Open Access Article
Open Access ArticleComparison of dry and wet milling pre-treatment methods for improving the anaerobic digestion performance of the Pennisetum hybrid
Dong Pengyu ab,
Li Lianhuaa,
Zhen Fenga,
Kong Xiaoying*a,
Sun Yongminga and
Zhang Yiab
ab,
Li Lianhuaa,
Zhen Fenga,
Kong Xiaoying*a,
Sun Yongminga and
Zhang Yiab
aGuangzhou Institute of Energy Conversion, CAS Key Laboratory of Renewable Energy, Chinese Academy of Sciences, Guangzhou 510640, China. E-mail: kongxy@ms.giec.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 22nd February 2017
Abstract
Two types of milling pre-treatment methods were used to study their effects on the anaerobic digestion performance of the Pennisetum hybrid. The physical structure of the Pennisetum hybrid could be significantly damaged with both milling pre-treatment methods. With an increase in treatment time, the pore volume, surface area, average pore diameter, and the crystallization index of the Pennisetum hybrid increased gradually. The anaerobic fermentation test showed that, among the dry and wet milling pre-treatments, the highest specific methane yield was obtained at 3 h dry milling pre-treated (358.07 mL g−1 VS) and 6 h wet milling pre-treated grass (315.87 mL g−1), which was 41.04% and 24.42% higher than that of the untreated Pennisetum hybrid (253.88 mL g−1 VS). The median diameter (D50) of the Pennisetum hybrid was 25.28% and 22.36% lower than that of the untreated Pennisetum hybrid after 3 h dry and 6 h wet milling pre-treatment. After wet milling pre-treatment, the fluidity of the Pennisetum hybrid was much better than conventional “smashing” pre-treatment, which may make feeding easier in large-scale biogas projects. Good fluidity and optimization of the degree of milling pre-treatment could, therefore, be applied on an industrial scale in the future.
1. Introduction
With the increasing use of energy resources and demand for sustainable development, finding new energy sources has an important role in the development of energy strategies.1 Global warming and environmental pollution also require exploration of alternative methods of energy production.2 Production of biogas by anaerobic fermentation has huge potential for meeting energy needs. Anaerobic digestion (AD) is a continuous biochemical process. AD can transform many types of biodegradable organic matter to clean, renewable biogas through the metabolism of microorganisms that can biodegrade polymers to CH4 and CO2.1 AD is usually divided into “thermophilic AD” (55–70 °C) and “mesophilic AD” (35–37 °C). Compared with thermophilic AD, mesophilic AD systems exhibit better process stability and higher richness of bacteria. Acidification may occur and then inhibit the production of biogas during thermophilic AD. Other disadvantages of thermophilic AD are: low-quality effluent; increased toxicity and susceptibility to environmental conditions; requirement of larger investment; poor methanogenesis; higher net energy input. In addition, this process is more sensitive to environmental changes than the mesophilic process.3 Although mesophilic systems afford low CH4 yields and suffer from poor biodegradability, the pre-treatment of raw materials can, to a certain degree, alleviate these problems.The sources of raw materials for use as fermentation feedstock are broad: maize straw, wheat straw, and cow dung. Disadvantages in using these raw materials for biogas production include high costs for transportation and storage. Dedicated energy crops make it possible for the bioenergy industry to have an adequate supply of renewable raw materials.4 Many perennial energy crops are planted in China's marginal lands: the Pennisetum hybrid, giant reeds, switchgrass, and elephant grass.5 The Pennisetum hybrid is promising feedstock for biogas production due to its higher volatile solid (VS) content.6 The dry matter content of its leaves accounts for ≈50% of the whole plant. It has strong regeneration ability and can be harvested several times during the growing season.7 It has high annual production of 90–120 ton hm−2.8 The Pennisetum hybrid can grow on marginal lands because it has high tolerance to hostile environments, and it is anticipated to have a positive impact on the environment.2,5
However, bioconversion is inefficient due to the presence of lignocellulosic materials that are difficult to degrade by bacterial hydrolysis.9 Lignocellulosic materials mainly consist of three types of polymers—cellulose, hemicellulose, and lignin—which are associated with each other, as well as smaller amounts of pectin, protein, extractives, and ash.10 The lignin is closely associated with hemicellulose because it covers cellulose and creates a physical barrier for hydrolytic enzymes.11 Furthermore, the cellulose arranges itself in crystalline structures, which are also difficult to degrade. Finally, large particles have a relatively small surface area where the microorganisms can attack the fibers and break down their structures.12 A reduction of the particle size represents an interesting pre-treatment option for solids to be used in biological processes because it is not necessary to add chemical substances and the system is relatively simple.13 Smaller particles allow higher kinetics during the biological processes through the release of dissolved organic matter.14 Also, smaller particles can stimulate hydrolysis by increasing the surface area and pore volume for the microorganisms to access,15 and cause a modification of the lignocellulosic structures through alteration of the cellulose crystallinity or lignin distribution.16
There are increasing numbers of studies on the pre-treatment methods of AD feedstock. Steam explosion is among the most widely applied thermal pre-treatment methods for enhancing CH4 production from lignocellulosic biomass. However, when a steam explosion is carried out, the release of furans and phenolic compounds may have an inhibitory effect on methanogens, leading to decreased gas production.17 “Ensiling” is the most common process used for farm-scale storage of energy crops. Chopped biomass undergoes anaerobic lactic fermentation, but loss of matter is a problem of ensiling pre-treatment.18 Enzymatic pre-treatments have been investigated at the laboratory scale. When enzymatic hydrolysis is applied upstream, AD occurs and there is a strong chance that released sugars are consumed by endogenous microorganisms. Romano et al.19 indicated that there was little increase (≤13%), no impact or even a decrease (≤10%) in biogas production. The combined effect of thermochemical disperser pre-treatment has been carried out and indicates that biogas production is comparatively higher. Production of volatile fatty acids (VFAs) can reach 675 mg L−1 after thermochemical disperser pre-treatment.20 Kavitha et al.21 investigated the synergistic effects of a combined thermochemical-sonic disintegration technique. However, limited literature is available on milling pre-treatment of the Pennisetum hybrid for biogas engineering.
In the present study, the Pennisetum hybrid was processed in a planetary ball mill at a rotational speed of 600 rpm and a ball![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) powder ratio of 10
powder ratio of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 at room temperature. The planetary ball mill had four ball-grinding jar holders installed on a single planetary disk. When the latter was rotated, the jar axis made planetary movements. With the balls ground and mixed grass at high speed, the grass in the jars was smashed. After pre-treatment, the particle size was reduced remarkably. The resulting slurry could be used as raw fermentation material and fed continuously into an anaerobic reactor for biogas production. The purpose of this study was to explore the effect of milling pre-treatments on the structure of the Pennisetum hybrid. Then, the treated Pennisetum hybrid was tested to identify the effect of treatment in mesophilic AD batch experiments at 35 °C. Then, microbial community analyses were done to assess the changes in microbial communities during AD.
1 at room temperature. The planetary ball mill had four ball-grinding jar holders installed on a single planetary disk. When the latter was rotated, the jar axis made planetary movements. With the balls ground and mixed grass at high speed, the grass in the jars was smashed. After pre-treatment, the particle size was reduced remarkably. The resulting slurry could be used as raw fermentation material and fed continuously into an anaerobic reactor for biogas production. The purpose of this study was to explore the effect of milling pre-treatments on the structure of the Pennisetum hybrid. Then, the treated Pennisetum hybrid was tested to identify the effect of treatment in mesophilic AD batch experiments at 35 °C. Then, microbial community analyses were done to assess the changes in microbial communities during AD.
2. Experimental
2.1 Substrates and inoculum
The Pennisetum hybrid (Pennisetum americanum × P. purpureum) was harvested from the Ruhu district of Guangdong Province (China) on 3 January 2016. The growth stage of the Pennisetum hybrid was set at 2 months so that we could obtain raw material with lower lignin content. No fertilizer was applied during the planting season. The pH, organic matter content, total nitrogen content, total phosphorus content, and total potassium content of the soil was 5.35 ± 0.1, 8.003 ± 0.4 g kg−1, 476.66 ± 4 mg kg−1, 313.33 ± 6 mg kg−1, and 17.46 ± 0.5 g kg−1, respectively. The grass was cut into pieces of length 20 mm using a hay cutter. Then, the grass was ensiled for 12 weeks in cylindrical plastic buckets (diameter, 0.8 m) wrapped in polyethylene foil and subsequently placed in the shade to facilitate bioconversion efficiency. After the silage, the grass was placed in an oven at 80 °C and removed after 5 h. This is because fresh grass is difficult to crush completely due to bast fiber in the stem; after dehydration the fiber can be crushed more easily. After crushing for 1 min, the substrates were homogenized and subsequently stored at 4 °C in a refrigerator.The anaerobic sludge used as the inoculum was collected from an AD at a cattle farm in Longmen County (Guangdong Province) and then cultured in a mesophilic AD reactor fed with pig manure. Before use, the inoculum was sieved through a 1 mm mesh to remove large particles and grit. The chemical characteristics of the Pennisetum hybrid and sludge are presented in Table 1.
| Analysis | Pennisetum hybrid silage | Inoculum |
|---|---|---|
| a Results are shown as mean ± sd (n = 3); TS = total solids; VS = volatile solids. | ||
| pH | — | 7.71 ± 0.04 |
| TS (%) | 21.93 ± 1.02 | 1.3729 ± 0.03 |
| VS (%) | 19.81 ± 0.54 | 0.7566 ± 0.02 |
| Total C (% TS) | 41.85 ± 0.31 | 30 ± 0.42 |
| Total N (% TS) | 0.78 ± 0.00 | 2.84 ± 0.11 |
C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N N |
53.65 ± 0.40 | 10.56 ± 0.58 |
| NH4–N (mg L−1) | — | 1168 ± 8 |
| Lignin (% TS) | 19.603 ± 0.497 | — |
| Cellulose (% TS) | 45.01 ± 1.30 | — |
| Hemicellulose (% TS) | 20.05 ± 0.82 | — |
| Calorific value (kJ g−1) | 13.20 ± 0.043 | — |
| Starch (% TS) | 15.2 ± 0.13 | — |
| Crude fat (%) | 2.0 ± 0.05 | — |
| Crude protein (%) | 5.2 ± 0.21 | — |
| Carbohydrate (% TS) | 71.3 ± 2.00 | — |
| Total sugar (% TS) | 3.2 ± 0.12 | — |
| Water (% TS) | 10.7 ± 0.38 | — |
2.2 Milling treatment
A planetary ball mill device was employed to pre-treat the grass hybrid. Milling pre-treatment was done in two ways: (i) “wet” milling (by addition of 1/3 volume of deionized water to the milling tank); (ii) “dry” milling (without the addition of water to the milling tank). Four milling times—3, 6, 9, and 12 h—were used for wet and dry milling (identified as “dry 3”, “dry 6”, “dry 9”, “dry 12”, “wet 3”, “wet 6”, “wet 9”, and “wet 12”, respectively). A 50 g (VS) sample was processed for each milling time. A total of 200 g (VS) samples was processed. The particle-size distribution was analyzed using a laser granulometer. The particle size of the substrate decreased and the surface area increased with milling time.2.3 AD
The AD equipment consisted of an anaerobic digester (1 L reagent bottle), a biogas collector (2 L Erlenmeyer flask), and a water discharge collector (1 L Erlenmeyer flask). The biogas generated in the digester was transported to the head space of the bottle using a glass pipe. Then, the water in the bottle was pressed out and overflowed into the receiver through another glass pipe. The volume of the water discharged from the bottle represented the volume of biogas generated in the digester.The inoculum and Pennisetum hybrid silage were added at a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 based on VS contents. All reactors were filled with 1000 mL of the inoculum, and 1.011 g L−1 of sodium bicarbonate was added to improve the buffer capacity. The inoculum without addition of any feedstock was used as a control. Reactor headspaces were flushed with N2 and placed in a mesophilic water bath at 35 ± 1 °C for 46 days. Each experimental run was carried out in duplicate. The biogas generated was measured every day for the first 7 days and every 3 days for subsequent days. At predetermined days (0, 1, 2, 3, 4, 5, 6, 7, 11, 15, 20, 27, and 39), the content of each reactor was mixed thoroughly and sampled for pH and VFA, and the microbial community composition was analyzed on days 0, 2, 5, 15, and 35. After termination, the total solids (TS) and volatile solids (VS) contents were analyzed.
1 based on VS contents. All reactors were filled with 1000 mL of the inoculum, and 1.011 g L−1 of sodium bicarbonate was added to improve the buffer capacity. The inoculum without addition of any feedstock was used as a control. Reactor headspaces were flushed with N2 and placed in a mesophilic water bath at 35 ± 1 °C for 46 days. Each experimental run was carried out in duplicate. The biogas generated was measured every day for the first 7 days and every 3 days for subsequent days. At predetermined days (0, 1, 2, 3, 4, 5, 6, 7, 11, 15, 20, 27, and 39), the content of each reactor was mixed thoroughly and sampled for pH and VFA, and the microbial community composition was analyzed on days 0, 2, 5, 15, and 35. After termination, the total solids (TS) and volatile solids (VS) contents were analyzed.
2.4 Analytical methods
Standard analytical methods were used to determine the TS and VS contents of the inoculum and silage feedstocks.22 C, N, and H contents were measured using a Vario EL element analyzer. Displacement of saturated brine solutions was used for calculation of daily biogas yield. Mixtures of silage samples (20 g) and deionized water (180 mL) were placed in a refrigerator at 4 °C for 24 h, and then filtered through four-layer gauze. A pH meter (pHS-3C) was used for pH determination. The concentration of NH3–N was determined using a Hach® test kit and spectrophotometer. The VFA contents were determined by a high-performance liquid chromatography system (e2695; Waters, USA) equipped with a refractive index detector, a Shodex sugar SH-1011 column with 0.005 M H2SO4 as the mobile phase at 0.5 mL min−1 and a column temperature of 50 °C. The gas composition (CH4, CO2, and N2) was determined using a gas chromatograph (hp5890) equipped with a thermal conductivity detector and Porpak5 column. The flow rate of the carrier gas (Ar) was 7.5 mL min−1, and the temperatures of the injection port, column, and detector, were 30 °C, 40 °C, and 120 °C, respectively.The special surface area, pore size, and total pore volume of the raw materials were analyzed with an automated surface and porosity analyzer (SI-MP-10/Pore Master 33; Quanta Chrome Instruments, USA).
2.5 Analyses of high-throughput microbial community sequencing data
3. Results and discussion
3.1 Change of physical structure after milling pre-treatment
The purpose of milling pre-treatment was to increase the surface area and digestibility of the Pennisetum hybrid. Milling could also increase the rates of degradation and volumetric biogas production during the AD process. Meanwhile, the smaller the particle size, the more likely it is that severe acidification will occur during the fermentation cycle. A decrease in pH can inhibit the activity of CH4-producing bacteria, which leads to a decrease in CH4 production. Hence, it is necessary to explore the optimal crushing degree of the Pennisetum hybrid to increase the amount of CH4 production while not causing severe acidification.Table 2 shows the particle-diameter distribution, pore volume, surface area, and average pore diameter of the Pennisetum hybrid before and after pre-treatment. The size of the surface area reflects the opportunities for contact with anaerobic microorganisms and raw materials, which determines the degree of difficulty for AD. The hydrolysis rate and the degree of cellulose hydrolysis are directly related to the size of the surface area of the raw materials.27 For the dry 3 and wet 6 pre-treatments, the surface area of the Pennisetum hybrid was 2.228 m2 g−1 and 2.678 m2 g−1, respectively, or 60.06% and 92.39%, higher than that of the untreated grass (1.392 m2 g−1). The median diameters (D50) of the Pennisetum hybrid were 234.46 μm, 131.75 μm, 106.12 μm, and 47.92 μm for dry 3, 6, 9, and 12, respectively, or 25.28%, 58.01%, 66.18%, and 84.73%, lower than that of the untreated Pennisetum hybrid (313.80 μm). The median diameters (D50) of the wet 3, 6, 9, and 12 were 290.87 μm, 243.64 μm, 165.96 μm, and 149.94 μm, respectively, or 7.3%, 22.36%, 47.11%, and 52.22%, lower than that of the untreated Pennisetum hybrid. With an increase in treatment time, the pore volume, surface area, and average pore diameter of the Pennisetum hybrid gradually increased. This effect helped to increase the accessibility of the substrate to microorganisms, thus improving the fermentation efficiency.
| Milling time (h) | 0 | 3 | 6 | 9 | 12 | |
|---|---|---|---|---|---|---|
| a Results are shown as means ± sd (n = 3); DX indicates that X% of the sample size is smaller than DX. | ||||||
| Dry milling | Pore volume (cm3 g−1) | 1.52 × 10−3 | 4.6 × 10−3 | 5.43 × 10−3 | 5.57 × 10−3 | 7.71 × 10−3 |
| Surface area (m2 g−1) | 1.392 | 2.228 | 2.532 | 2.68 | 3.895 | |
| Average pore diameter (nm) | 4.37502 | 10.3018 | 8.26448 | 8.09834 | 7.91347 | |
| D10 (μm) | 100.62 | 33.25 | 25.815 | 21.945 | 11.79 | |
| D50 (μm) | 313.8 | 234.46 | 131.75 | 106.12 | 47.92 | |
| D90 (μm) | 1056.45 | 959.8 | 535.52 | 469.22 | 202.25 | |
| Wet milling | Pore volume (cm3 g−1) | 1.52 × 10−3 | 5.99 × 10−3 | 6.63 × 10−3 | 9.11 × 10−3 | 9.41 × 10−3 |
| Surface area (m2 g−1) | 1.392 | 1.936 | 2.678 | 4.202 | 4.777 | |
| Average pore diameter (nm) | 4.37502 | 9.90184 | 12.3670 | 8.95684 | 7.63025 | |
| D10 (μm) | 100.62 | 95.89 | 88.49 | 78.08 | 65.57 | |
| D50 (μm) | 313.8 | 290.87 | 243.64 | 165.96 | 149.94 | |
| D90 (μm) | 1056.45 | 1033.49 | 810.59 | 759.95 | 684.73 | |
Milling treatment changed the surface structure of the Pennisetum hybrid and changed its structural characteristics. X-ray diffraction can be used to investigate the change of crystallinity in raw materials. With an increase in treatment time, the cellulose crystallinity index (CrI) of the Pennisetum hybrid increased gradually. This may have been due to the removal of amorphous components, such as hemicellulose and lignin, during the pre-treatment process, which leads to a relative increase in the proportion of crystalline cellulose. The characteristic peak did not shift or diffract, so the milling treatment did not change the cellulose crystal form of the Pennisetum hybrid.28 The cellulose in the untreated Pennisetum hybrid was inlaid and wrapped with the hemicellulose and lignin yet, due to the reduction of cellulose and lignin in the treated Pennisetum hybrid, the shape of the characteristic peak became sharp, the width of the half peak was reduced, and the response was enhanced. This shows that the cellulose content of the Pennisetum hybrid increased after the milling treatment, which improved the CrI. A clear relationship between the CrI and the degree of damage to the material structure has been shown in a previous study,29 which is in agreement with our experimental results: with an increase in the degree of damage to the material structure, the CrI also increased.
3.2 Biogas production
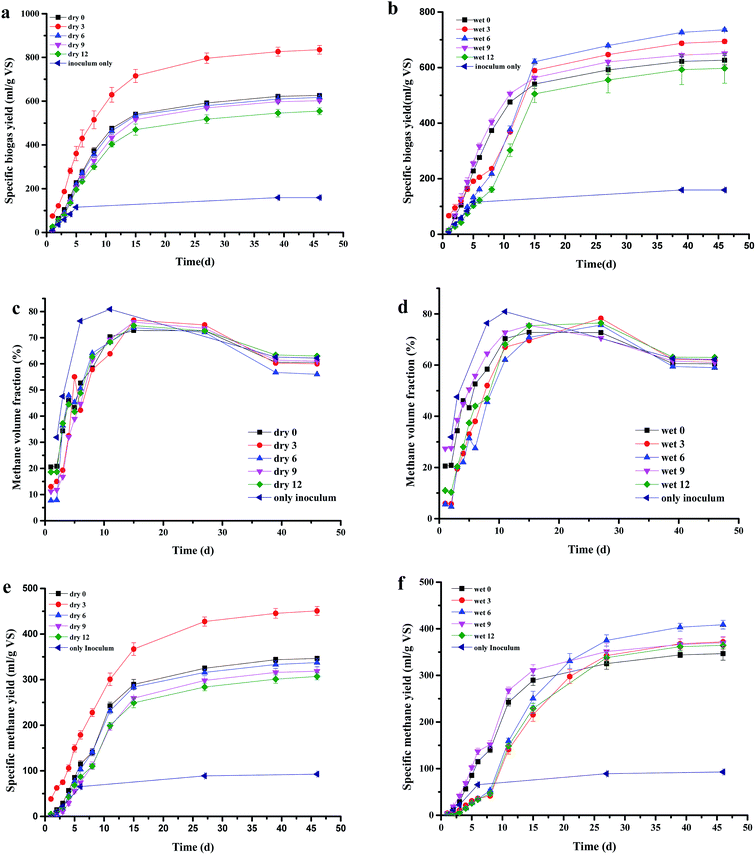
As indicated in Fig. 1a, the specific biogas yields of untreated grass, and the dry 3, 6, 9, and 12 treatments reached 467.33, 676.17, 458.51, 443.13, and 395.5 mL g−1-VS, respectively. The highest cumulative biogas yield (676.17 mL g−1-VS) was obtained with the dry 3 treatment, at 44.68% higher than that of the un-milled samples. The biogas production decreased for the other pre-treatments compared with that of the untreated Pennisetum hybrid. Also, Fig. 1b shows that the specific biogas yields of untreated grass, and the wet 3, 6, 9, and 12 treatments reached 467.33, 535.17, 577.00, 492.00, and 438.17 mL g−1-VS, respectively. The wet 6 treatment had the highest yield, which was 23.47% higher than that of the untreated grass. This phenomenon could be because the pre-treated Pennisetum hybrid has a larger specific surface area and smaller particle size, which results in the acidification of the reactor. Thus, a reduction in gas production in the corresponding reactor occurs. These results indicated that >6 h for dry milled grass and >9 h for wet milled grass does not increase the biogas yield. Furthermore, >6 h for dry milled grass and 9 h for wet milled grass inhibited CH4 production.Biogas CH4 contents are shown in Fig. 1c and d. The CH4 contents of the dry milled grass digesters increased gradually. On day 15, CH4 contents reached about 74–77%, and then decreased gradually afterwards. Also, Fig. 1d shows that, in the wet milled grass digesters, the highest CH4 contents (78.27%) during the 46 day digestion were obtained with the wet 3 AD on day 27. After day 27, the CH4 contents of all digesters fluctuated between 60% and 75%.
The specific CH4 yields for the pre-treated Pennisetum hybrid during the 46 days of AD are presented in Fig. 1e and f. Among the four dry milled grass pre-treatments, the highest specific CH4 yield was obtained for the dry 3 AD (358.07 mL g−1-VS), which was 41.04% higher than that of the untreated grass AD (253.88 mL g−1-VS), followed by dry 6 (244.78 mL g−1-VS), dry 9 (225.74 mL g−1-VS), and dry 12 (214.26 mL g−1-VS), respectively. The specific CH4 yields for the wet 3, 6, 9, and 12 AD reached 278.99, 315.87, 277.12, and 271.55 mL g−1-VS, respectively. The wet 6 AD had the largest specific CH4 yield, at 24.42% higher than that of the untreated grass (253.88 mL g−1-VS).
Several analyses have been developed to investigate the effects of pre-treatment on the feedstock of AD and mainly to describe the improvements in the CH4 yield. “Partial composting” has been investigated as a pre-treatment step in AD. Mshandete et al.30 obtained a 26% higher CH4 yield from sisal pulp pre-treated during 9 h of composting under aerobic conditions. Alkaline pre-treatment is a suitable method for solubilizing lignin. Sambusiti et al.31 investigated the effect of alkaline (NaOH) pre-treatment on ensiled sorghum forage in semi-continuous digesters. It was observed that pre-treatment with 10 g NaOH/100 g TS increased the CH4 yield by 25% compared with untreated sorghum. Steam explosion of different lignocellulosic feedstocks has been thoroughly studied and applied at the laboratory scale. Forgacs et al.32 studied the co-digestion of steam-exploded citrus waste with municipal solid wastes in continuous reactors and found CH4 production of 0.56 m3 CH4 per kg VS d. Enzymatic pre-treatments have been investigated at the laboratory scale and biochemical methane potential tests showed that the addition of the enzyme to laboratory-scale batch anaerobic tests can lead to an increase in biogas yield of ≈10%.33 Several research studies have shown that combined pre-treatment is efficient at enhancing the extent and rate of sludge AD. According to Kavitha et al.,34 though combined thermochemical-sonic disintegration combination pre-treatment enhanced the preliminary rate of CH4 generation as a result of increased solubilization, it caused only a non-significant increase in CH4 generation. Rani et al.35 studied the influence of combined alkaline and disperser pre-treatment on sludge disintegration, and found that biogas production was 76% higher than that of control at optimized conditions. Our investigation indicated that milling pre-treatment of the Pennisetum hybrid was effective in improving CH4 yield compared with other single pre-treatments.
3.3 Changes in pH and VFA
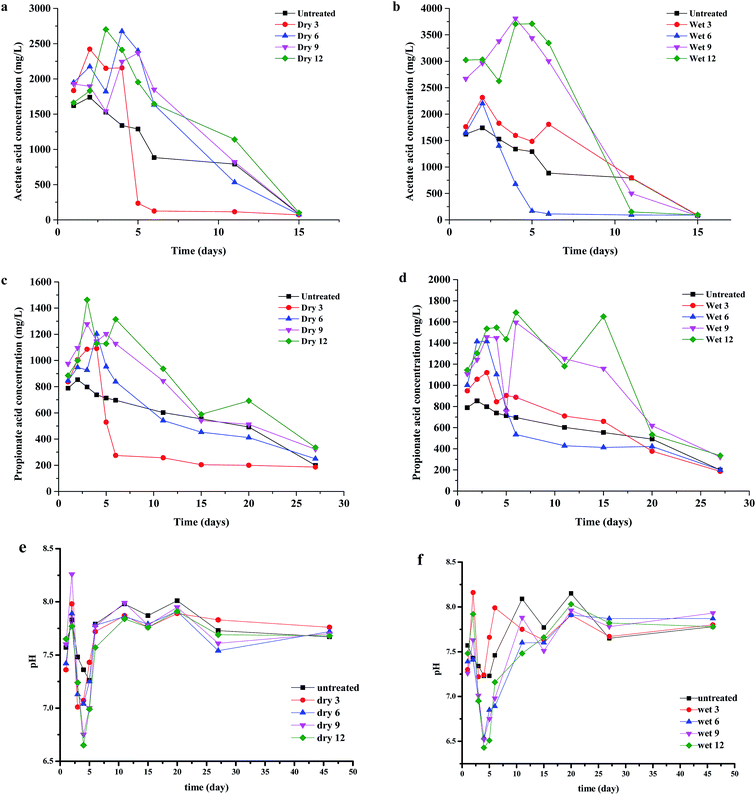
During AD, the pH value is a significant parameter that is highly sensitive to the buffering capacity and VFA concentration. The pH values had a completely different trend than VFA concentrations. As shown in Fig. 2e and f, pH values of 6.6–6.7 and 6.4–6.6 in the dry and wet AD systems were detected at day 4, and at that time the acetic acid reached maximum yields of 2500–3000 mg L−1 and 3500–4000 mg L−1, respectively. The CH4 yields from the dry 6, dry 9, dry 12, wet 9, and wet 12 AD were relatively lower, which could have been caused by the acidification of the digestion and inhibition of methanogenesis, resulting in falling production of CH4. Thereafter, the pH values appeared to increase from 7.6 to 7.9, whereas the VFA concentration decreased, which indicates that VFA was being transformed to CH4.The evolution of VFAs has a very significant role in guaranteeing the effect of AD, and strongly influences the pH value, alkalinity, and methanogen activity.16,36 Rapid hydrolysis and acidogenesis is a major challenge in AD, and can lead to the accumulation of VFAs. Irreversible acidification and inhibition of methanogenesis would result in failure of the digester.37,38 The variations in VFAs during AD of the milled Pennisetum hybrid are shown in Fig. 2a–d. The data indicate that the dominant VFA was acetic acid. After starting the test, the concentrations of VFAs for all AD reactors increased rapidly. Among them, as shown in Fig. 2a and c, the concentrations of acetic acid and propionic acid in dry 3 rapidly dropped to about 70–80 mg L−1 and 200–300 mg L−1, respectively, after a brief increase. The concentrations of acetic acid and propionic acid in dry 6, 9, and 12 reached a maximum of about 2700 mg L−1 and 1400 mg L−1 on day 4, and then dropped slowly until the digestion process was complete. The decrease in the VFAs concentrations indicates that the production of VFAs is slower than its consumption by methanogenesis. This finding suggests that the hydrolysis of lignocellulose is the rate-limiting step for the AD of the Pennisetum hybrid. Constant CH4 contents of 50–60% were achieved. Changes in the concentrations of acetic acid and propionic acid are shown in Fig. 2b and d, respectively. The highest concentrations of acetic acid and propionic acid were detected in the wet 9 and wet 12 AD reactors, with values of 3500–4000 mg L−1 and 1500–1800 mg L−1, respectively. Conversely, the wet 6 AD system did not show accumulation of acetic acid or propionic acid after 5 days, with values of 80–100 mg L−1 and 400–500 mg L−1, respectively. This phenomenon led to a decline in CH4 production for the dry 6, dry 9, dry 12, wet 9, and wet 12 AD systems. This could have been because of acid accumulation, at 3000 mg L−1 acetic acid and 1500 mg L−1 propionic acid, and a drop in pH to 6.5.
3.4 Microbial community analyses
The compositions of the microbial communities were analyzed dynamically to assess how milling pre-treatment impacted the microbial activity and the adaptation of microbial communities to specific processing conditions. As shown in Table 3, the cumulative recovered reading of the dry and wet samples was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 762
762![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 583, and the total amount of OTUs was 95
583, and the total amount of OTUs was 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 709. OTUs are indicators of the quantity of bacterial communities. The OTUs of the dry milled grass AD were 48
709. OTUs are indicators of the quantity of bacterial communities. The OTUs of the dry milled grass AD were 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 516, slightly higher than that of the wet milled grass (47
516, slightly higher than that of the wet milled grass (47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 193). The Chao1 algorithm was also used to estimate the number of OTUs in microbial communities. The Chao1 and OTUs estimators showed similar trends, and indicated that the bacterial communities for wet milled grass AD had lower richness than those for dry milled grass AD. The diversity of a microbial community can also be reflected by Shannon and Simpson indices. A higher Shannon index indicates a higher diversity of the microbial community, whereas a lower Simpson index indicates higher diversity. The Shannon indices for dry milled grass AD were 4.06–4.66, and for wet milled grass AD they were 3.58–4.57. The Simpson indices were 0.11–0.06 and 0.15–0.04, respectively. The Shannon and Simpson indices showed that dry milled grass AD had a higher diversity of the microbial community compared with wet milled grass AD. The phenomenon mentioned above explained why the CH4 yield of dry milled grass AD was better than that for wet milled grass AD. The coverage values of all samples were >0.95, indicating that most of the bacteria were within the range of detection.
193). The Chao1 algorithm was also used to estimate the number of OTUs in microbial communities. The Chao1 and OTUs estimators showed similar trends, and indicated that the bacterial communities for wet milled grass AD had lower richness than those for dry milled grass AD. The diversity of a microbial community can also be reflected by Shannon and Simpson indices. A higher Shannon index indicates a higher diversity of the microbial community, whereas a lower Simpson index indicates higher diversity. The Shannon indices for dry milled grass AD were 4.06–4.66, and for wet milled grass AD they were 3.58–4.57. The Simpson indices were 0.11–0.06 and 0.15–0.04, respectively. The Shannon and Simpson indices showed that dry milled grass AD had a higher diversity of the microbial community compared with wet milled grass AD. The phenomenon mentioned above explained why the CH4 yield of dry milled grass AD was better than that for wet milled grass AD. The coverage values of all samples were >0.95, indicating that most of the bacteria were within the range of detection.
| Fermentation time (days) | Sample | Reads | OTUs | Shannon | Chao1 | Coverage | Simpson |
|---|---|---|---|---|---|---|---|
| 1 | Dry 3 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 806 806 |
3154 | 4.16182 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 076.96 076.96 |
0.956779 | 0.069527 |
| Dry 6 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 881 881 |
3094 | 4.061444 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 653.25 653.25 |
0.964466 | 0.073349 | |
| Dry 9 | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 319 319 |
3317 | 4.262944 | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 708.37 708.37 |
0.953398 | 0.058401 | |
| Dry 12 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 566 566 |
2468 | 3.934603 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 266.44 266.44 |
0.963786 | 0.091761 | |
| Wet 3 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 342 342 |
2954 | 4.28821 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 914.84 914.84 |
0.955445 | 0.052661 | |
| Wet 6 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 106 106 |
2912 | 4.217762 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 153.36 153.36 |
0.958488 | 0.050612 | |
| Wet 9 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 060 060 |
3049 | 3.577421 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 001.14 001.14 |
0.964694 | 0.153 | |
| Wet 12 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 422 422 |
2867 | 4.181939 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 505.93 505.93 |
0.959511 | 0.055752 | |
| 4 | Dry 3 | 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 738 738 |
2593 | 4.552737 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 772.70 772.70 |
0.958022 | 0.037974 |
| Dry 6 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
2846 | 4.091292 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 290.80 290.80 |
0.962997 | 0.064667 | |
| Dry 9 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 840 |
3317 | 4.176026 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 842.01 842.01 |
0.961984 | 0.060175 | |
| Dry 12 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 216 216 |
3004 | 4.144411 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 862.06 862.06 |
0.961885 | 0.056986 | |
| Wet 3 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 723 723 |
2715 | 4.204539 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 759.27 759.27 |
0.961339 | 0.04807 | |
| Wet 6 | 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 341 341 |
2691 | 4.350349 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 441.16 441.16 |
0.958655 | 0.043056 | |
| Wet 9 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 353 353 |
2745 | 4.06387 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 539.09 539.09 |
0.964797 | 0.075542 | |
| Wet 12 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 180 180 |
3172 | 4.442022 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 673.33 673.33 |
0.955536 | 0.036447 | |
| 15 | Dry 3 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 941 941 |
3064 | 4.652357 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 903.78 903.78 |
0.961118 | 0.036078 |
| Dry 6 | 57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 035 035 |
3070 | 4.046908 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 617.67 617.67 |
0.958973 | 0.080478 | |
| Dry 9 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790 790 |
3351 | 4.288492 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 883.22 883.22 |
0.954147 | 0.063166 | |
| Dry 12 | 59![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 693 693 |
3052 | 4.031164 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 938.78 938.78 |
0.961419 | 0.075797 | |
| Wet 3 | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 819 819 |
1742 | 4.598299 | 8076.64 | 0.956217 | 0.032259 | |
| Wet 6 | 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790 790 |
2798 | 4.565135 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 479.78 479.78 |
0.956413 | 0.040131 | |
| Wet 9 | 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 524 524 |
2972 | 4.347882 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 072.07 072.07 |
0.958 | 0.051466 | |
| Wet 12 | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 758 758 |
3035 | 4.234921 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 465.01 465.01 |
0.959129 | 0.080019 | |
| 35 | Dry 3 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 840 |
2675 | 4.204925 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 471.70 471.70 |
0.957899 | 0.084436 |
| Dry 6 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 967 967 |
3280 | 4.299125 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 595.23 595.23 |
0.956177 | 0.082486 | |
| Dry 9 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 836 836 |
3321 | 4.379049 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 150.71 150.71 |
0.961689 | 0.065638 | |
| Dry 12 | 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 822 822 |
2910 | 4.060932 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 422.52 422.52 |
0.9652 | 0.111108 | |
| Wet 3 | 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 160 160 |
3605 | 4.660527 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 655 655 |
0.964396 | 0.033939 | |
| Wet 6 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 420 420 |
3342 | 4.663317 | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 567.63 567.63 |
0.954549 | 0.055168 | |
| Wet 9 | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 765 765 |
3223 | 4.485514 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 220.17 220.17 |
0.957258 | 0.065835 | |
| Wet 12 | 54![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 130 130 |
3371 | 4.207647 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 607.11 607.11 |
0.954591 | 0.102073 |
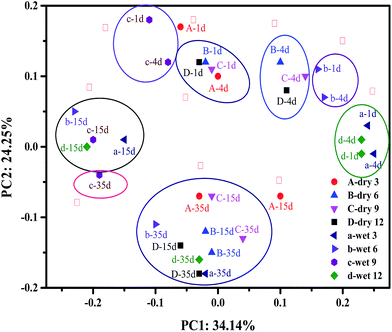
Principal component analyses indicate changes in the structure of a microbial community during AD. As shown in Fig. 3, principal components 1 (PC1) and 2 (PC2) accounted for 34.14% and 24.25% of the total change, respectively. Samples were aggregated into ten groups and identified by Roman numerals. The dry milled treatment samples were divided into groups I, III, IV, IX, and X and a part of group VIII (A-35d, B-35d, D-35d), whereas the other groups contained the wet milled treatment samples. Samples for days 1 and 4 of the wet milled grass (c and b) fell into groups II and V, respectively, whereas a and d fell into group VI, indicating that there was no significant change in the microbial-community structure during the first 4 days of digestion. The microbial-community structure of dry milled grass AD changed little compared with that of wet milled grass AD during the first 4 days of fermentation, as shown in groups I, III, and IV. This phenomenon indicated that there was no significant difference in the starting speed of all AD reactors. It is worth noting that sample A-4d was located in group III, not in group IV like the other day 4 samples. This may have been because dry 3 did not experience serious acidification on day 4. Groups VIII and X indicated that the bacterial-community structure of the dry milled grass AD reached a relatively steady state after 15 days of digestion, whereas the wet milled grass entered stable fermentation much later. This result was consistent with the results for CH4 production and VFAs.
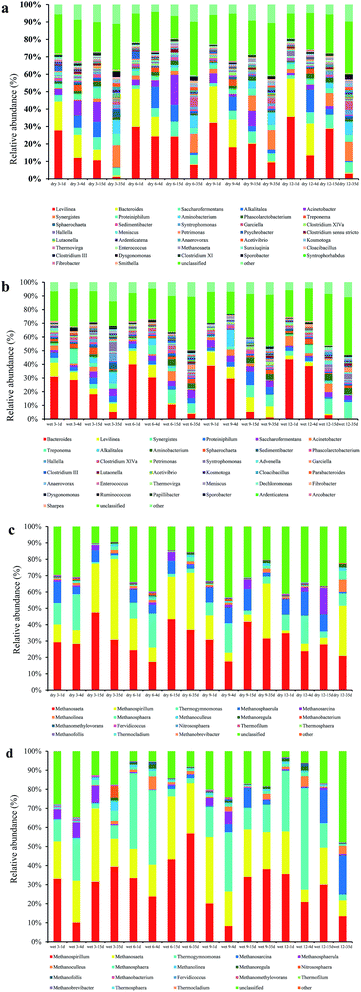
During the AD, various bacterial (Fig. 4a and b) and archaeal (Fig. 4c and d) community structures at the genus level were detected. The dominant genus was the same for all AD processes, but the relative abundance of each genus was different depending on the pre-treatment type. Dominant bacteria included Levilinea, Bacteroides, Saccharofermentans, Alkalitalea, Acinetobacter, Synergistes, and Proteiniphilum. Dominant archaea included Methanosaeta, Methanospirillum, Thermogymnomonas, Methanosphaerula, Methanosarcina, and Methanolinea.
As shown in Fig. 4a, the most abundant bacteria at the genus level were Levilinea in samples from dry milled grass AD, followed by Bacteroides, at relative abundances of 2.33–27.76% and 0.4–24.94%, respectively. The two most abundant genera in the samples of wet milling treatment were Bacteroides and Levilinea (Fig. 4b), with relative abundance of 0.18–42.01% and 0.14–37.12%, respectively. Levilinea abundance was drastically reduced on day 4 of fermentation in dry 3 (11.98%), more so than in dry 6 (9.13%), dry 9 (8.26%), or dry 12 (8.18%). This may have been caused by acidification of the sample. As shown in Fig. 4b, the relative abundance of Bacteroides decreased with increasing fermentation time.
Some of the archaea, such as Methanosaeta, Methanospirillum, Thermogymnomonas, Methanosphaerula, Methanosarcina, and Methanolinea, occupied dominant positions in the fermentation process (Fig. 4c and d). The most abundant archaea at the genus level were Methanospirillum (up to 56.83% for wet 6 at 35 days), followed by Methanosaeta (up to 50.77% for dry 3 at 35 days), in dry and wet milled grass AD. For samples from dry and wet milled grass AD, after 15 days of digestion the relative abundance of Methanospirillum increased significantly; however, the relative abundance of Thermogymnomonas decreased significantly after 15 days of fermentation. This may have been due to the close correlation of Methanospirillum to the methanogenic phase of AD. Thermogymnomonas was closely related to the hydrolysis of cellulose.
4. Conclusions
The use of a planetary ball mill can effectively reduce the particle size of the Pennisetum hybrid. Dry milling was more efficient than wet milling for a given processing time. The effects of milling were visually evident, and the pore diameters, pore volumes, and specific surface areas of the grass were altered. The CrI increased with increasing treatment time. The particle-size reduction significantly increased the digestibility of the raw material. Excessive breakage caused acidification, as detected by increasing concentrations of VFAs and changes in microbiological populations. This investigation showed a 41.04% increase in the specific CH4 yields after 3 h of dry milling pre-treatment, and indicated that the milling pre-treatment of the Pennisetum hybrid was effective in improving the CH4 yield. In addition, wet milling pre-treatment can improve the mobility of raw materials. Good fluidity and optimization of the degree of milling pre-treatment could, therefore, be applied on an industrial scale.Acknowledgements
This material is based upon work that is supported by the Science and Technology Service Network Initiative (KFJ-Ew-STS-138) and National Science and Technology Support Program: Key technology and engineering demonstration of lignocellulosic biomass energy conversion (2013BAD22B03). The authors wish to thank Mr Lianhua Li (Guangzhou Institute of Energy Conversion, CAS Key Laboratory of Renewable Energy, Chinese Academy of Sciences) for reading the manuscript and providing useful suggestions.References
- A. Mshandete, et al., Effect of particle size on biogas yield from sisal fibre waste, Renewable Energy, 2006, 31(14), 2385–2392 CrossRef CAS.
- S. Palit, A. K. Chowdhuri and B. K. Mandal, Environmental impact of using biodiesel as fuel in transportation: a review, International Journal of Global Warming, 2011, 3(3), 232–256 CrossRef.
- C. L. Mao, et al., Review on research achievements of biogas from anaerobic digestion, Renewable Sustainable Energy Rev., 2015, 45, 540–555 CrossRef CAS.
- Billion-Ton Update: Biomass Supply for a Bioenergy and bio-products Industry, O. R. N. Laboratory, U. S. D. o. Energy, 2011 Search PubMed.
- J. Z. Zhang, et al., Biomass to bio-ethanol: The evaluation of hybrid Pennisetum used as raw material for bio-ethanol production compared with corn stalk by steam explosion joint use of mild chemicals, Renewable Energy, 2016, 88, 164–170 CrossRef CAS.
- C. Somerville, et al., Feedstocks for Lignocellulosic Biofuels, Science, 2010, 329(5993), 790–792 CrossRef CAS PubMed.
- S. Xiulan, C. Ping and Z. Yulei, study on the suitable cutting dates and stubble heights of the new strainNo. 1 of hybrid Pennisetum in late autumn, Chinese Journal of Grassland, 2010, 329(4), 35–41 Search PubMed.
- C. Zhitong, H. Shuilin and H. Yibin, Research progress of Pennisetum rich, Acta Agrestia Sin., 2010, 18(5), 740–748 Search PubMed.
- M. J. Taherzadeh and K. Karimi, Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review, Int. J. Mol. Sci., 2008, 9(9), 1621–1651 CrossRef CAS PubMed.
- E. Brun, Improved anaerobic digestion of energy crops and agricultural residues, in Department of Environmental Engineering, in Technical, University of Denmark, 2010 Search PubMed.
- T. Noike, Characteristics of Carbohydrate Degradation and the Rate-Limiting Step in Anaerobic-Digestion, Biotechnol. Bioeng., 1985, 27(10), 1482–1489 CrossRef CAS PubMed.
- I. Angelidaki and B. K. Ahring, Methods for increasing the biogas potential from the recalcitrant organic matter contained in manure, Water Sci. Technol., 2000, 41(3), 189–194 CAS.
- A. Barakat, H. de Vries and X. Rouau, Dry fractionation process as an important step in current and future lignocellulose biorefineries: A review, Bioresour. Technol., 2013, 134, 362–373 CrossRef CAS PubMed.
- J. A. Muller, Comminution of organic material, Chem. Eng. Technol., 2003, 26(2), 207–217 CrossRef CAS.
- Z. H. Hu, H. Q. Yu and R. F. Zhu, Influence of particle size and pH on anaerobic degradation of cellulose by ruminal microbes, Int. Biodeterior. Biodegrad., 2005, 55(3), 233–238 CrossRef CAS.
- F. Monlau, et al., Lignocellulosic Materials Into Biohydrogen and Biomethane: Impact of Structural Features and Pretreatment, Crit. Rev. Environ. Sci. Technol., 2013, 43(3), 260–322 CrossRef CAS.
- F. Monlau, et al., Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review, Biotechnol. Adv., 2014, 32(5), 934–951 CrossRef CAS PubMed.
- A. Pakarinen, et al., Evaluation of preservation methods for improving biogas production and enzymatic conversion yields of annual crops, Biotechnol. Biofuels, 2011, 4(1), 20 CrossRef CAS PubMed.
- R. T. Romano, et al., The effect of enzyme addition on anaerobic digestion of Jose Tall Wheat Grass, Bioresour. Technol., 2009, 100(20), 4564–4571 CrossRef CAS PubMed.
- S. Kavitha, et al., Enhancing the functional and economical efficiency of a novel combined thermo chemical disperser disintegration of waste activated sludge for biogas production, Bioresour. Technol., 2014, 173, 32–41 CrossRef CAS PubMed.
- S. Kavitha, et al., Combined thermo-chemo-sonic disintegration of waste activated sludge for biogas production, Bioresour. Technol., 2015, 197, 383–392 CrossRef CAS PubMed.
- Standard Methods for the Examination of Water and Wastewater, A. P. H. Association, 1998 Search PubMed.
- A. Rademacher, et al., Characterization of microbial biofilms in a thermophilic biogas system by high-throughput metagenome sequencing, FEMS Microbiol. Ecol., 2012, 79(3), 785–799 CrossRef CAS PubMed.
- H. Tamaki, et al., Analysis of 16S rRNA Amplicon Sequencing Options on the Roche/454 Next-Generation Titanium Sequencing Platform, PLoS One, 2011, 6(9), e25263 CAS.
- J. G. Caporaso, et al., QIIME allows analysis of high-throughput community sequencing data, Nat. Methods, 2010, 7(5), 335–336 CrossRef CAS PubMed.
- R. C. Edgar, et al., UCHIME improves sensitivity and speed of chimera detection, Bioinformatics, 2011, 27(16), 2194–2200 CrossRef CAS PubMed.
- M. Wiman, et al., Rheological Characterization of Dilute Acid Pretreated Softwood, Biotechnol. Bioeng., 2011, 108(5), 1031–1041 CrossRef CAS PubMed.
- H. B. Zhao, et al., Studying cellulose fiber structure by SEM, XRD, NMR and acid hydrolysis, Carbohydr. Polym., 2007, 68(2), 235–241 CrossRef CAS.
- S. Park, et al., Cellulose crystallinity index: measurement techniques and their impact on interpreting cellulase performance, Biotechnol. Biofuels, 2010, 3(1), 10 CrossRef PubMed.
- A. Mshandete, et al., Enhancement of anaerobic batch digestion of sisal pulp waste by mesophilic aerobic pre-treatment, Water Res., 2005, 39(8), 1569–1575 CrossRef CAS PubMed.
- C. Sambusiti, et al., Benefit of sodium hydroxide pretreatment of ensiled sorghum forage on the anaerobic reactor stability and methane production, Bioresour. Technol., 2013, 144, 149–155 CrossRef CAS PubMed.
- G. Forgacs, et al., Methane production from citrus wastes: process development and cost estimation, J. Chem. Technol. Biotechnol., 2012, 87(2), 250–255 CrossRef CAS.
- U. Schimpf, et al., Improving the efficiency of large-scale biogas processes: pectinolytic enzymes accelerate the lignocellulose degradation, Energy Environ., 2013, 4, 53–60 Search PubMed.
- S. Kavitha, et al., Impact of thermo-chemo-sonic pretreatment in solubilizing waste activated sludge for biogas production: Energetic analysis and economic assessment, Bioresour. Technol., 2016, 219, 479–486 CrossRef CAS PubMed.
- R. U. Rani, et al., Combined treatment of alkaline and disperser for improving solubilization and anaerobic biodegradability of dairy waste activated sludge, Bioresour. Technol., 2012, 126, 107–116 CrossRef PubMed.
- H. B. Moller, S. G. Sommer and B. K. Ahring, Biological degradation and greenhouse gas emissions during pre-storage of liquid animal manure, J. Environ. Qual., 2004, 33(1), 27–36 CrossRef CAS PubMed.
- Y. Y. Wang, et al., Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria, Biomass Bioenergy, 2009, 33(5), 848–853 CrossRef CAS.
- A. Veeken and B. Hamelers, Effect of temperature on hydrolysis rates of selected biowaste components, Bioresour. Technol., 1999, 69(3), 249–254 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |