reactions are as follow:
| Positive pulse: 4H+ + 2GO + 4e− = 2GN + 2H2O |
| Negative pulse: 2Cu2+ + 2e− + 2OH− = Cu2O + H2O |
| Cu2+ + 2OH− = CuO + H2O |
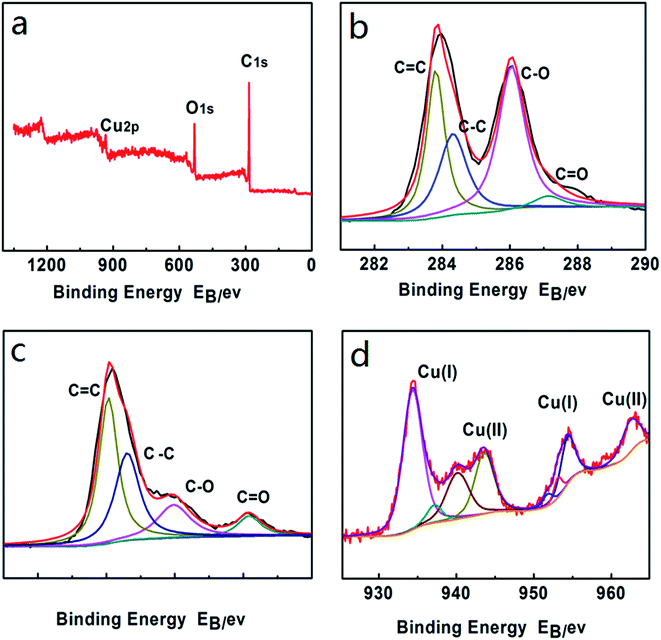 | ||
| Fig. 3 Wide scan XPS spectra of Cu2O–CuO–GN (a), deconvoluted XPS spectra of GO (b) and Cu2O–CuO–GN (c and d). | ||
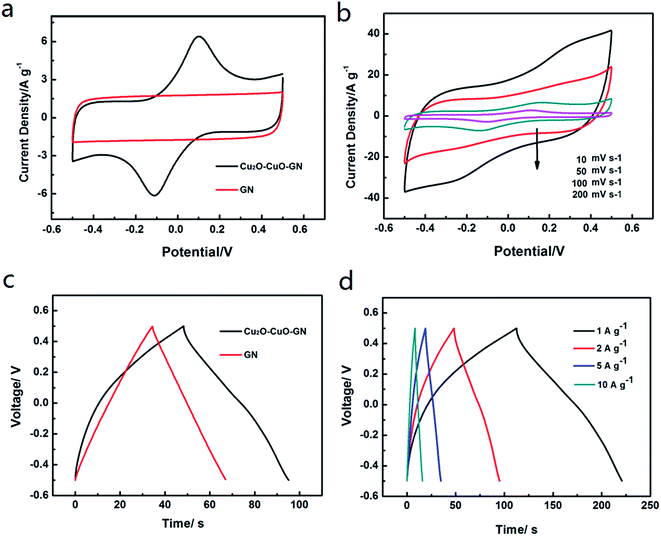
Fig. 4a shows CV curves of Cu2O–CuO–GN and GN at a scan rate of 10 mV s−1. It can be seen that the curve of Cu2O–CuO–GN electrode has a rectangular-like shape with a broad redox peaks, indicating the contribution of both EDLC behavior and pseudocapacitive characteristics. The curve of Cu2O–CuO–GN displays a pair of redox peaks which can be attributed to reversible Cu(I)/Cu(II) redox reactions involving one electron transfer process. The mechanism of redox reactions occurring at the Cu2O–CuO–GN electrode can be proposed as:34
| Cu2+ + e− → Cu+ |
The CV of GN exhibits a symmetric rectangular shape, indicating an ideal electric double layer capacitive behavior. Also, the current density of Cu2O–CuO–GN nanohybrid is higher than that of GN due to the positive effect of Cu2O–CuO on energy storage capability.
Fig. 4b displays the CV curves of the Cu2O–CuO–GN electrode at different scan rates. The prominent increase of the current is observed by increasing scan rate which indicates the capacitive behavior of Cu2O–CuO–GN electrode. During the increasing scan rates, inner active sites cannot completely contribute in the redox transitions and the capacitance of the electrode decreases. Also, the redox peaks tend to disappear with increasing scan rate, suggesting a kinetic limitation for the reaction between the redox sites at scan rate beyond 200 mV s−1. Moreover, anodic and cathodic peaks shift towards the positive and negative potentials, respectively, which is due to the resistance of the electrode. Fig. 4c shows the DC curves of Cu2O–CuO–GN and GN electrodes at a current density of 2 A g−1. The curve of GN electrode shows a typical triangular symmetrical distribution, indicating that GN has good electro-chemical double-layer capacitive behavior, which is in agreement with the results obtained from the CV curve. The Cu2O–CuO–GN electrode exhibits a nonlinear discharge–charge curve, the typical behaviors of the combination of EDLC of GN and pseudocapacitance from the redox reaction of Cu2O–CuO. On the basis of the DC curves, it can be concluded that covering the Cu2O–CuO nanoparticles on the GN network can introduce new paths for electron transfer and therefore improve the conductivity and capacitance behavior of the electrodes. Providing intact contact between the thin GN sheets and the Cu2O–CuO nanoparticles in a molecular scale, the composite material Cu2O–CuO–GN showed higher capacitance than those of pure GN. In addition, the electrochemical behaviors of GN and Cu2O–CuO–GN were also analyzed by using a three-electrode system, in which a platinum disk was used as the counter electrode and a saturated calomel electrode was used as the reference electrode (see ESI†).
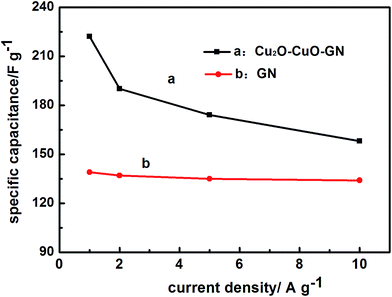
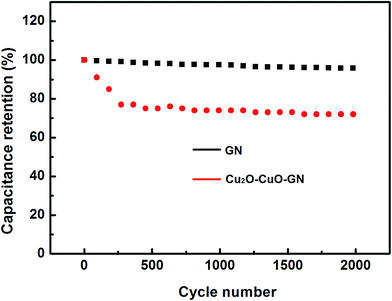
To further examination the rate capability of the Cu2O–CuO–GN nanohybrid, DC experiments were performed at different current densities, as shown in Fig. 4d. At high current densities, inner active sites cannot contribute in the redox transitions due to the diffusion effect of ions within the electrode. The variations of Cs at different current densities were shown in Fig. 5. When the current densities were increased up to 10 A g−1, the Cu2O–CuO–GN and GN electrodes maintained nearly 72% and 96% of their initial Cs values, respectively. At a given current density, e.g., 1 A g−1, the Cs of electrode was 222 F g−1 for Cu2O–CuO–GN, larger than 143 F g−1 for GN. Cycle ability is another important criterion to evaluate the compatibility during prolonged cycling. In this regard, GN and Cu2O–CuO–GN were cycled at current density of 2 A g−1 and the cycling profiles are given in Fig. 6. The capacitances of the Cu2O–CuO–GN supercapacitor show a decrease during the first 200 cycles and then keep stable. The capacitance remains about 72% of its initial values after 2000 cycles, indicating that Cu2O–CuO–GN is a suitable nanohybrid in practical energy storage systems.
The electrochemical performance of the Cu2O–CuO–GN and GN were further explored by EIS measurements, the results were shown in Fig. 7A along with an equivalent circuit model (Fig. 7B). Here, Rs is the total resistance of electrolyte, electrode, current collector and separator. C and Rct are the capacitance and charge transfer resistance. W is the Warburg impedance related to the diffusion/transport of ions in the electrolyte to the surface of the electrode. The impedance spectrum is characterized by a well-defined semicircle at high and intermediate frequencies and a straight line inclined at a constant angle relative to the real axis at low frequencies. The high-frequency semicircle corresponds to the equivalent series internal resistance, while the semicircle in the medium-frequency region represents the charge-transfer resistance at the electrode/electrolyte interface. The inclined line is associated with ions diffusion within the electrode materials. After fitting the impedance spectrum, the internal resistance value of Cu2O–CuO–GN electrode was found to be 2.8 Ω. This value was very close to the GN electrode (2.3 Ω). All of those illustrated that the material of Cu2O–CuO–GN may be developed as a suitable material for electrochemical capacitors applications.
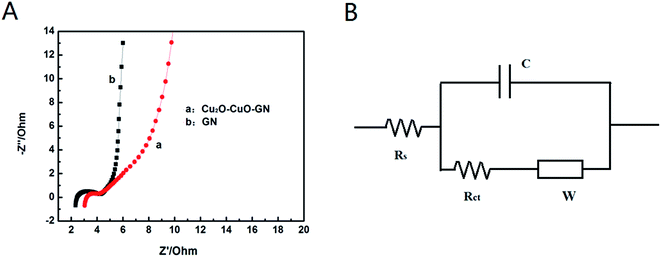 | ||
| Fig. 7 (A) Nyquist plot of GN and Cu2O–CuO–GN (B) corresponding equivalent circuit used for Nyquist plot simulation. | ||
4. Conclusions
In summary, we have demonstrated a simple and efficient electrochemical method for preparing Cu2O–CuO–GN nanohybrid by applying positive and negative pulse electric signal on GO and Cu(NO3)2 mixture dispersion. XRD pattern of nanohybrid confirms the formation of Cu2O–CuO loaded on the ERGO. TEM and SEM image analysis reveals the successful decoration of GN with Cu2O–CuO. In addition, the Cu2O–CuO–GN nanohybrid shows a higher specific capacitance (222 F g−1 at 1 A g−1) than that of pure GN (143 F g−1 at 1 A g−1) prepared under the same conditions. Therefore, this method is a promising candidate to provide Cu2O–CuO–GN nanohybrid for high-performance GN-based electrode materials.Acknowledgements
Financial support was provided by the Doctoral Startup and Research Fund (BSJJ2015212) of Jinzhong University, the Foundation of Key Laboratory of Materials for Energy Conversion and Storage of Shanxi Province (MECS2016001).References
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang and S. V. Dubonos, et al., Science, 2004, 306, 666 CrossRef CAS PubMed.
- A. K. Geim, Science, 2009, 324, 1530 CrossRef CAS PubMed.
- C. Lee, X. Wei, J. W. Kysar and J. Hone, Science, 2008, 321, 385 CrossRef CAS PubMed.
- A. A. Balandin, S. Ghosh, W. Bao, I. Calizo, D. Teweldebrhan and F. Miao, et al., Nano Lett., 2008, 8, 902 CrossRef CAS PubMed.
- D. Deng, K. S. Novoselov, Q. Fu, N. Zheng, Z. Tian and X. Bao, Nat. Nanotechnol., 2016, 11, 218 CrossRef CAS PubMed.
- H. Sun, L. Wu, W. Wei and X. Qu, Mater. Today, 2013, 16, 433 CrossRef CAS.
- S. S. Varghese, S. Lonkar, K. K. Singh, S. Swaminathan and A. Abdala, Sens. Actuators, B, 2015, 218, 160 CrossRef CAS.
- J. Xu, L. Wang and Y. Zhu, Langmuir, 2012, 28, 8418 CrossRef CAS PubMed.
- M. Pumera, Energy Environ. Sci., 2011, 4, 668 CAS.
- D. A. C. Brownson, D. K. Kampouris and C. E. Banks, J. Power Sources, 2011, 196, 4873 CrossRef CAS.
- X. Wang, G. Sun, P. Routh, D. H. Kim, W. Huang and P. Chen, Chem. Soc. Rev., 2014, 43, 7067 RSC.
- W. Zhang, B. Quan, C. Lee, S. K. Park, X. Li, E. Choi, G. Diao and Y. Piao, ACS Appl. Mater. Interfaces, 2015, 7, 2404 CAS.
- W. Zhang, M. Chen, X. Gong and G. Diao, Carbon, 2013, 61, 154 CrossRef CAS.
- B. You, L. Wang, L. Yao and J. Yang, Chem. Commun., 2013, 49, 5016 RSC.
- C. Zhu, J. Zhai and S. Dong, Nanoscale, 2014, 6, 10077 RSC.
- N. A. Kumar and J. B. Baek, Chem. Commun., 2014, 50, 6298 RSC.
- H. Zhang, J. Feng and M. Zhang, Mater. Res. Bull., 2008, 43, 3221 CrossRef CAS.
- K. X. Yao, X. M. Yin, T. H. Wang and H. C. Zeng, J. Am. Chem. Soc., 2010, 132, 6131 CrossRef CAS PubMed.
- Y. Li, S. Chang, X. Liu, J. Huang, J. Yin, G. Wang and D. Cao, Electrochim. Acta, 2012, 85, 393 CrossRef CAS.
- G. H. Dong, Z. H. Ai and L. Z. Zhang, Water Res., 2014, 66, 22 CrossRef CAS PubMed.
- D. Y. Fu, G. Y. Han, Y. Z. Chang and J. H. Dong, Mater. Chem. Phys., 2012, 132, 673 CrossRef CAS.
- W. Lv, F. Sun, D. M. Tang, H. T. Fang, C. Liu, Q. H. Yang and H. M. Cheng, J. Mater. Chem., 2011, 21, 9014 RSC.
- C. Dong, Y. Wang, J. Xu, G. Cheng, W. Yang, T. Kou, Z. Zhang and Y. Ding, J. Mater. Chem. A, 2014, 2, 18229 CAS.
- Y. S. Lee, D. Chua, R. E. Brandt, S. C. Siah, J. V. Li, J. P. Mailoa, S. W. Lee, R. G. Gordon and T. Buonassisi, Adv. Mater., 2014, 26, 4704 CrossRef CAS PubMed.
- D. Liu, Z. Yang, P. Wang, F. Li, D. Wang and D. He, Nanoscale, 2013, 5, 1917 RSC.
- K. Wang, X. Dong, C. Zhao, X. Qian and Y. Xu, Electrochim. Acta, 2015, 152, 433 CrossRef CAS.
- J. Zhu, G. Zeng, F. Nie, X. Xu, S. Chen, Q. Han and X. Wang, Nanoscale, 2010, 2, 988 RSC.
- C. Xu, X. Wang, L. Yang and Y. Wu, J. Solid State Chem., 2009, 182, 2486 CrossRef CAS.
- Y. Yang, C. Tian, J. Wang, L. Sun, K. Shi, W. Zhou and H. Fu, Nanoscale, 2014, 6, 7369 RSC.
- H. Meng, W. Yang, K. Ding, L. Feng and Y. Guan, J. Mater. Chem. A, 2015, 3, 1174 CAS.
- S. Pei, J. Du, Y. Zeng, C. Liu and H. M. Cheng, Nanotechnology, 2009, 20, 235707 CrossRef PubMed.
- S. Hong, S. Jung, S. Kang, Y. Kim, X. Chen and S. Stankovich, et al., J. Nanosci. Nanotechnol., 2008, 8, 424 CrossRef CAS PubMed.
- W. S. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- V. D. Patake, S. S. Joshi, C. D. Lokhande and O. S. Joo, Mater. Chem. Phys., 2009, 114, 6 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra26535a |
| This journal is © The Royal Society of Chemistry 2017 |