Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
CO2/N2 triggered switchable Pickering emulsions stabilized by alumina nanoparticles in combination with a conventional anionic surfactant†
Maodong Xuab,
Wanqing Zhanga,
Xiaomei Peia,
Jianzhong Jiang a,
Zhenggang Cui
a,
Zhenggang Cui *a and
Bernard P. Binks
*a and
Bernard P. Binks *c
*c
aThe Key Laboratory of Synthetic and Biological Colloids, Ministry of Education, School of Chemical and Material Engineering, Jiangnan University, 1800 Lihu Road, Wuxi, Jiangsu 214122, P. R. China. E-mail: cuizhenggang@hotmail.com
bSchool of Biological and Chemical Engineering, Anhui Polytechnic University, Wuhu 241000, P. R. China
cSchool of Mathematics and Physical Sciences, University of Hull, Hull, HU6 7RX, UK. E-mail: b.p.binks@hull.ac.uk
First published on 7th June 2017
Abstract
Stable n-decane-in-water Pickering emulsions were prepared using positively charged alumina nanoparticles in combination with a trace amount of the anionic surfactant sodium dodecyl sulfate (SDS) as a stabilizer. Particles were hydrophobized in situ by adsorption of surfactant enhancing their surface activity. Emulsions can be readily demulsified by addition of an equal amount of a switchable surfactant, N′-dodecyl-N,N-dimethylacetamidine (DDAA), which can be transformed between a surface-active amidinium/cationic form and a surface-inactive amidine/neutral form by bubbling CO2 or N2, respectively. Following addition of cationic DDAA which prefers to form ion pairs with SDS, desorption of SDS from particle surfaces occurs and alumina particles are rendered hydrophilic resulting in demulsification of the emulsion. However, by bubbling N2 into the demulsified mixture, DDAA molecules are converted to the amidine/neutral form leading to collapse of the ion pairs and re-establishment of the in situ hydrophobization of particles. Stable Pickering emulsions can be prepared again following homogenization. This simple demulsification/re-stabilization cycle can be repeated several times. Experimental evidence including measurement of the adsorption isotherm, zeta potentials, extent of particle adsorption at droplet interfaces in emulsions and microscopy is given to support the postulated mechanisms.
1. Introduction
It is well known that Pickering emulsions stabilized by surface-active colloid particles have usually very good long term stability,1–3 in contrast to conventional emulsions stabilized by surfactants or polymers which are kinetically stable.4 On the other hand, however, demulsification of Pickering emulsions is relatively difficult due to the high desorption energy of particles from the oil–water interface,1,2 which is a challenge in cases where only temporary stability is required such as in emulsion polymerization, oil transport and fossil fuel production.5,6 Switchable or stimuli-responsive Pickering emulsions which can be transformed between stable and unstable by some simple triggers are therefore of interest and have received recent attention.6It is obvious that the formation of switchable or stimuli-responsive emulsions relies on developments in the corresponding particulate stabilizers, which can be transformed between surface-active and surface-inactive via appropriate triggers.5,6 For this purpose particles have to be coated or grafted with functional groups which can be transformed between hydrophilic and lipophilic in response to appropriate triggers.6 Currently several triggers have been developed, including light irradiation,7–10 CO2/N2,11–14 temperature,15–17 pH,18–24 redox,25,26 magnetic field,27,28 and dual triggers like pH–temperature,29,30 light–temperature31 and magnetic field intensity–temperature.32
Among these triggers, the pH and redox triggers require addition of chemicals which accumulate in the system and may produce unexpected effects on products especially in systems undergoing multiple cycles.6 These triggers however are convenient to achieve with a variety of suitable chemicals. Light irradiation is environmentally benign, but the efficiency of demulsification may be significantly inhibited by the turbidity or opacity of the emulsions.6 The temperature trigger which avoids addition of chemicals is a good choice in cases where the products are thermo-resistant and the energy consumption is not a problem. The CO2/N2 trigger originally developed for surfactants33–35 is more attractive and has been extended to colloidal particles5,11–14 since both CO2 and N2 are environmentally benign and there is no accumulation in systems after use. Rigorous switching conditions however are sometimes required such as very low or high temperature.5 Tang et al.6 have recently reviewed the different triggers applicable to Pickering emulsions including their advantages and disadvantages. Nevertheless the design and synthesis of these functional particles are in general complicated.
It has been reported that commercial inorganic nanoparticles which are originally surface-inactive can be made surface-active by in situ hydrophobization.36–41 A typical example is silica nanoparticles which are negatively charged in neutral water and can be hydrophobized in situ by a trace amount of cationic surfactant.36,38,39 The positively charged surfactant ions adsorb at the particle–water interface via electrostatic interaction with head-on configuration, forming a hydrophobic monolayer on particle surfaces increasing the particle hydrophobicity and thereby endowing particles with surface activity.39 The coated particles can then assemble at either oil–water or air–water interfaces to stabilize Pickering emulsions and foams respectively.36–41 Moreover the wettability or surface activity of the particles can be controlled by adjusting the length and/or the number of the surfactant alkyl chain(s) as well as the concentration of surfactant enabling stabilization of either oil-in-water (o/w) or water-in-oil Pickering emulsions along with emulsion phase inversion.37,42 In systems where the in situ hydrophobization can be removed via some triggers, switchable or stimuli-responsive surface-active particles can be obtained.5 Using this protocol we have recently prepared switchable Pickering emulsions and foams with the CO2/N2 trigger5,43 using silica nanoparticles in combination with a CO2/N2 switchable surfactant. In addition, stimuli-responsive Pickering emulsions and foams were demonstrated triggered by ion pair formation and pH using silica nanoparticles in combination with conventional cationic/anionic and zwitterionic surfactants.44–47 In all cases the concentration of surfactant required is as low as 10% of the critical micelle concentration (cmc), which is economically beneficial for practical applications.
In contrast to silica nanoparticles, alumina nanoparticles are positively charged in neutral water,48,49 and there is no direct interaction between them and the CO2/N2 switchable surfactants. On the other hand it is possible to hydrophobize in situ these nanoparticles using anionic amphiphiles such as anionic surfactant,48 short chain fatty acids50 and functional polymers51 to endow them with surface activity. In this paper we report on CO2/N2 triggered switchable Pickering emulsions prepared by using positively charged nanoparticles in combination with the anionic surfactant sodium dodecyl sulfate (SDS) and equal moles of the switchable surfactant N′-dodecyl-N,N-dimethylacetamidine (DDAA). In neutral water, alumina nanoparticles are hydrophobized in situ by adsorption of SDS enabling them to adsorb at the oil–water interface and stabilize an o/w emulsion. Once DDAA in amidinium/cationic form is added into the emulsion, it prefers to form ion pairs with SDS via electrostatic attraction.44,45 SDS ions desorb from particle surfaces reversing their hydrophobization resulting in demulsification of the emulsion. However, upon bubbling N2 into the demulsified system, DDAA molecules are converted to the amidine/neutral form. The ion pairs disassemble and the in situ hydrophobization of particles is re-established such that a stable Pickering emulsion is reformed following re-homogenization. Demulsification can be achieved again by bubbling CO2 into the system. This demulsification/re-stabilization cycle can be realized several times without any detrimental effects on the emulsion.
2. Experimental
2.1 Materials
Hydrophilic alumina (Al2O3) nanoparticles with a purity of >99.8 wt% were purchased from Sigma. Their primary particle diameter is 13 nm with a surface area of 85–115 m2 g−1 according to the supplier. Electron microscopy images of the particles are shown in Fig. S1.† Sodium dodecyl sulfate (SDS) with a purity >99% was purchased from Sigma. N′-Dodecyl-N,N-dimethylacetamidine (DDAA) was synthesized in-house and was purified by passing through a column filled with silica resulting in a purity >98%.5 It can be converted to N′-dodecyl-N,N-dimethylacetamidinium bicarbonate by bubbling CO2 in pure water at low temperature 0–5 °C.5 n-Decane and n-hexane with a purity of >98% were purchased from Shanghai Aladdin Bio-Chem Technology Co. Ltd., China. Ultrapure water with a resistance of 18.2 MΩ cm and a pH of 6.1 was produced from a Simplicity Pure Water System (Merck Millipore, Shanghai). All other chemicals used were analytically pure and were purchased from Sinopharm Chemical Reagent Co. They were used as received unless specified.2.2 Preparation and characterization of emulsions
A known mass of alumina nanoparticles was weighed into a glass bottle of 25 mm (d) × 65 mm (h) of volume 25 mL. 7 mL of either pure water or aqueous SDS solution was added, followed by ultrasonic dispersing at an output of 50 W for 1 min using an ultrasound probe (JYD-650, Shanghai) of tip diameter 0.6 cm. Then 7 mL of oil (n-decane) was added and the mixture was homogenized using an ultraturrax homogenizer (IKA T18 basic, S18N-10G head) at 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 2 min, with the emulsion type being identified by the drop test.39 The concentration of alumina and surfactant is expressed as weight percentage (wt%) and moles per liter (mol L−1) in the aqueous phase respectively. Emulsions were allowed to stand at room temperature to observe their stability by taking photographs of the vessels and micrographs of the droplets using a VHX-1000 microscope system (Keyence Co.). In systems without particles, oil was directly emulsified with surfactant solution as described above.
000 rpm for 2 min, with the emulsion type being identified by the drop test.39 The concentration of alumina and surfactant is expressed as weight percentage (wt%) and moles per liter (mol L−1) in the aqueous phase respectively. Emulsions were allowed to stand at room temperature to observe their stability by taking photographs of the vessels and micrographs of the droplets using a VHX-1000 microscope system (Keyence Co.). In systems without particles, oil was directly emulsified with surfactant solution as described above.
2.3 Demulsification/re-stabilization cycle of the Pickering emulsions
A stable n-decane-in-water (7 mL/7 mL) Pickering emulsion stabilized by 0.5 wt% alumina nanoparticles in combination with 0.3 mM SDS was prepared in the container of a glass bubbling device shown in Fig. S2† as described above. Then DDAA in amidinium/cationic form of equal moles to SDS in the emulsion was added as a concentrated aqueous solution (30 mM) to induce demulsification. The phase separated demulsified system was then bubbled with N2 at 65 °C for 80 min at a flow rate of 160 mL min−1 followed by homogenization to re-form a stable Pickering emulsion. This latter emulsion was subsequently bubbled with CO2 at 0–5 °C for 80 min at a flow rate of 160 mL min−1 to induce demulsification. In this way, the demulsification/re-stabilization cycle of the Pickering emulsion was effected by alternately bubbling with CO2 and N2 followed by homogenization.2.4 Measurements
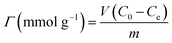 | (1) |
Unless specified otherwise, all experiments were carried out at 20–25 °C.
3. Results and discussion
3.1 Pickering emulsions stabilized by alumina nanoparticles plus SDS
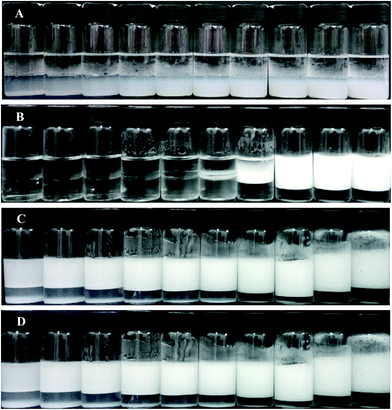
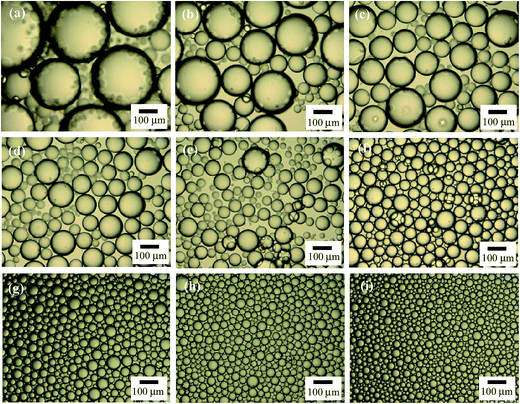
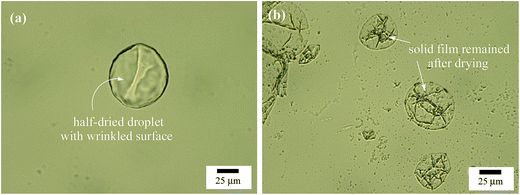
Alumina nanoparticles are positively charged at acidic and neutral pH and negatively charged at alkaline pH. The isoelectric point of the particles was measured to be 10.6, as shown in Fig. S3,† a little higher than that reported in the literature of 9.5.52,53 This means that the particles are positively charged in pure water (pH = 6.1). Although the particles have a primary diameter of 13 nm according to the supplier and the TEM image, they aggregate to some extent to give an average particle diameter of around 190 nm and a polydispersity of 0.12 at concentrations between 0.01 wt% and 0.1 wt% when dispersed ultrasonically in pure water (measured by light scattering, Fig. S4†).In pure water, the alumina nanoparticles are therefore highly hydrophilic and surface-inactive, as evidenced by Fig. 1(A) where the particles alone do not stabilize an emulsion at particle concentrations between 0.1 and 1.0 wt%. Particles prefer to remain dispersed in the aqueous phase and exhibit partial sedimentation. The anionic surfactant SDS alone does not stabilize an n-decane-in-water emulsion either at concentrations below 0.6 mM (ca. 0.1 cmc), as shown in Fig. 1(B). However, when 0.5 wt% alumina nanoparticles co-exist with a trace amount of SDS (<0.6 mM), stable n-decane-in-water emulsions are formed as shown in Fig. 1(C), where creaming occurs soon after preparation but coalescence was not observed 24 h after homogenization. The appearance of the emulsion remained almost unchanged a month later except that some sedimentation of particles in the aqueous phase occurred as shown in Fig. 1(D). The extent of sedimentation decreased with increasing surfactant concentration until none was evidenced above 0.3 mM SDS. The micrographs shown in Fig. 2(a–f) indicate that the droplet size of the emulsions decreases with increasing SDS concentration, but the droplets are in general larger than >50 μm, in contrast to those in emulsions stabilized by SDS alone at high concentration which are <50 μm as shown in Fig. 2(g–i). The larger droplet size of Pickering emulsions compared with conventional emulsions has been observed in systems stabilized by silica nanoparticles in combination with a trace amount of cationic surfactant5,39,44 or zwitterionic surfactant at low pH.47 We thus believe emulsions stabilised by alumina nanoparticles and a trace amount of SDS are also Pickering emulsions. This can be further evidenced by the micrographs of oil droplets shown in Fig. 3, where the n-decane has been replaced by a more volatile oil n-hexane and the emulsion is allowed to evaporate. After some time, a wrinkled skin or particle shell can be seen on the surface of the droplet (a), whereas after further time clear patches of solid film were observed (b). These features are characteristic of Pickering emulsions stabilised by particles alone5,47 and did not appear in emulsions of SDS alone.
3.2 Demulsification/re-stabilization of Pickering emulsions
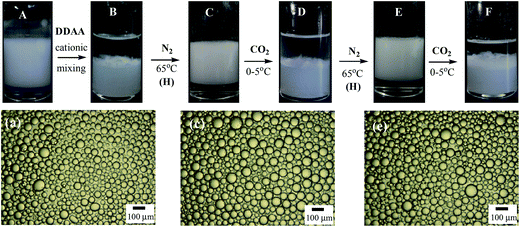
The Pickering emulsions have very good long term stability to coalescence, which however poses a difficulty for demulsification. In cases where the particulate emulsifiers are inorganic nanoparticles hydrophobized in situ by suitable amphiphiles, demulsification can be achieved easily by removing the in situ hydrophobization. For example, the hydrophobization of silica nanoparticles by cationic surfactants can be removed by either switching off the cationic surfactant to its uncharged form5,43 or by addition of an oppositely charged anionic surfactant which prefers to form ion pairs with it resulting in desorption of the cationic surfactant from particle surfaces.44,45 For the Pickering emulsion stabilized by alumina nanoparticles hydrophobized in situ by SDS, addition of a cationic surfactant should be effective but in order to re-stabilize the emulsion additional SDS should be added. However, the accumulation of both surfactants in the system may finally negate the stimuli-responsiveness of the system in multiple cycles. Here we use a switchable surfactant DDAA, which can be transformed between amidinium/cationic and amidine/neutral forms by bubbling CO2 and N2 into water5 as shown in eqn (2) to introduce the CO2/N2 trigger to the system. An otherwise stable Pickering emulsion containing 0.5 wt% alumina nanoparticles and 0.3 mM SDS was initially prepared in the container of a glass bubbling device shown in Fig. S2.† This is composed of a column container and a bubbling tube together with an air distributor where the upper side of the bubbling tube can be connected to a condenser to reduce the evaporation loss of liquid during bubbling at elevated temperature. Then DDAA in amidinium/cationic form of equal moles to the SDS in the emulsion was added into the stable Pickering emulsion as a concentrated aqueous solution (30 mM) and demulsification ensued. The demulsified mixture was then bubbled with N2 at 65 °C and re-homogenised followed by bubbling the emulsion with CO2 at 0–5 °C alternately to trigger multiple demulsification/re-stabilization events, as shown in Fig. 4.
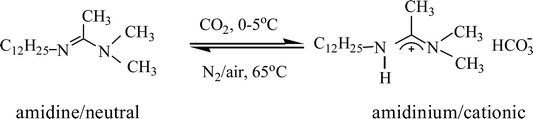 | (2) |
It is seen from Fig. 4 that the initial stable Pickering emulsion (A) has an average droplet diameter of ca. 50 μm as shown in micrograph (a), and demulsification occurs when DDAA in the amidinium/cationic form is added to the emulsion with gentle mixing (B). The re-stabilization of the emulsion can be achieved by bubbling N2 into the system for 80 min at 65 °C followed by homogenization (C). The droplet size of the re-homogenized emulsion (c) is only slightly larger than that of the original emulsion. Demulsification of this stable Pickering emulsion can be achieved by bubbling CO2 into the emulsion for 80 min at 0–5 °C (D) where a small volume of oil and water (0.7 mL each) is added to compensate the slight evaporation during bubbling at elevated temperature. The re-stabilization/demulsification steps can be cycled by bubbling N2 and CO2 alternately (E and F) and a similar droplet size can be reached for the stable emulsion (e). Although in principle the demulsification/emulsification transformation can be cycled many times, it is found that the demulsification efficiency is reduced with increasing cycle number. This is because the switching on of DDAA surfactant is not complete by bubbling CO2 (ref. 5) and hydrolysis of DDAA is possible at elevated temperature. The extent of hydrolysis of 10.1% during a demulsification/emulsification cycle was roughly estimated by measuring the concentration of DDAA remaining in aqueous solution after bubbling N2 at 65 °C for 3 h followed by bubbling CO2 at 0–5 °C for another 3 h for a DDAA solution at an initial concentration of 13.5 mM using HPLC-MS. The cationic amidinium available in the emulsion thus decreases gradually with time resulting in incomplete demulsification. This problem can be solved however by addition of more DDAA into the system since DDAA in either form does not interact with alumina particles and therefore demulsification/emulsification is not affected. In this way switchable Pickering emulsions triggered by CO2/N2 are obtained.
3.3 Probing into the mechanism
As previously reported5,36–47 charged inorganic nanoparticles can be hydrophobized in situ by adsorption of oppositely charged surfactant via electrostatic interaction in water. The alumina nanoparticles are positively charged as evidenced by the zeta potential of +40.8 mV when dispersed in pure water (pH of dispersion = 5.85) as shown in Fig. S3,† and can therefore be hydrophobized by adsorption of anionic surfactant SDS. In fact the hydrophobization of alumina particles by negatively charged short amphiphiles50 and functional polymer51 has been reported.The adsorption of SDS at the alumina particle–water interface can be assessed by measuring the adsorption isotherm of the surfactant as well as the zeta potential of the particles dispersed in aqueous SDS solutions. Fig. 5 shows the adsorption isotherm at 25 °C obtained via surface tension measurements shown in Fig. 6. The surface tension of the solutions increases on addition of alumina particles, indicating a decrease of the surfactant concentration in the aqueous phase due to adsorption by the particles. It was found that the surface tension (γ) of aqueous SDS solutions without particles as a function of SDS concentration (C) can be well fitted by the Szyszkowski equation, γo − γ = 2RTΓ∞![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(1 + KC) with Γ∞ = 3.27 × 10−10 mol cm−2 and K = 1.33 × 103 L mol−1, where γo is the surface tension of pure water, R is the gas constant, T is the absolute temperature, K is a constant reflecting adsorption free energy and Γ∞ is the saturated adsorbed amount. The equilibrium concentration (Ce = C here) of SDS in the dispersion can then be calculated directly based on the measured surface tension of the dispersion. It is found that the adsorption of SDS on particle surfaces increases with an increase in the equilibrium surfactant concentration. At low concentration (ca. below 0.5 mM) monolayer adsorption occurs via electrostatic interaction between the negative charge of SDS and positive charge on particle surfaces rendering particles partially hydrophobic. For example, at an initial concentration of 0.3 mM (equilibrium concentration = 0.028 mM where SDS alone cannot stabilize an emulsion), the adsorbed amount of SDS is around 0.054 mmol g−1 equivalent to a cross-sectional area per SDS molecule of 3.08 nm2, which is much higher than that of SDS at the air–water interface (0.52 nm2 per molec.)4 at saturated adsorption, suggesting monolayer adsorption. Upon increasing further the SDS concentration, double layer or hemi-micelle adsorption occurs via chain–chain interactions between SDS molecules rendering particles hydrophilic again. As an example, at an equilibrium concentration of 5.4 mM (close to cmc = 6.2 mM, Fig. 6), the adsorption reaches 0.921 mmol g−1, corresponding to a cross-sectional area of 0.18 nm2 per molec. (≪ 0.52 nm2 per molec.).
ln(1 + KC) with Γ∞ = 3.27 × 10−10 mol cm−2 and K = 1.33 × 103 L mol−1, where γo is the surface tension of pure water, R is the gas constant, T is the absolute temperature, K is a constant reflecting adsorption free energy and Γ∞ is the saturated adsorbed amount. The equilibrium concentration (Ce = C here) of SDS in the dispersion can then be calculated directly based on the measured surface tension of the dispersion. It is found that the adsorption of SDS on particle surfaces increases with an increase in the equilibrium surfactant concentration. At low concentration (ca. below 0.5 mM) monolayer adsorption occurs via electrostatic interaction between the negative charge of SDS and positive charge on particle surfaces rendering particles partially hydrophobic. For example, at an initial concentration of 0.3 mM (equilibrium concentration = 0.028 mM where SDS alone cannot stabilize an emulsion), the adsorbed amount of SDS is around 0.054 mmol g−1 equivalent to a cross-sectional area per SDS molecule of 3.08 nm2, which is much higher than that of SDS at the air–water interface (0.52 nm2 per molec.)4 at saturated adsorption, suggesting monolayer adsorption. Upon increasing further the SDS concentration, double layer or hemi-micelle adsorption occurs via chain–chain interactions between SDS molecules rendering particles hydrophilic again. As an example, at an equilibrium concentration of 5.4 mM (close to cmc = 6.2 mM, Fig. 6), the adsorption reaches 0.921 mmol g−1, corresponding to a cross-sectional area of 0.18 nm2 per molec. (≪ 0.52 nm2 per molec.).
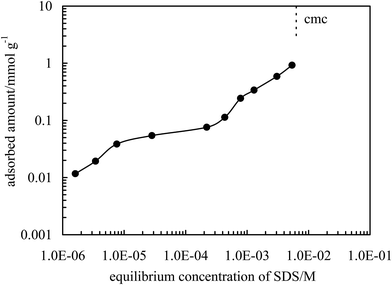 | ||
| Fig. 5 Adsorption isotherm of SDS at the alumina nanoparticle–water interface (pH = 5.8) as a function of the equilibrium concentration of SDS at 25 °C. | ||
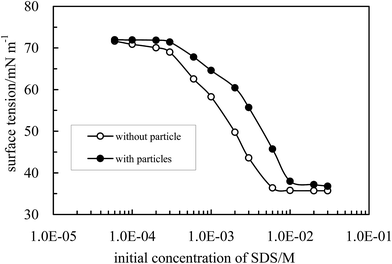 | ||
| Fig. 6 Air–water surface tension of aqueous SDS with and without 0.5 wt% alumina nanoparticles as a function of initial SDS concentration at 25 °C. | ||
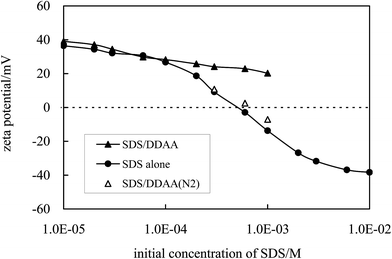
In accordance with the adsorption of SDS on particle surfaces, the zeta potential of alumina particles dispersed in aqueous SDS solutions decreases with increasing SDS concentration, as shown in Fig. 7 (filled circles). The inherent positive charges on particle surfaces are neutralized by the negatively charged surfactant. Particles exhibiting a zeta potential around zero were formed at an initial SDS concentration of ca. 0.5 mM, where the adsorption of SDS reaches 0.07 mmol g−1 corresponding to a cross-sectional area of 2.37 nm2 per molecule (monolayer adsorption). The zero zeta potential is supported by the flocculation of the particles (0.1 wt%) dispersed in 0.3 mM SDS as shown in Fig. S4† where the average particle size increases from ca. 190 nm in pure water to nearly 600 nm and the polydispersity increases from 0.135 to 0.301. At higher SDS concentrations, the zeta potential of the particles becomes negative as a result of double layer or hemi-micelle adsorption of SDS molecules. It is believed the SDS molecules initially adsorb on particle surfaces with a head-on configuration driven by electrostatic attraction. The formation of a monolayer reduces the hydrophilicity of the particles and endows them with sufficient surface activity enabling their adsorption at the oil–water interface in stabilizing emulsions. In the previous section we provided micrographs (Fig. 3) which clearly show the adsorption of the particles at droplet surfaces. In addition we have determined the amount of particles adsorbed at the oil–water interfaces in emulsions by measuring the concentration of particles which are non-adsorbed in the aqueous phase40 as shown in Table 1. It is seen that at an initial SDS concentration of 0.01 mM, only 23% of the particles adsorb to the oil–water interfaces. However, this increases to 88% at 0.1 mM SDS and further increases to 97% at 1 mM SDS. In contrast, when SDS is replaced by DDAA in amidinium/cationic form the percentage of particles adsorbed is very low (<5%) and does not change with surfactant concentration. This is because the cationic surfactant does not induce hydrophobization of the alumina nanoparticles being of the same charge sign.
| Alumina + SDS | Alumina + DDAA | ||||
|---|---|---|---|---|---|
| CSDS/mM | Cp/wt% | % adsorbed | CDDAA/mM | Cp/wt% | % adsorbed |
| 0.01 | 0.385 ± 0.006 | 23.0 ± 0.2 | 0.01 | 0.479 ± 0.001 | 4.2 ± 0.3 |
| 0.1 | 0.060 ± 0.002 | 88.0 ± 3.3 | 0.1 | 0.477 ± 0.003 | 4.6 ± 0.3 |
| 1.0 | 0.016 ± 0.002 | 96.8 ± 12.5 | 1.0 | 0.476 ± 0.001 | 4.8 ± 0.3 |
When DDAA in amidinium/cationic form is added to the emulsion stabilized by alumina nanoparticles hydrophobized in situ by SDS, the cationic surfactant tends to form ion pairs with SDS which are not soluble in water and which precipitate from solution at concentrations above 0.06 mM, as shown in Fig. S5.† Desorption of SDS from particle surfaces occurs and alumina particles are rendered hydrophilic again. Particles return to the aqueous phase from emulsion droplet interfaces followed by droplet coalescence and ensuing demulsification. This is evidenced by the fact that the zeta potential of alumina particles dispersed in aqueous solutions of equimolar mixtures of SDS and DDAA (in amidinium/cationic form) is highly positive (Fig. 7) and no stable Pickering emulsion was produced when n-decane was homogenized with the dispersion, as shown in Fig. 8. When the DDAA surfactant is switched off to the amidine/neutral form by bubbling with N2, the ion pairs collapse and the SDS molecules are released. As evidence, the zeta potential of the particles dispersed in equimolar mixtures of SDS/DDAA (in amidinium/cationic form) decreases to close to the values in dispersions with SDS alone after bubbling with N2, as shown by the open triangles in Fig. 7. The slightly higher zeta potential values than that in systems of SDS alone may be due to incomplete switching off of the DDAA5 so that the concentration of free SDS in the system following bubbling N2 is a little lower than that in a dispersion with SDS alone at specified initial concentration. The in situ hydrophobization of the alumina particles can then take place again and a stable Pickering emulsion is formed after homogenization.
4. Conclusions
We have demonstrated that switchable Pickering n-decane-in-water emulsions can be prepared by using positively charged alumina nanoparticles in combination with a trace amount of a conventional anionic surfactant (SDS) and a CO2/N2 switchable surfactant such as DDAA. When the switchable surfactant is switched off to the amidine/neutral form by bubbling with N2 at 65 °C, the hydrophilic alumina nanoparticles are hydrophobized in situ by adsorption of SDS molecules endowing them with sufficient surface activity to stabilise a Pickering oil-in-water emulsion. On the other hand when the emulsion was bubbled with CO2 at low temperature, DDAA is switched to the amidinium/cationic form and prefers to form ion pairs with SDS molecules. The in situ hydrophobization is removed due to desorption of SDS molecules from particle surfaces resulting in demulsification of the emulsion. The emulsification/demulsification cycle can be repeated several times. This protocol is applicable to other positively charged colloid particles in preparing switchable Pickering emulsions.Acknowledgements
Financial support from the National Natural Science Foundation of China (NSFC 21573096, 21473080) and from the Fundamental Research Funds for the Central Universities (No. JUSRP51405A) is gratefully acknowledged. Financial support from MOE & SAFEA for the 111 Project (B13025) is also gratefully acknowledged.References
- R. Aveyard, B. P. Binks and J. H. Clint, Emulsions stabilized solely by colloidal particles, Adv. Colloid Interface Sci., 2003, 100–102, 503–546 CrossRef CAS.
- Colloidal Particles at Liquid Interfaces, ed. B. P. Binks and T. S. Horozov, Cambridge University Press, Cambridge, 2006 Search PubMed.
- Y. Chevalier and M. A. Bolzinger, Emulsions stabilized with solid nanoparticles: Pickering emulsions, Colloids Surf., A, 2013, 439, 23–34 CrossRef CAS.
- Surfactants and Interfacial Phenomena, ed. M. J. Rosen and J. T. Kunjappu, Wiley, Hoboken, 4th edn, 2012 Search PubMed.
- J.-Z. Jiang, Y. Zhu, Z.-G. Cui and B. P. Binks, Switchable Pickering emulsions stabilized by silica nanoparticles hydrophobized in situ by a switchable surfactant, Angew. Chem., Int. Ed., 2013, 52, 12373–12376 CrossRef CAS PubMed.
- J.-T. Tang, P. J. Quinlan and K. C. Tam, Stimuli-responsive Pickering emulsions: recent advances and potential applications, Soft Matter, 2015, 11, 3512–3529 RSC.
- J. Eastoe, M. Sánchez-Dominguez, P. Wyatta and R. K. Heenan, A photo-responsive organogel, Chem. Commun., 2004, 2608–2609 RSC.
- C. Kördel, C. S. Popeney and R. Haag, Photoresponsive amphiphiles based on azobenzene-dendritic glycerol conjugates show switchable transport behavior, Chem. Commun., 2011, 47, 6584–6586 RSC.
- L. Li, M. Rosenthal, H. Zhang, J. J. Hernandez, M. Drechsler, K. H. Phan, S. Rütten, X. Zhu, D. A. Ivanov and M. Möler, Light-switchable vesicles from liquid-crystalline homopolymer–surfactant complexes, Angew. Chem., Int. Ed., 2012, 51, 11616–11619 CrossRef CAS PubMed.
- T. T. Y. Tan, A. Ahsan, M. R. Reithofer, S. W. Tay, S. Y. Tan, T. S. A. Hor, J. M. Chin, B. K. J. Chew and X. B. Wang, Photoresponsive liquid marbles and dry water, Langmuir, 2014, 30, 3448–3454 CrossRef CAS PubMed.
- C. Liang, Q.-X. Liu and Z.-H. Xu, Surfactant-free switchable emulsions using CO2-responsive particles, ACS Appl. Mater. Interfaces, 2014, 6, 6898–6904 CAS.
- P.-W. Liu, W.-Q. Lu, W.-J. Wang, B.-G. Li and S.-P. Zhu, Highly CO2/N2-switchable zwitterionic surfactant for Pickering emulsions at ambient temperature, Langmuir, 2014, 30, 10248–10255 CrossRef CAS PubMed.
- Q. Zhang, G.-Q. Yu, W.-J. Wang, H.-M. Yuan, B.-G. Li and S.-P. Zhu, Preparation of N2/CO2 triggered reversibly coagulatable and redispersible latexes by emulsion polymerization of styrene with a reactive switchable surfactant, Langmuir, 2012, 28, 5940–5946 CrossRef CAS PubMed.
- J. Pinaud, E. Kowal, M. Cunningham and P. Jessop, 2-(Diethyl)aminoethyl methacrylate as a CO2-switchable comonomer for the preparation of readily coagulated and redispersed polymer latexes, ACS Macro Lett., 2012, 1, 1103–1107 CrossRef CAS.
- B. P. Binks, R. Murakami, S. P. Armes and S. Fujii, Temperature-induced inversion of nanoparticle-stabilized emulsions, Angew. Chem., Int. Ed., 2005, 44, 4795–4798 CrossRef CAS PubMed.
- T. Saigal, H.-C. Dong, K. Matyjaszewski and R. D. Tilton, Pickering emulsions stabilized by nanoparticles with thermally responsive grafted polymer brushes, Langmuir, 2010, 26, 15200–15209 CrossRef CAS PubMed.
- J. O. Zoppe, R. A. Venditti and O. J. Rojas, Pickering emulsions stabilized by cellulose nanocrystals grafted with thermo-responsive polymer brushes, J. Colloid Interface Sci., 2012, 369, 202–209 CrossRef CAS PubMed.
- A. J. Morse, S. P. Armes, K. L. Thompson, D. Dupin, L. A. Fielding, P. Mills and R. Swart, Novel Pickering emulsifiers based on pH-responsive poly[2-(diethylamino)ethyl methacrylate] latexes, Langmuir, 2013, 29, 5466–5475 CrossRef CAS PubMed.
- H. Liu, C.-Y. Wang, S.-W. Zou, Z.-J. Wei and Z. Tong, Simple reversible emulsion system switched by pH on the basis of chitosan without any hydrophobic modification, Langmuir, 2012, 28, 11017–11024 CrossRef CAS PubMed.
- F. Tu and D. Lee, Shape-changing and amphiphilicity-reversing Janus particles with pH-responsive surfactant properties, J. Am. Chem. Soc., 2014, 136, 9999–10006 CrossRef CAS PubMed.
- S. Fujii, M. Okada and T. Furuzone, Hydroxyapatite nanoparticles as stimulus-responsive particulate emulsifiers and building block for porous materials, J. Colloid Interface Sci., 2007, 315, 287–296 CrossRef CAS PubMed.
- H. Yang, T. Zhou and W. Zhang, A strategy for separating and recycling solid catalysts based on the pH-triggered Pickering-emulsion inversion, Angew. Chem., Int. Ed., 2013, 52, 7455–7459 CrossRef CAS PubMed.
- J. I. Amalvay, G. F. Unali, Y. Li, S. Granger-Bevan, S. P. Armes, B. P. Binks, J. A. Rodrigues and C. P. Whitby, Synthesis of sterically stabilized polystyrene latex particles using cationic block copolymers and macromonomers and their application as stimulus-responsive particulate emulsifiers for oil-in-water emulsions, Langmuir, 2004, 20, 4345–4354 CrossRef.
- S. Fujii, Y. Cai, J. V. M. Weaver and S. P. Armes, Syntheses of shell crosslinked micelles using acidic ABC triblock copolymers and their application as pH-responsive particulate emulsifiers, J. Am. Chem. Soc., 2005, 127, 7304–7305 CrossRef CAS PubMed.
- T. Saji, K. Hoshino and S. Aoyagui, Reversible formation and disruption of micelles by control of the redox state of the head group, J. Am. Chem. Soc., 1985, 107, 6865–6868 CrossRef CAS.
- M. Schmittel, M. Lal, K. Graf, G. Jeschke, I. Suske and J. Salbeck, N,N′-Dimethyl-2,3-dialkylpyrazinium salts as redox-switchable surfactants? Redox, spectral, EPR and surfactant properties, Chem. Commun., 2005, 5650–5652 RSC.
- S. Lam, E. Blanco, S. K. Smoukov, K. P. Velikov and O. D. Velev, Magnetically responsive Pickering foams, J. Am. Chem. Soc., 2011, 133, 13856–13859 CrossRef CAS PubMed.
- E. Blanco, S. Lam, S. K. Smoukov, K. P. Velikov, S. A. Khan and O. D. Velev, Stability and viscoelasticity of magneto-Pickering foams, Langmuir, 2013, 29, 10019–10027 CrossRef CAS PubMed.
- T. Ngai, S. H. Behrens and H. Auweter, Novel emulsions stabilized by pH and temperature sensitive microgels, Chem. Commun., 2005, 331–333 RSC.
- J. Tang, M. F. X. Lee, W. Zhang, B. Zhao, R. M. Berry and K. C. Tam, Dual responsive Pickering emulsion stabilized by poly[2-(dimethylamino)ethyl methacrylate] grafted cellulose nanocrystals, Biomacromolecules, 2014, 15, 3052–3062 CrossRef CAS PubMed.
- A. L. Fameau, S. Lam and O. D. Velev, Multi-stimuli responsive foams combining particles and self-assembling fatty acids, Chem. Sci., 2013, 4, 3874–3881 RSC.
- B. Brugger and W. Richtering, Magnetic, thermosensitive microgels as stimuli-responsive emulsifiers allowing for remote control of separability and stability of oil-in-water emulsions, Adv. Mater., 2007, 19, 2973–2978 CrossRef CAS.
- Y. X. Liu, P. G. Jessop, M. Cunningham, C. A. Eckert and C. L. Liotta, Switchable surfactants, Science, 2006, 313, 958–960 CrossRef CAS PubMed.
- C. Liang, J. R. Harjani, T. Robert, E. Rogel, D. Kuehne, C. Ovalles, V. Sampath and P. G. Jessop, Use of CO2-triggered switchable surfactant for the stabilization of oil-in-water emulsions, Energy Fuels, 2012, 26, 488–494 CrossRef CAS.
- M. Mihara, P. Jessop and M. Cunningham, Redispersible polymer colloids using carbon dioxide as an external trigger, Macromolecules, 2011, 44, 3688–3693 CrossRef CAS.
- B. P. Binks, J. A. Rodrigues and W. J. Frith, Synergistic interaction in emulsions stabilized by a mixture of silica nanoparticles and cationic surfactant, Langmuir, 2007, 23, 3626–3636 CrossRef CAS PubMed.
- Z.-G. Cui, K.-Z. Shi, Y.-Z. Cui and B. P. Binks, Double phase inversion of emulsions stabilized by a mixture of CaCO3 nanoparticles and sodium dodecyl sulphate, Colloids Surf., A, 2008, 329, 67–74 CrossRef CAS.
- B. P. Binks and J. A. Rodrigues, Influence of surfactant structure on the double inversion of emulsions in the presence of nanoparticles, Colloids Surf., A, 2009, 345, 195–201 CrossRef CAS.
- Z.-G. Cui, L.-L. Yang, Y.-Z. Cui and B. P. Binks, Effects of surfactant structure on the phase inversion of emulsions stabilized by mixtures of silica nanoparticles and cationic surfactant, Langmuir, 2010, 26, 4717–4724 CrossRef CAS PubMed.
- Z.-G. Cui, Y.-Z. Cui, C.-F. Cui, Z. Chen and B. P. Binks, Aqueous foams stabilized by in situ surface activation of calcium carbonate nanoparticles via adsorption of anionic surfactant, Langmuir, 2010, 26, 12567–12574 CrossRef CAS PubMed.
- Z.-G. Cui, C.-F. Cui, Y. Zhu and B. P. Binks, Multiple phase inversion of emulsions stabilized by in situ surface activation of CaCO3 nanoparticles via adsorption of fatty acids, Langmuir, 2012, 28, 314–320 CrossRef PubMed.
- B. P. Binks and J. A. Rodrigues, Double inversion of emulsions by using nanoparticles and a di-chain surfactant, Angew. Chem., Int. Ed., 2007, 46, 5389–5392 CrossRef CAS PubMed.
- Y. Zhu, J.-Z. Jiang, Z.-G. Cui and B. P. Binks, Responsive aqueous foams stabilized by silica nanoparticles hydrophobized in situ with a switchable surfactant, Soft Matter, 2014, 10, 9739–9745 RSC.
- Y. Zhu, J.-Z. Jiang, K.-H. Liu, Z.-G. Cui and B. P. Binks, Switchable Pickering emulsions stabilized by silica nanoparticles hydrophobized in situ with a conventional cationic surfactant, Langmuir, 2015, 31, 3301–3307 CrossRef CAS PubMed.
- Y. Zhu, X.-M. Pei, J.-Z. Jiang, Z.-G. Cui and B. P. Binks, Responsive aqueous foams stabilized by silica nanoparticles hydrophobised in situ with a conventional surfactant, Langmuir, 2015, 31, 12937–12943 CrossRef CAS PubMed.
- K.-H. Liu, Q. Lin, Z.-G. Cui, X.-M. Pei and J.-Z. Jiang, pH-Responsive Pickering emulsions stabilized by silica nanoparticles in combination with n-dodecyl-β-aminopropionate, Chem. J. Chin. Univ., 2017, 38, 85–93 CAS.
- K.-H. Liu, J.-Z. Jiang, Z.-G. Cui and B. P. Binks, pH-Responsive Pickering emulsions stabilized by silica nanoparticles in combination with a conventional zwitterionic surfactant, Langmuir, 2017, 33, 2296–2305 CrossRef CAS PubMed.
- B. P. Binks and J. A. Rodrigues, Enhanced stabilization of emulsions due to surfactant-induced nanoparticle flocculation, Langmuir, 2007, 23, 7436–7439 CrossRef CAS PubMed.
- M. Schmitt, S. Limage, D. O. Grigoriev, J. Krägel, V. Dutschk, S. Vincent-Bonnieu, R. Miller and M. Antoni, Transition from spherical to irregular dispersed phase in water/oil emulsions, Langmuir, 2014, 30, 4599–4604 CrossRef CAS PubMed.
- I. Akartuna, A. R. Studart, E. Tervoort, U. T. Gonzenbach and L. J. Gauckler, Stabilization of oil-in-water emulsions by colloidal particles modified with short amphiphiles, Langmuir, 2008, 24, 7161–7168 CrossRef CAS PubMed.
- E. Garcia-Tunon, S. Barg, R. Bell, J. V. M. Weaver, C. Walter, L. Goyos and E. Saiz, Designing smart particles for the assembly of complex macroscopic structures, Angew. Chem., Int. Ed., 2013, 52, 7805–7808 CrossRef CAS PubMed.
- I. A. Siddiquey, T. Furusawaa, M. Satoa, N. M. Bahadurc and N. Suzukia, Fabrication of silica coated Al2O3 nanoparticles via a fast and facile route utilizing microwave irradiation, Mater. Chem. Phys., 2011, 130, 583–586 CrossRef CAS.
- C. Wu, L. Wang, D. Harbottle, J. Masliyah and Z. Xu, Studying bubble–particle interactions by zeta potential distribution analysis, J. Colloid Interface Sci., 2015, 449, 399–408 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra03722h |
| This journal is © The Royal Society of Chemistry 2017 |