Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Ultrathin Ag nanoparticles anchored on urchin-like WO3·0.33H2O for enhanced photocatalytic performance†
Haoqi Ren a,
Xufeng Goub and
Qing Yang*b
a,
Xufeng Goub and
Qing Yang*b
aDepartment of Chemistry, Fudan University, Shanghai 200433, China. E-mail: 13307110326@fudan.edu.cn
bSchool of Material Science and Engineering, Xi'an University of Technology, Xi'an 710048, China. E-mail: yangqing@xaut.edu.cn
First published on 20th February 2017
Abstract
Ultrathin Ag nanoparticles anchored on urchin-like WO3·0.33H2O can be successfully achieved by a novel and facile self-catalytic reduction approach, and displayed a better solar-driven photocatalytic performance than that of the individual WO3·0.33H2O.
As an important n-type semiconductor, tungsten oxide hydrate (WO3·0.33H2O) has been demonstrated to be a promising material for the photocatalytic degradation of organic dyes.1–3 In the past few years, many efforts have been employed into the shape-controlled synthesis of WO3·0.33H2O crystals. Although morphological WO3·0.33H2O, such as nanoplates, nanorods, and hierarchical architectures, has been successfully synthesized,4–8 individual WO3·0.33H2O is not an efficient photocatalyst because of its weak light-harvesting and relatively low conduction band (CB) level.9,10
So far, two general strategies have been developed to improve the photocatalytic activity of WO3·0.33H2O. One is the controllable synthesis of WO3·0.33H2O with high-active building blocks.4–8 The other one is the modification of WO3·0.33H2O, such as doping and hybridizing.11,12 Especially, a heterogeneous junction originating from the intimate contact of a metal with a semiconductor, is very useful for improving the photocatalytic performance of the pristine semiconductor, because it can effectively limit the recombination of photo-generated electrons and holes.1 Consequently, the combination of the above two strategies is a perspective pathway to effectively improve the photocatalytic activity of WO3·0.33H2O. However, it has been rarely reported on the metal/WO3·0.33H2O for enhanced photocatalytic activity. Therefore, to precisely control the structure of hybrid metal/WO3·0.33H2O composite, and thorough understanding of the formation and photocatalytic mechanisms are imperative.
Herein, we report a novel and facile self-catalytic reduction approach for the achievement of ultrathin silver (Ag) nanoparticles anchored on urchin-like WO3·0.33H2O assembly of rod-like building blocks. When evaluated the photocatalytic performance, the as-prepared Ag/WO3·0.33H2O composite exhibited a better photodegradation of Rhodamine B (RhB) than that of the individual WO3·0.33H2O under simulated solar light irradiation. The preparation of this novel Ag/WO3·0.33H2O hybridizing architecture could provide a good opportunity to understand the foundational significance of metal nanoparticle for improving the photocatalytic activity of semiconductor.
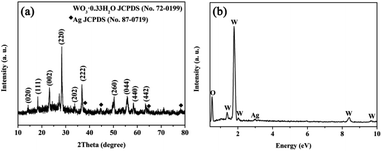
Fig. 1a is the powder X-ray diffraction (XRD) pattern of the as-synthesized Ag/WO3·0.33H2O composite. The main diffraction peaks can be indexed by the orthorhombic WO3·0.33H2O (JCPDS card no. 72-0199, and the thermogravimetric analysis (TGA) can be used for further determining the stoichiometry of the hydrated water in the oxide, see Fig. S2†). Moreover, it can be seen that there are slight diffraction peaks at 38.1°, 44.3°, 64.4° and 77.5° (marked by rhomboic symbol), respectively, which are indexed by the cubic Ag (JCPDS card no. 87-0719). Electron energy dispersive X-ray (EDX) analysis can also be used to characterize the chemical composition, and the tungsten, silver and oxygen element are detected (see Fig. 1b), further indicating the formation of Ag/WO3·0.33H2O composite.
Fig. 2 shows the scanning electron microscope (SEM) images of the as-synthesized Ag/WO3·0.33H2O composite with different magnifications. A low-magnification SEM image (see Fig. 2a) displays that the as-synthesized Ag/WO3·0.33H2O composite exhibits a three dimensional (3D) urchin-like architectures. A high-magnification SEM image (see Fig. 2b) presents that the hierarchical architecture is assembled by a number of outward radioactive rods-like building blocks with a length of 200–300 nm. Fig. 3a is a typical low-magnification transmission electron microscope (TEM) image of a rod-like building block of the urchin-like Ag/WO3·0.33H2O, and it can be found that some uniform and monodispersed Ag nanoparticles with an average size of 7–9 nm are obviously anchored onto the rod-like WO3·0.33H2O building block. The detailed microstructures of the rod-like WO3·0.33H2O building block and Ag nanoparticle are further investigated by the high resolution transmission electron microscope (HRTEM), as shown in Fig. 3b. The lattice fringes are marked by white lines and arrows. The corresponding lattice spacing of the rod is about 0.63 nm, which is in good agreement with the d value of the (110) facet of WO3·0.33H2O crystal. The lattice spacing of the Ag nanoparticles is about 0.23 nm, which is corresponding to the (111) facet of Ag crystal. Based on the above information, ultrathin Ag nanoparticles anchored on urchin-like WO3·0.33H2O assemblies is successfully achieved in this work.
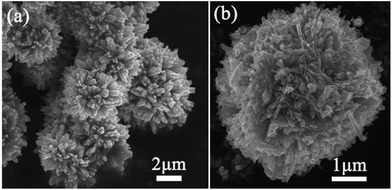 | ||
| Fig. 2 SEM images of the as-synthesized Ag/WO3·0.33H2O. (a) Low-magnification, (b) high-magnification. | ||
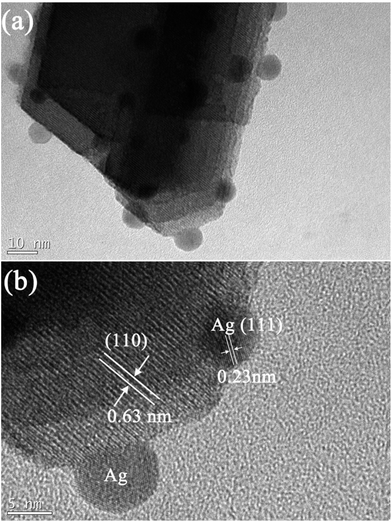 | ||
| Fig. 3 (a) A typical TEM image of a rod-like building block of the urchin-like Ag/WO3·0.33H2O, (b) the corresponding HRTEM image. | ||
The formation of Ag/WO3·0.33H2O composite is based on a self-catalytic reduction route, which might be attributed to the in situ reduction of AgNO3 precursors by the low-valence W5+ site in WO3·0.33H2O. The valence state of tungsten element was measured by X-ray photoelectron spectroscopy (XPS, see ESI, Fig. S1†). The peaks at binding energies of 35.3 eV and 37.4 eV correspond to W4f7/2 and W4f5/2 of W6+, while the peak at binding energy of 36.0 eV corresponds to that of W5+.13 On account of the fact that the redox pair value of W6+/W5+ (+0.26 V, vs. SHE) is much lower than that of a redox Ag+/Ag pair (+0.799 V, vs. SHE), the Ag nanoparticles can be reduced in theory. A two-step formation process is proposed as follows, (i) the formation of urchin-like WO3·0.33H2O precursors by a surfactant-free hydrothermal heating the mixture of tungsten powder/H2O2/NaCl, (ii) the preparation of Ag/WO3·0.33H2O in an aqueous solution at room temperature with a multi-step injection of AgNO3 through an in situ redox reaction between weakly reductive W5+ sites and oxidative AgNO3 in aqueous solution. Once the Ag+ ions contacted with the low-valence W5+ sites, they would be gradually reduced and in situ nucleated on the surface of WO3·0.33H2O, finally leading to the formation of ultrathin Ag nanoparticles by a Ostwald ripening process, which is similar to that occurred in the previous ref. 14 However, in this present work, the morphology and crystalline phase of pristine WO3·0.33H2O precursors can be saved after depositing Ag nanoparticles.
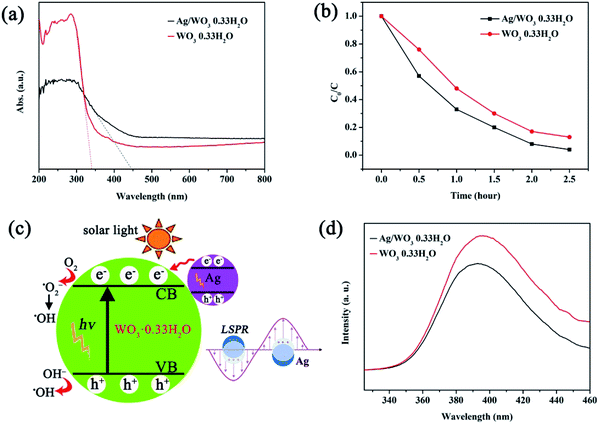
In order to investigate the change of optical property induced by the introduction of Ag nanoparticles, UV-vis diffuse reflectance measurement was carried out. It can be seen that the absorption edge of the as-synthesized Ag/WO3·0.33H2O sample displays an obvious red shift compared to the pristine WO3·0.33H2O (see Fig. 4a), which can be attributed to the localized surface plasmon resonance (LSPR) of Ag nanoparticles.15 It is noted that the increase of light-harvesting is beneficial to enhance the photocatalytic performance.16
The photocatalytic activity of the as-synthesized Ag/WO3·0.33H2O sample was evaluated by measuring the photodegradation of RhB solution under solar light irradiation. For comparison, the photodegradation activity of the pristine WO3·0.33H2O without Ag nanoparticles was also carried out. The characteristic absorption peak at 553 nm of RhB was used to monitor the photocatalytic degradation process. A better photocatalytic activity of Ag/WO3·0.33H2O composite than that of the individual WO3·0.33H2O can be directly seen in the curves shown in Fig. 4b. The red curve (pristine WO3·0.33H2O) indicated that the photodegradation of RhB decreased rapidly with the extension of exposure time, and about 87% of the RhB was degraded after 2.5 hour. However, it can be found that black curve (Ag/WO3·0.33H2O) decreased further compared to the above red curve, displaying a noticeable photocatalytic degradation of RhB, and about 96% of the RhB was degraded after 2.5 hour. Therefore, the decomposition of the RhB aqueous solution after 2.5 hour in the presence of above two samples is ordered as follows: Ag/WO3·0.33H2O (96%) > original WO3·0.33H2O (87%). Fig. S3† shows the recycled experiment of the photodegradation of RhB. It was observed that about 92% of RhB was photodegraded after three successive cycles.
Based on the above photocatalytic results, it is proposed that the as-synthesized Ag/WO3·0.33H2O composite with a better photodegradation activity can be attributed to the introduction of Ag nanoparticles on the original WO3·0.33H2O semiconductor, which results in the increase of light-harvesting (see Fig. 4a) and the generation of a synergistic effect between Ag plasmonic photocatalysis and WO3·0.33H2O semiconductor. The photocatalytic mechanism might be explained as follows. After anchoring Ag nanoparticles onto the urchin-like WO3·0.33H2O, a metal–semiconductor heterojunction would be formed. Since the n type WO3·0.33H2O semiconductor has a higher work function (ΦWO3·0.33H2O = 5.32 eV, see Fig. S4, ESI†) than Ag nanoparticle (ΦAg = 4.25 eV),17 thus the Fermi level of WO3·0.33H2O is lower than that of Ag, and the photoexcited electrons would transfer from Ag to WO3·0.33H2O. This phenomenon is similar to the case occurred in a previous report.17 Under solar light illumination, LSPR-excited electrons would generate and occupy on the surface of Ag owing to their strong LSPR, as demonstrated by Fig. 4a. Besides this LSPR effect, the WO3·0.33H2O semiconductor would also be directly photoexcited under solar light illumination, thus electrons in the valence band (VB) could excited to the CB, with simultaneous formation of holes in the VB. Combination with the injected LSPR electrons from Ag nanoparticles, the photoexcited electrons in the CB would initiate the photocatalytic reaction. As a result, the interfacial junction between Ag and WO3·0.33H2O could obviously facilitate the charge separation in LSPR-excited Ag nanoparticles and form long-life charge carriers.17,18 During the photocatalytic process, these photoexcited electrons could be trapped by the adsorbed O2 and generate the superoxide anion radicals (˙O2−), which were further reduced to ˙OH radicals.19 It has been demonstrated that the ˙OH radical was in favor of oxidizing organic contaminates owing to its high oxidative capacity.19 Furthermore, the photoexcited holes might be captured by OH−, leading to the formation of hydroxyl radical species (˙OH).19 A schematic illustration of the synergistic effect between Ag and WO3·0.33H2O is shown in Fig. 4c. This synergistic effect is proposed to arise from LSPR enhancement on Ag,17 which could produce more photoexcited electrons in the CB of WO3·0.33H2O under solar light irradiation, thus suppressing the recombination of electron–hole pairs. Therefore, the photocatalytic activity of Ag/WO3·0.33H2O would be obviously improved compared to the pristine WO3·0.33H2O.
In principle, an efficient electron–hole separation can strongly suppress the charge recombination, which was confirmed by the photoluminescence (PL) spectrum, because the recombination generally induced luminescence.11,18 As shown in Fig. 4d, the relatively strong photoluminescence is quenched in the Ag/WO3·0.33H2O sample, indicating that the recombination of electron–hole pairs can be more efficiently suppressed, resulting in the enhancement of charge separation. This is agreement with the above photocatalytic result (see Fig. 4b).
In summary, ultrathin Ag nanoparticles anchored on urchin-like WO3·0.33H2O can be successfully achieved by a novel and facile self-catalytic reduction approach. The as-prepared Ag/WO3·0.33H2O composite presents a better photodegradation of RhB than that of the individual WO3·0.33H2O under solar light irradiation, which is attributed to a synergistic effect between Ag plasmonic photocatalysis and WO3·0.33H2O semiconductor. The synergistic effect is thought to arise from LSPR enhancement on Ag, which produces more photoexcited electrons in the CB of WO3·0.33H2O under solar light irradiation, suppressing the recombination of electron–hole pairs. The results provide a convincing evidence for the designate synthesis of metal–semiconductor heterojunction with improved functionality.
Acknowledgements
Haoqi Ren deeply appreciates Prof. Hong Yang offered the chance of summer internship at University of Illinois at Urbana-Champaign, and thanks Prof. Yang and Mr Kai-Chieh Tsao for the useful discussion and suggestions for this work. This work was financially supported by the innovation funds from youth league committee in Fudan University, and the National Science Foundation of China (NSFC No. 51471132).Notes and references
- M. D'Arienzo, L. Armelao, C. M. Mari, S. Polizzi, R. Ruffo, R. Scotti and F. Morazzoni, J. Am. Chem. Soc., 2011, 133, 5296–5304 CrossRef PubMed
.
- X. He, C. Hu, Q. Yi, X. Wang, H. Hua and X. Li, Catal. Lett., 2012, 142, 637–645 CrossRef CAS
.
- J. C. Shi, G. J. Hu, R. Cong, H. J. Bu and N. Dai, New J. Chem., 2013, 37, 1538–1544 RSC
.
- X. Y. He, C. G. Hu, Q. N. Yi, X. Wang, H. Hua and X. Y. Li, Catal. Lett., 2012, 142, 637–645 CrossRef CAS
.
- B. X. Liu, J. S. Wang, J. S. Wu, H. Y. Li, H. Wang, Z. F. Li, M. L. Zhou and T. Y. Zuo, Mater. Lett., 2013, 91, 334–337 CrossRef CAS
.
- X. Q. Gao, C. Yang, F. Xiao, Y. Zhu, J. D. Wang and X. T. Su, Mater. Lett., 2012, 84, 151–153 CrossRef CAS
.
- J. Y. Li, J. F. Huang, J. P. Wu, L. Y. Cao, Q. J. Li and K. Yanagisawa, CrystEngComm, 2013, 15, 7904–7913 RSC
.
- Y. Zheng, G. Chen, Y. G. Yu, J. X. Sun, Y. S. Zhou and J. Pei, CrystEngComm, 2014, 16, 6107–6113 RSC
.
- M. S. Bazarjani, M. Hojamberdiev, K. Morita, G. Zhu, G. Cherkashinin, C. Fasel, T. Herrmann, H. Breitzke, A. Gurlo and R. Riedel, J. Am. Chem. Soc., 2013, 135, 4467–4475 CrossRef PubMed
.
- F. Wang, C. D. Valentin and G. Pacchioni, J. Phys. Chem. C, 2012, 116, 10672–10679 CAS
.
- Y. Zheng, G. Chen, Y. G. Yu, Y. Zhou and F. He, Appl. Surf. Sci., 2016, 362, 182–190 CrossRef CAS
.
- X. Y. He, C. G. Hu, Y. Xi, K. Y. Zhang and H. Hua, Mater. Res. Bull., 2014, 5, 91–94 CrossRef
.
- C. Lian, X. L. Xiao, Z. Chen, Y. X. Liu, E. Y. Zhao, D. S. Wang, C. Chen and Y. D. Li, Nano Res., 2016, 9, 435–441 CrossRef CAS
.
- G. C. Xi, J. H. Ye, Q. Ma, N. Su, H. Bai and C. Wang, J. Am. Chem. Soc., 2012, 134, 6508–6511 CrossRef CAS PubMed
.
- S. K. Cushing, J. T. Li, F. K. Meng, T. R. Senty, S. Suri, M. J. Zhi, M. Li, A. D. Bristow and N. Q. Wu, J. Am. Chem. Soc., 2012, 134, 15033–15041 CrossRef CAS PubMed
.
- X. M. Zhang, Y. L. Chen, R. S. Liu and D. P. Tsai, Rep. Prog. Phys., 2013, 76, 046401 CrossRef PubMed
.
- J. Jiang, H. Li and L. Z. Zhang, Chem.–Eur. J., 2012, 18, 6360–6369 CrossRef CAS PubMed
.
- S. Bai, J. Jiang, Q. Zhang and Y. J. Xiong, Chem. Soc. Rev., 2015, 44, 2893–2939 RSC
.
- Q. Xiang, G. F. Meng, H. B. Zhao, Y. Zhang, H. Li, W. J. Ma and J. Q. Xu, J. Phys. Chem. C, 2010, 114, 2049–2055 CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, TGA analysis, XPS spectrum as well as ultraviolet photoemission spectrum of the urchin-like WO3·0.33H2O, and recycled photodegradation performance of the Ag/WO3·0.33H2O composites. See DOI: 10.1039/c6ra28015c |
| This journal is © The Royal Society of Chemistry 2017 |