Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Managing nucleophilic addition reactions to tune the physical properties of 2-substituted pentamethylBODIPY derivatives†
S. R. Sritharana,
B. A. Husseina,
D. D. Machina,
M. A. El-Aooitia,
J. A. Adjeia,
J. K. Singha,
J. T. H. Paua,
J. S. Dhindsaa,
A. J. Loughb and
B. D. Koivisto *a
*a
aDepartment of Chemistry and Biology, Ryerson University, 350 Victoria St. Toronto ON, M5B2K3, Canada. E-mail: bryan.koivisto@ryerson.ca
bDepartment of Chemistry, University of Toronto, Toronto ON, M5S 3H6, Canada
First published on 27th January 2017
Abstract
The synthesis and characterization of a family of 2-substituted BODIPY dyes are reported herein. The dyes have been prepared using nucleophilic addition reactions (Wittig reactions, imine, Knoevenagel & aldol condensations) which are typically more challenging for BODIPY ring systems. Structure–property relationships have also been delineated by evaluating the physicochemical properties including; absorption & emission spectroscopy, electrochemical studies (CV, DPV) and theoretical calculations (TD-DFT).
Introduction
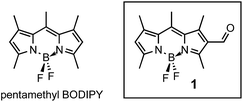
BODIPY derivatives have received considerable attention in recent literature owing to their unique and tunable optical properties poised for a variety of applications.1–3 BODIPYs, with their demiporphyrin-like structure, have been explored as biological probes,4,5 photovoltaic materials,6–8 photosynthetic mimics,9 laser dyes10 and as promising candidates for photodynamic therapy.11 As such the ability to tune the optical properties (both absorbance and fluorescence) of these dyes is particularly significant depending on the nature of the desired application. Despite BODIPYs being stable in a wide range of environments (including physiological conditions), their ability to withstand classical chemical transformations is not trivial owing to their; (1) low energy LUMO orbitals which makes the π-system of the dipyrrin core susceptible to nucleophilic attack, and (2) the hard acidic nature of the boron centre can readily react with hard bases like hydroxide, and alkoxides. Combined, these behaviours can lead to decomposition and less desirable byproducts.Owing to intense and distinct π–π* optical transitions another significant challenge with BODIPYs is broadening and red-shifting the absorption profile for the desired application. Among others, Ziessel and co-workers have exploited the 3,5 positions of BODIPY to successfully red-shift the absorption profile.12 While these type of symmetric modifications are suitable for applications involving absorption/fluorescence, they do not permit effective energy extraction because these dyes lack a suitable symmetry-breaking condition required for photo-induced charge redistribution. As such, we sought to develop a suitable and general process where one could advantageously use nucleophilic substitution reactions to electronically desymmetrize the BODIPY core for any desired application. To this end, we prepared derivatives based on the less studied pentamethylBODIPY (Fig. 1); these methylated derivatives are more stable to nucleophilic attack then unsubstituted BODIPYs and have a smaller hydrodynamic volume than BODIPYs substituted at the meso-position (a desired feature when considering biological applications). Furthermore, we hypothesized that having access to the readily modifiable 2,6-positions could permit greater charge separation in the excited state and allow us to effectively control the absorption spectrum of these dyes. Herein, we describe a general protocol for the preparation of a family of asymmetrically substituted BOPDIPYs and the structure–property relationships as a result of their varied derivatization.
Synthesis and characterization
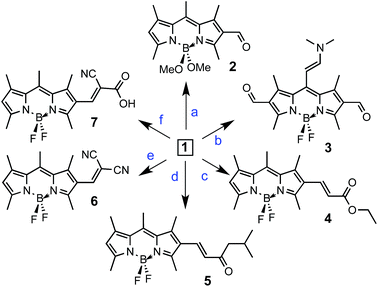
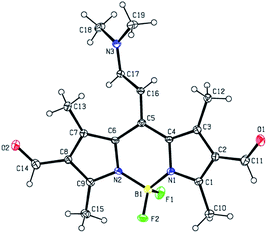
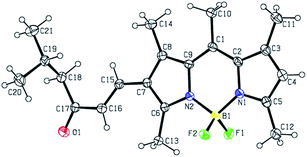
Showcasing the variety of nucleophilic addition reactions possible, a number of derivatives were prepared starting with 1 (Fig. 1). FormylBODIPY, 1 has been a popular building block in our group and its efficient synthesis has been trivialized (see ESI†) and optimized for study in the undergraduate laboratory. Scheme 1 highlights a selection of nucleophilic transformations possible. The first example in this subset, is actually a nucleophilic substitution where the fluorines have been replaced with methoxy moieties to yield derivative 2. The second example is another misfit in the set, where an attempt to further formylate 1 (using Vilsmeier–Haack conditions) actually resulted in a rather peculiar product, 3, that was eventually identified using X-ray crystallography (Fig. 2). Evidence for similar meso condensations have been previously reported, and in that report product formation was surmised to be a strain driven process.13 However, in our case we surmise that the significantly lower pKa (owing to resonance stabilization) of the meso position permitted a Knoevenagel-like condensation with the Vilsmeier reagent (see proposed mechanism in the ESI†).Scheme 1 also depicts the successful nucleophilic addition reactions to install conjugated carbonyl derivatives via Wittig (4), aldol (5) and Knoevenagel condensations14 (6 & 7). The Wittig reaction went smoothly so long as a stabilized phosphonium ylide was prepared and isolated in advance. Product 5 was not planned and remained a puzzling curiosity until it was identified using X-ray crystallography (Fig. 3). An aldol condensation with small (but significant) amounts of acetone led to product 5. Finally, strong electron withdrawing groups can also be installed using Knoevenagel condensations to yield 6 and 7.
Scheme 2 highlights the derivatization of 1, into a variety of Schiff-bases15 (8–15) and hydrazones (16 & 17) using nucleophilic addition reactions.
Within the Schiff-base family of BODIPY dyes, the intent was to explore the effect of adding electron donating or withdrawing groups in order to tune the optical properties of the dye (8–11). Similarly, the bromide (12) was prepared to showcase the possibility of further modification using Pd-catalyzed cross coupling. Imine (13) was prepared to assess the impact of breaking conjugation with the BODIPY core, while 14 & 15 sought to marry the unique properties of ferrocene and azo-compounds with the BODIPY core, respectively. Depending on the electronic nature of the amine, the synthesis required slightly different conditions. In our hands, electron donating amines reacted smoothly, while less electron donating groups required more strenuous conditions in order to shift the equilibrium towards the imine. As a result, these reactions typically resulted in lower yields (owing to BODIPY decomposition) and disappointingly, only a minimal amount of imine 11 was isolated. It should be noted that these Schiff-bases seem to be stable in the solid state, but do hydrolyse easily under weakly acidic conditions in solution.
Results and discussion
The physicochemical properties for compounds 1–17 (in DCM solutions), have be compared and contrasted in Table 1 and Fig. 4–8. BODIPY derivatives typically show rich electrochemical behaviour in addition to their unique optical properties. When comparing the effect of the strong electron-withdrawing groups on the physicochemical properties, malononitrile is expected to be a significantly stronger electron withdrawing group, when compared to enone 4 and aldehyde 1 (Table 1). This is seen in the observation that the oxidation of 6 occurs closer to the solvent decomposition window, while the oxidation potential of 1 rests approximately 80 mV below 6. When comparing the effect of electron withdrawing groups on the optical properties, red-shifting of the absorption profile is observed for both derivatives 4 and 6 by 24 and 14 nm, respectively, compared to the formylBODIPY 1 (Fig. 4). This is not surprising considering the TD-DFT calculations, where the dominant transitions (denoted by the solid arrow) remain the same for enone 4 and malononitrile 6; however, the electron density shifts more towards the BODIPY core when comparing the HOMO−1 to LUMO transition. While the optical properties of formylBODIPY, 1, are different from 4 and 6, the electron withdrawing nature of the enone 4 and malononitrile 6 are only modestly different from each other.| BODIPY dye | E1/2a (V vs. NHE) Eox | λmax emissionb (nm) | UV-Visc λmax nm (ε × 10−4 M−1 cm−1) | Dominant FMO transition from TD-DFT | |
|---|---|---|---|---|---|
| a Data collected using 0.1 M NBu4PF6 DCM solutions at 100 mV s−1 and referenced to a [Fc]/[Fc]+ internal standard followed by conversion to NHE; [Fc]/[Fc+] = +765 mV vs. NHE in DCM.b Emission in DCM solutions corresponding to excitation at absorption maxima.c Low energy visible transitions from UV-Vis in DCM.d No discernible oxidation in DCM.e Insoluble in DCM. | |||||
| 1 | 0.81 | 508 | 497(8.3) | HOMO to LUMO | |
| 2 | 0.68 | — | 494(2.7) | HOMO to LUMO | |
| 3 | 0.85 | — | 475(5.4) | HOMO to LUMO | |
| 4 | 0.79 | 540 | 521(5.5) | HOMO to LUMO | |
| 5 | 0.74 | — | 522(5.7) | HOMO to LUMO | |
| 6 | 0.89 | — | 511(2.3) | HOMO to LUMO | |
| 7 | —d | 534 | 513(3.8) | HOMO to LUMO | |
| 8 | 0.77 | — | 515(5.7) | HOMO−1 to LUMO | |
| 9 | 0.26 | — | 522(5.5) | HOMO−1 to LUMO | |
| 10 | 0.77 | — | 520(6.1) | HOMO−1 to LUMO | |
| 11 | 0.78 | — | 514(0.5) | HOMO to LUMO | |
| 12 | 0.78 | — | 517(0.9) | HOMO to LUMO | |
| 13 | 0.74 | 526 | 511(0.5) | HOMO to LUMO | |
| 14 | 0.00 | 579 | 511(3.5) | HOMO to LUMO | |
| 15 | 0.78 | — | 518(6.6) | HOMO−2 to LUMO | |
| 16 | —e | — | 545(0.6) | HOMO to LUMO−1 | |
| 17 | 1.03 | — | 498(3.4) | HOMO−1 to LUMO | |
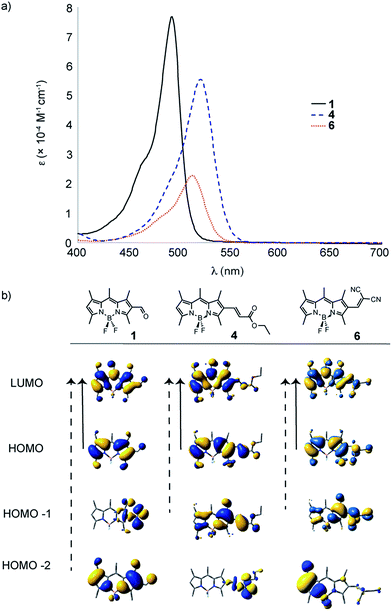 | ||
| Fig. 4 UV-Vis spectra (in DCM) for target molecules 1, 4 and 6; comparing the effect of electronic withdrawing character after nucleophilic addition reactions with formylBODIPY 1. | ||
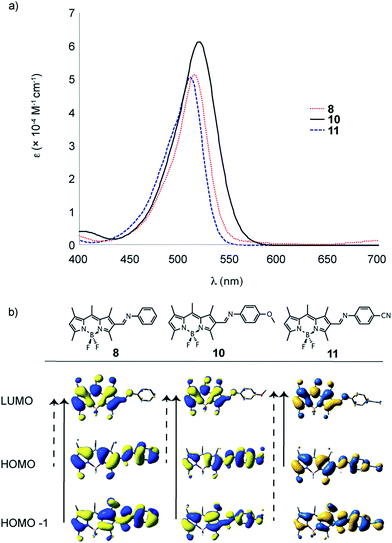 | ||
| Fig. 5 UV-Vis spectra (in DCM) for target molecules 8, 10 and 11; comparing the effect of Schiff-base formation on a family of BODIPY dyes. | ||
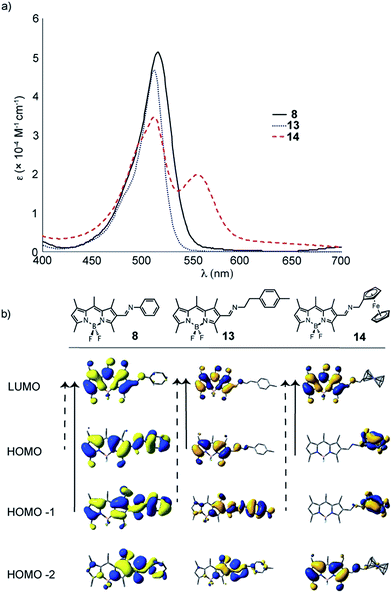 | ||
| Fig. 6 UV-Vis spectra (in DCM) for target molecules 8, 13 and 14; comparing the effect of extended/broken conjugation in Schiff-base – base BODIPY dyes. | ||
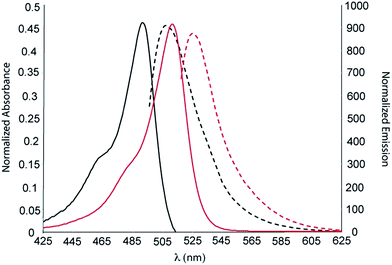 | ||
| Fig. 8 UV-Vis absorption (solid lines) and normalized emission (dotted line) for molecules 1 (black) and 13 (red) (in DCM). | ||
When exploring the effect of varying the electronic nature of the Schiff-base, little change is observed in both the optical and electronic properties when varying the para-substitution. While this para-substituent seems to have a significant effect on the reactivity in making the derivative, comparing the effect of electron donating and withdrawing groups attached to the BODIPY they are nearly at parity when considering oxidation potential. Moreover, only a modest red-shift in the absorption profile is observed for 10 and a slight blue-shift is observed in the absorption profile of 11, consistent with perturbation of the frontier orbitals (Fig. 5). When comparing the TD-DFT calculations, the dominant transitions (denoted by the dashed arrow) differ throughout the series.
When comparing the effect of extended versus broken conjugation in Schiff-base BODIPY derivatives 8 and 13, a modest hypsochromic shift in the absorption profile is observed for 13 (Fig. 6); however, when a redox active substituent (ferrocene, 14) is added a new charge transfer band emerges, despite a lack of conjugation. This is consistent with electrochemistry and DFT showing that the HOMO resides on the ferrocene portion of the molecule. Interestingly, this thermally induced charge transfer band leads to instability in dye 14, and these ferrocene derivatives do not persist for significant periods of time in solution.16–18 This has opened an entire secondary research theme in our group and we are currently examining this phenomenon in further detail (and will be the focus of a future report).
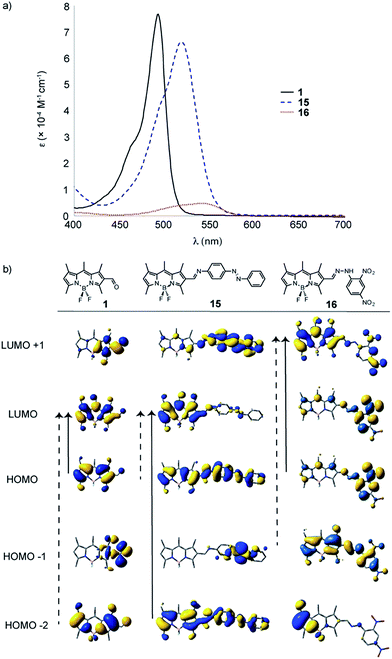
When aldehyde 1 is appended with azo-imine or hydrazone derivatives (15, 16, and 17 respectively, Fig. 7 and S3†), more substantial changes to the absorption properties are observed. When comparing the optical properties of the Schiff-base BODIPY derivatives, red-shifting of the absorption profile is observed for 15 by 21 nm and 16 by 21 and 48 nm, respectively, compared to the formylBODIPY 1 (Fig. 6), but poor solubility of 16 in DCM (or anything!) leads to anomalous extinction coefficients. It should be noted that the hydrazones have broad absorptions (tailing out beyond 600 nm). Based on the TD-DFT calculations, and owing to the structural diversity, the dominant transitions (denoted by the dashed arrow) differ throughout the series. When comparing the electrochemical properties, oxidation potentials are also varied and a second oxidation corresponding to the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond is observed for 15 (not listed in table). As a side note, the hydrazones seem to be more stable to hydrolysis than their imine counterparts, which may suggest this is a more desirable route to alter the physical properties of BODIPY.
N bond is observed for 15 (not listed in table). As a side note, the hydrazones seem to be more stable to hydrolysis than their imine counterparts, which may suggest this is a more desirable route to alter the physical properties of BODIPY.
Finally, most of the derivatives prepared in this paper are not fluorescent; however, Fig. 8 (& Table 1) highlight some of the emissive differences in the family. Generally, the conjugated imines and hydrazones are not fluorescent, and when comparing the fluorescence of 1 & 13 (Fig. 8), there is little difference in the Stokes shift (14 & 15 nm, respectively).
Conclusions
The successful preparation of these unique, unsymmetrically-substituted derivatives suggests that despite the BODIPYs intolerance to many nucleophilic addition reactions, this current set of reactions is tolerated (although some unpredicted products do form). It also appears that functionalizing the β-position is an effective way to alter the physicochemical properties (and stability) of this family of dyes, with the following caveats; (a) conjugated Schiff-base modification has little effect on the physical properties, and (b) fluorescence is quenched in this Schiff-base family of molecules. The non-conjugated Schiff-base ferrocene derivative is an intriguing anomaly in this family as it readily decomposes, likely owing to the reduction of the BODIPY17 and the formation of radical intermediates, although this will be elaborated on in greater detail in a forthcoming report.Acknowledgements
BDK would like to acknowledge NSERC and Ryerson University for financial support. In addition, BDK would like to thank former students of CHY 307/399 for their contribution in preparing building blocks necessary for this work.Notes and references
- J. Bañuelos, Chem. Rec., 2016, 16, 335–348 CrossRef PubMed.
- A. Bessette and G. S. Hanan, Chem. Soc. Rev., 2014, 43, 3342–3405 RSC.
- A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS PubMed.
- M. J. Ouairy, M. J. Ferraz, R. G. Boot and M. P. Baggelaar, Chem. Commun., 2015, 51, 6161–6163 RSC.
- D. P. Murale, S. C. Hong, J. Yun, C. N. Yoon and J.-s. Lee, Chem. Commun., 2015, 51, 6643–6646 RSC.
- M. Mao and Q.-H. Song, Chem. Rec., 2016, 16, 719–733 CrossRef CAS PubMed.
- M. Mao, X. L. Zhang, X. Q. Fang, G. H. Wu, Y. Ding, X. L. Liu, S. Y. Dai and Q. H. Song, Org. Electron., 2014, 15, 2079–2090 CrossRef CAS.
- C. Bonnier, D. D. Machin, O. Abdi and B. D. Koivisto, Org. Biomol. Chem., 2013, 11, 3756–3760 CAS.
- M. Yousaf, A. J. Lough, E. Schott and B. D. Koivisto, RSC Adv., 2015, 5, 57490–57492 RSC.
- Y. Ni and J. Wu, Org. Biomol. Chem., 2014, 12, 3774–3791 CAS.
- A. Kamkaew, S. H. Lim, H. B. Lee, L. V. Kiew, L. Y. Chung and K. Burgess, Chem. Soc. Rev., 2013, 42, 77–88 RSC.
- G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184–1201 CrossRef CAS PubMed.
- N. Shivran, S. Mula, T. K. Ghanty and S. Chattopadhyay, Org. Lett., 2011, 13, 5870–5873 CrossRef CAS PubMed.
- S. Zhu, J. Zhang, G. Vegesna, A. Tiwari, F.-T. Luo, M. Zeller, R. Luck, H. Li, S. Green and H. Liu, RSC Adv., 2012, 2, 404–407 RSC.
- Y. Jia and J. Li, Chem. Rev., 2015, 115, 1597–1621 CrossRef CAS PubMed.
- Y. V. Zatsikha, E. Maligaspe, A. A. Purchel, N. O. Didukh, Y. F. Wang, Y. P. Kovtun, D. A. Blank and V. N. Nemykin, Inorg. Chem., 2015, 54, 7915–7928 CrossRef CAS PubMed.
- B. Verbelen, S. Boodts, J. Hofkens, N. l. Boens and W. Dehaen, Angew. Chem., Int. Ed., 2015, 54, 4612–4616 CrossRef CAS PubMed.
- S. M. Chen, W. Chen, W. Shi and H. M. Ma, Chem.–Eur. J., 2012, 18, 925–930 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1522318 and 1522319. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6ra28114a |
| This journal is © The Royal Society of Chemistry 2017 |