Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hydrogenation/oxidation induced efficient reversible color switching between methylene blue and leuco-methylene blue†
Ya-Nan Liu,
Xiao Zhou,
Xin Wang,
Kuang Liang,
Zheng-Kun Yang,
Cong-Cong Shen,
M. Imran,
Shafaq Sahar and
An-Wu Xu *
*
Division of Nanomaterials and Chemistry, Hefei National Laboratory for Physical Sciences at Microscale Department, University of Science and Technology of China, Hefei 230026, P. R. China. E-mail: anwuxu@ustc.edu.cn
First published on 9th June 2017
Abstract
In this paper, we present the use of graphitic carbon nitride (g-C3N4) supported palladium nanoparticles (Pd/g-C3N4) for reversible color switching of methylene blue (MB). g-C3N4 with a high polymeric degree could improve the dispersity of Pd nanoparticles, contributing to fast color switching of MB as the agglomeration of metal nanoparticles is significantly prevented. Moreover, strong metal-support interaction (SMSI) between Pd nanoparticles and g-C3N4 support promotes the adsorption and subsequent dissociation of molecular hydrogen and oxygen, thus leading to efficient reversible conversion between MB and leuco-methylene blue (LMB). Our obtained Pd/g-C3N4 nanocatalyst exhibits outstanding hydrogenation activity of blue MB to colorless LMB with a turnover frequency as high as 165 h−1 at room temperature, moreover, colorless LMB can quickly switch back to MB upon exposing the same reaction system to oxygen for oxidation. It is noted that our color switching system exhibits remarkable reversibility and stability without obvious fatigue even after 10 consecutive cycles. This novel redox-driven reversible color switching system could find potential in food packaging, sensing and organic transformations.
1. Introduction
Over the last few decades, materials have become increasingly important in the development of human society. One of the key innovations in this field is the emergence of color switching materials that react upon exposure to certain stimuli. Color-switching materials have attracted a great deal of attention due to their application prospects in rewritable paper, sensors, optical data storage and security feature technologies.1–4 Significant efforts were previously devoted to develop various systems with improved stability to enhance switching rates. Pal et al. have reported reversible color switching of organic redox dyes over various catalysts. In these cases, the color changes are strongly influenced by the pH of the solution or the type of reducing agent contained in the system.5–10 With the continuing search for color switching system, the self-assembled monolayer of azobenzene on Au (111) shows good reversibility for photoinduced cis–trans photoisomerization; polymer coated TiO2 nanocrystals also prove to be an efficient color switching system without relying on external sacrificial electron donors.11,12 However, the chromophores experience competing thermal back relaxation and sometimes other side reactions for reversible color switching, and then hinder the further application of color switching system. Therefore, for a clean and reversible color switching system, molecular hydrogen, oxygen, suitable dyes and efficient catalysts are required.Methylene blue (MB), a positively charged heterocyclic aromatic thiazine dye, has long been used as staining agent in industry.13 In reducing environment, methylene blue can be reduced to colorless leuco-methylene blue (LMB) through hydrogenation reaction, which can switch back to initial blue color by oxidative dehydrogenation upon exposure to oxidizing environment.
Recent years have witnessed a growing interest in palladium based nanomaterials due to their exceptional properties and potential applications in hydrogenation and oxidation reactions.14,15 Meanwhile, it is well established in the literature that the nature of support plays a key role in the catalytic performance of Pd-containing catalyst.16 For a given reaction, the activity, selectivity and stability of the catalyst could be improved by use of an appropriate support. Recently, graphitic carbon nitride (g-C3N4), a metal-free conjugated polymer, has attracted ever-growing attention in recent years.17,18 g-C3N4 may find a much wider range of applications as compared to carbon materials due to the incorporation of nitrogen atoms in the carbon, which can improve its chemical, electrical and functional properties.19 The structure of g-C3N4 is a two-dimensional (2D) framework of tri-s-triazine connected via tertiary amines, which endows its high thermal and chemical stability.20 Based on the specific properties of g-C3N4, our aim is to use g-C3N4 as a support to load noble metal nanoparticles, which not only stabilizes noble metal nanoparticles against aggregation, but also induces new physicochemical properties due to the synergistic effect and strong metal-support interaction (SMSI) between noble metal nanoparticles and g-C3N4, resulting in the enhancement of the performance of nanocomposites.15,21–23 Therefore, Pd supported on g-C3N4 material could be a nice candidate and hold great potential in catalytic applications for hydrogenation and oxidative dehydrogenation reactions in redox dye based color switching system.
In this study, we report the use of highly dispersed palladium nanoparticles supported onto g-C3N4 nanosheets for efficient reversible color switching of methylene blue (MB). The obtained Pd/g-C3N4 catalyst can rapidly bleach the blue color of MB by hydrogenation process with 1 bar hydrogen, while the colorless leuco-methylene blue (LMB) can quickly switch back to original blue color (MB) through oxidative dehydrogenation by 1 bar oxygen. This one-pot hydrogenation/oxidation color switching system can now be enabled efficiently without relying on other complicated stimuli. Finally, based on experimental results, a possible hydrogenation/oxidation reaction mechanism is proposed.
2. Results and discussion
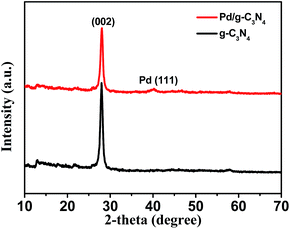
g-C3N4 was synthesized from dicyandiamide by heat treatment, and Pd/g-C3N4 nanocatalyst was prepared by impregnation and H2 reduction (see Experimental). The X-ray diffraction (XRD) patterns of g-C3N4 and Pd/g-C3N4 samples are shown in Fig. 1. For bare g-C3N4, the XRD pattern displays two pronounced diffraction peaks; one peak at 13.10° is associated with the characteristic inter-layer structural packing motif of tri-s-triazine units, and the other peak at 27.39° is ascribed to the interlayer stacking of conjugated aromatic system.24 After Pd nanoparticles (NPs) loading on g-C3N4 nanosheets, a weak peak at 40.16° is observed from XRD pattern, which is readily assigned to the (111) plane of Pd. The morphology and structure of obtained Pd/g-C3N4 were further investigated by transmission electron microscopy (TEM) and high resolution TEM (HRTEM). As shown in Fig. S1,† The as-synthesized g-C3N4 displays free-standing 2D lamellar structure. The HRTEM image furtherreveals the formation of Pd/g-C3N4 nanocatalyst, the ultra small Pd NPs with an average size of ca. 5.07 nm are uniform dispersed on the surface of g-C3N4 nanosheets (Fig. 2a and b). Fig. 2c presents the HRTEM image of a single particle Pd NP. The contrast line profile is plotted under the corresponding image (see Fig. 2d). The average d-spacing distance is measured to be ∼0.23 nm, assigned to the (111) planes of Pd. All these observations demonstrate the formation of Pd NPs on g-C3N4 nanosheets. In addition, the actual Pd weight content of Pd/g-C3N4 sample was determined to be 1.78 wt% by inductively-coupled plasma mass spectrometry (ICP-MS) and is close to the nominal content in the experiment.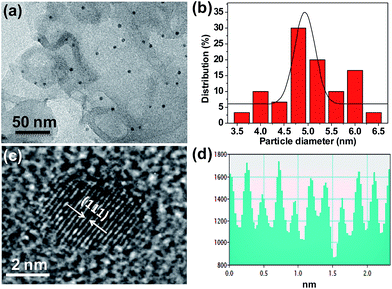 | ||
| Fig. 2 TEM image of Pd/g-C3N4 (a), and the size distribution of Pd NPs (b), HRTEM image (c) and contrast intensity profile indicates the lattice parameter of Pd (d). | ||
To further reveal information about the oxidization states and the compositions of obtained Pd/g-C3N4 sample, X-ray photoelectron spectroscopy (XPS) measurements were performed. The representative XPS survey scan (Fig. 3a) exhibits the existence of C, N and Pd elements. The high resolution C 1s spectrum shows two peaks at binding energy of 284.95 eV and 288.30 eV (Fig. 3b). The weaker one centered at 284.95 eV can be attributed to C–C coordination, while the other peak located at 288.30 eV is assigned to the carbon atom bonded to three nitrogen atoms (C–N–C) in g-C3N4 lattice.25 From N 1s spectrum (Fig. 3c), it can be seen that the broad peak of N 1s is deconvoluted into three Gaussian components having centres at 398.75 eV, 399.40 eV and 401.15 eV. The component at 398.75 eV is due to the C–N–C bonding, while the components at 399.40 eV and 401.15 eV are ascribed to the tertiary nitrogen N–(C)3 and N–H respectively.26,27 These π-bonded planar C–N–C-layers as well as their incompletely condensed amino groups in g-C3N4 are conducive to stabilize highly dispersed Pd NPs and prevent their aggregation and oxidation.15 The 3d XPS spectrum of Pd NPs supported on carbon nitride presents a doublet corresponding to Pd 3d5/2 and Pd 3d3/2 (Fig. 3d). The deconvoluted Gaussian components at 335.75 eV and 341.05 eV are assigned to metallic Pd0, in agreement with XRD result. While the components at binding energy of 337.85 eV and 343.15 eV are attributed to Pd2+.28,29 XPS data reveals that Pd0 (56.67%) is the main species of Pd on the surface of g-C3N4, as clearly observed from TEM images. Notably, the corresponding peaks of Pd0 in Pd/g-C3N4 sample shift to higher-energy side to a certain extent (about 0.9 eV) as compared to bare Pd0.30 This binding energy shift is due to a fact that strong metal support interaction (SMSI) occurs between Pd NPs and g-C3N4 support,31 which is beneficial to the activation of H2 and O2, thus boosts the catalytic performance. The existence of Pd2+ may be attributed to the following reasons: (a) the Pd–N bond formed at the interface of Pd/g-C3N4; (b) the reduction of Pd2+ was not entirely completed; (c) the external metal Pd0 is likely oxidized to Pd2+ at ambient conditions.28,32,33
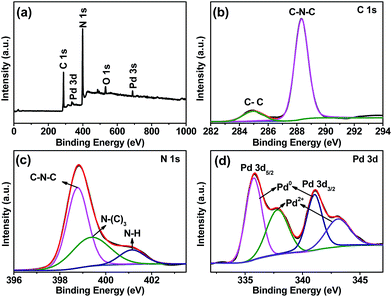 | ||
| Fig. 3 XPS survey spectrum of as-prepared Pd/g-C3N4 (a), and high-resolution XPS spectra of C 1s (b), N 1s (c) and Pd 3d (d). | ||
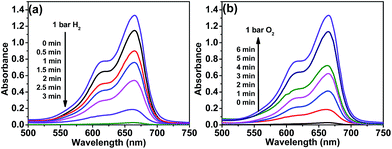
The reversible color switching was conducted in one-pot reaction system, MB was used as a model redox dye to evaluate the catalytic activities of Pd/g-C3N4 catalyst. The hydrogenation of MB was performed over Pd/g-C3N4 catalysts under a hydrogen pressure of 1 bar at room temperature. In the process of hydrogenation, the color of the aqueous MB solution rapidly changed from blue to colorless with increasing the reaction time and completely disappeared after 3 min, corresponding to the reduction of MB to LMB. Fig. 4a shows the time dependent UV/vis spectra of the reaction solution, the absorption peak of MB at 664 nm decreases in intensity within a short period of time in the presence of hydrogen (H2). The outstanding hydrogenation activity of Pd/g-C3N4 is attributed to the synergistic effect of active Pd species and g-C3N4 support. g-C3N4 nanosheets not only stabilize Pd NPs against aggregation, but also enhance the adsorption of π-conjugated MB substrate via the π–π interaction. The hydrogenation of MB over Pd/g-C3N4 hybrid catalyst was assessed by the turnover frequency (TOF, defined as the moles of MB molecules per mole of Pd catalyst per hour), which was calculated to be as high as 165 h−1.
After the completion of hydrogenation reaction, H2 atmosphere was replaced by O2 to evaluate the oxidative dehydrogenation performance of Pd/g-C3N4. It is noted that the LMB is spontaneously and quickly oxidized back to MB in the presence of Pd/g-C3N4 when the system is exposed to oxygen or air. To make the system compatible with the needs in practical applications, hydroxyethyl cellulose (HEC) was added in the reaction system. The HEC can stabilize LMB through hydrogen bonding between the abundant –OH groups on HEC molecules and the –N(CH3)2 groups on LMB (Fig. S2†), and then efficiently slow down the fast recoloration process (oxidative dehydrogenation process) at ambient conditions.1 When the system was exposed to oxygen atmosphere of 1 bar pressure at room temperature, the absorption peak intensity of MB gradually increased and recovered to the original intensity within 6 min (Fig. 4b). The colorless solution (LMB) quickly switched back to original blue color (MB) by activated O2 over Pd/g-C3N4. In the process of oxidative dehydrogenation, highly dispersed Pd NPs on g-C3N4 nanosheets play a crucial role in effectively oxidizing LMB to MB, because the adsorption and dissociation of O2 on the surface of Pd NPs is highly favorable.34
To evaluate the stability of our color switching system for potential application, the hydrogenation and oxidation processes were repeated for 10 consecutive cycles by sequentially exposing the system to hydrogen and oxygen atmosphere. The absorbance of methylene blue (∼664 nm) for 10 repeated hydrogenation/oxidative dehydrogenation cycles was plotted and shown in Fig. 5. The result demonstrates complete reversibility with only a slight decrease in absorption intensity after repeated cycles. Overall, the outstanding hydrogenation/dehydrogenation performance makes the system suitable for potential applications due to its excellent reversibility and low environmental toxicity.
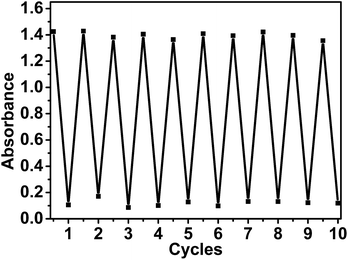 | ||
| Fig. 5 The absorption intensity at about 664 nm of the mixture recorded continuously in the switching cycles between blue color and colorless states. | ||
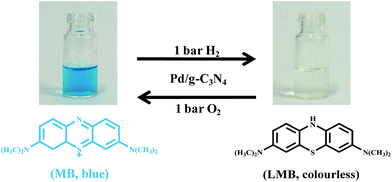
To ensure the possible use of our color switching system in practical applications, a simple lab demonstration was performed (Fig. 6). As the color switching system was exposed to hydrogen atmosphere (1 bar H2), the color of the system changed from blue to colorless corresponding to the hydrogenation of MB to LMB. While the colorless solution was exposed to an oxidative environment (1 bar O2) in the presence of Pd/g-C3N4 catalyst, the blue color gradually appeared and the system completely recovers to its pristine blue color in 6 min, which is much faster than that without Pd/g-C3N4 catalyst (Fig. S3†). The principle behind the application is that the oxidation of LMB is stopped in the absence of oxygen, but it can be rapidly re-oxidized back to its original blue color (MB) in the existence of oxygen and Pd/g-C3N4 catalyst. The inclusion of this color switching system in food packaging could serve as oxygen indicator to ensure the packaging is properly sealed and the quality of food can be guaranteed.35 This color switching system can also be used as security ink for anti-counterfeiting, information coding applications. The information can be written by using aqueous dispersion of Pd/g-C3N4/MB/HEC which will become colorless after hydrogenation process, while the visible images or words can be regenerated from the printed ink upon exposure to oxidative environment for a period of time.
Based on the above results, a possible mechanism of one-pot reversible color switching over Pd/g-C3N4 is proposed (Scheme 1).14,15,36–38 Depending on the nature of the catalyst's active sites, MB molecules can interact with –NH and –NH2 groups on the surface of g-C3N4 through strong S⋯H–N or π⋯π interactions.36,39 The π⋯π interaction between the π electron of graphitic carbon nitride and the aromatic ring of MB, is beneficial for adsorption of MB molecules on the surface of g-C3N4. It is well documented that altering the electronic structure of metal nanocatalyst is an effective strategy to optimize the catalytic performance.40 For Pd/g-C3N4 catalyst, the nitrogen atoms in g-C3N4 could enrich the electron density of the metallic Pd due to the interfacial polarization between Pd and g-C3N4,41 consequently accelerating the reactions of color switching. As shown in Scheme 1, MB can easily adsorbed on the surface of the catalyst via the π⋯π interaction, molecular hydrogen would initially bind to the atoms of Pd nanoparticles through its H–H σ-bond and then undergo homolytic dissociation into H atoms with partially negative charge (Hδ−).42,43 The electron–electron repulsion effect in Hδ− and electron-rich Pd NPs is beneficial for the rapid desorption of Hδ−. Subsequently, MB molecules can efficiently react with the active H and LMB formed via the synergistic interactions between Pd NPs and g-C3N4 support. Meanwhile, Pd is also an efficient catalyst for oxidative reactions in the presence of molecular O2. This reaction proceeds through the following way: O2 is first adsorbed on the surface of Pd NPs, and electron transfer from Pd surface to the ground state of O2 occurs, the change of the charge density on Pd surface is expected to form the Pd–O bond.44 Then, the hydrogen atom of N–H bond in LMB interacts with the negatively charged oxygen atoms, leading to fast oxidation of LMB to MB. The reaction of hydride species with the activated oxygen regenerates Pd0 species along with the formation of H2O2; and H2O2 eventually decomposes to O2 and H2O. In Pd/g-C3N4 hybrid structure, the loaded Pd atoms are preferentially deposited on nitrogen sites, which can increase the electron density on Pd surface and promotes the oxidation reaction, resulting in an increased recoloration reaction rates.41 In this work, taken together, strong metal support interaction between metallic Pd and g-C3N4 support plays an important role in highly efficient reversible color switching of methylene blue/leuco-methylene blue.
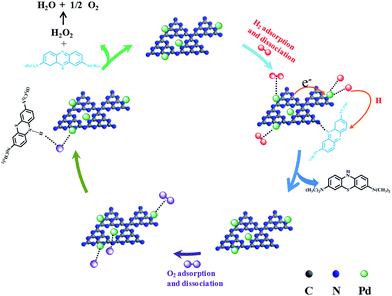 | ||
| Scheme 1 Proposed reaction processes of color switching of methylene blue over Pd/g-C3N4 nanocatalyst. | ||
3. Conclusions
In this study, Pd/g-C3N4 nanocatalyst has been successfully synthesized, and used for one-pot reversible hydrogenation and oxidation reactions. g-C3N4 nanosheets with a higher polymeric degree could improve the dispersity and stability of Pd nanoparticles. Methylene blue is used as model redox dye to investigate the hydrogenation and oxidative dehydrogenation reactions. The obtained Pd/g-C3N4 shows superior hydrogenation of MB to colorless LMB which can rapidly switch back to MB by re-oxidation process in the presence of oxygen or air under ambient conditions. The strong metal support interaction between Pd NPs and graphitic carbon nitride boosts the adsorption and subsequent dissociation of hydrogen and oxygen molecules, resulting in highly efficient reversible color switching between MB and LMB. More importantly, the Pd/g-C3N4/MB/HEC switching system exhibit remarkable reversibility and durability as the absorption intensity of MB at 664 nm shows only a slight decrease even after 10 consecutive switching cycles. Compared to the previous color switching systems, the current system has same advantages in terms of high efficiency, recyclability and easy preparation, and could provide opportunities in food packaging, printing inks for writing and erasing paper, and so on.Acknowledgements
The special funding support from the National Natural Science Foundation of China (51572253, 21271165), Scientific Research Grant of Hefei Science Center of CAS (2015SRG-HSC048), and cooperation between NSFC and Netherlands Organization for Scientific Research (51561135011) is acknowledged.References
- W. S. Wang, N. Xie, L. He and Y. D. Yin, Nat. Commun., 2014, 5, 5459 CrossRef CAS PubMed.
- H. L. Dong, H. F. Zhu, Q. Meng, X. Gong and W. P. Hu, Chem. Soc. Rev., 2012, 41, 1754–1808 RSC.
- Y. D. Liu, X. G. Han, L. He and Y. D. Yin, Angew. Chem., Int. Ed., 2012, 51, 6373–6377 CrossRef CAS PubMed.
- Y. Galagana and W. F. Su, J. Photochem. Photobiol., A, 2008, 195, 378–383 CrossRef.
- M. Basu, A. K. Sinha, M. Pradhan, S. Sarkar, A. Pal, C. Mondal and T. Pal, J. Phys. Chem. C, 2012, 116, 25741–25747 CAS.
- C. Ray, S. Dutta, S. Sarkar, R. Sahoo, A. Roy and T. Pal, RSC Adv., 2013, 3, 24313–24320 RSC.
- J. Pal, M. Ganguly, S. Dutta, C. Mondal, Y. Negishi and T. Pal, CrystEngComm, 2014, 16, 883–893 RSC.
- S. Pande, S. Jana, S. Basu, A. K. Sinha, A. Datta and T. Pal, J. Phys. Chem. C, 2008, 112, 3619–3626 CAS.
- T. Pal, S. De, N. R. Jana, N. Pradhan, R. Mandal and A. Pal, Langmuir, 1998, 14, 4724–4730 CrossRef CAS.
- T. Pal and R. Chaiti, Metal Nanoparticles for Catalysis, 2014, vol. 17, pp. 203–218 Search PubMed.
- H. Jacob, S. Ulrich, U. Jung, S. Lemke, T. Rusch, C. Schutt, F. Petersen, T. Strunskus, O. Magnussen, R. Herges and F. Tuczek, Phys. Chem. Chem. Phys., 2014, 16, 22643–22650 RSC.
- W. S. Wang, Y. F. Ye, J. Feng, M. F. Chi, J. H. Guo and Y. D. Yin, Angew. Chem., Int. Ed., 2015, 127, 1337–1342 CrossRef.
- M. Wainwright, M. N. Byrne and M. A. Gattrell, J. Photochem. Photobiol., B, 2006, 84, 227–230 CrossRef CAS PubMed.
- F. F. Wang, Z. S. Lu, L. Yang, Y. X. Zhang, Q. H. Tang, Y. M. Guo, X. M. Ma and Z. X. Yang, Chem. Commun., 2013, 49, 6626–6628 RSC.
- Y. Wang, J. Yao, H. R. Li, D. S. Su and M. Antonietti, J. Am. Chem. Soc., 2011, 133, 2362–2365 CrossRef CAS PubMed.
- Z. L. Zheng, C. Sun, R. Dai, S. Y. Wang, X. Wu, X. An and X. M. Xie, Catal. Sci. Technol., 2016, 6, 6472–6475 CAS.
- Y. Zheng, L. H. Lin, B. Wang and X. C. Wang, Angew. Chem., Int. Ed., 2015, 54, 12868–12884 CrossRef CAS PubMed.
- G. G. Liu, T. Wang, H. B. Zhang, X. G. Meng, D. Hao, K. Chang, P. Li, T. Kako and J. H. Ye, Angew. Chem., Int. Ed., 2015, 54, 13561–13565 CrossRef CAS PubMed.
- F. Raziq, C. M. Li, M. Humayun, Y. Qu, A. Zada, H. T. Yu and L. Q. Jing, Mater. Res. Bull., 2015, 70, 494–499 CrossRef CAS.
- Y. Wang, X. C. Wang and M. Antonietti, Angew. Chem., Int. Ed., 2012, 51, 68–89 CrossRef CAS PubMed.
- S. J. Liang, Y. Z. Xia, S. Y. Zhu, S. Zheng, Y. H. He, J. H. Bi, M. H. Liu and L. Wu, Appl. Surf. Sci., 2015, 358, 304–312 CrossRef CAS.
- F. F. Wang, S. Shao, C. L. Liu, C. L. Xu, R. Z. Yang and W. S. Dong, Chem. Eng. J., 2015, 264, 336–343 CrossRef CAS.
- S. J. Tauster, Acc. Chem. Res., 1987, 20, 389–394 CrossRef CAS.
- F. Goettmann, A. Fischer, M. Antonietti and A. Thomas, Angew. Chem., Int. Ed., 2006, 45, 4467–4471 CrossRef CAS PubMed.
- S. X. Min and G. X. Lu, J. Phys. Chem. C, 2012, 116, 19644–19652 CAS.
- E. Raymundo-Piñero, D. Cazorla-Amorós, A. Linares-Solano, J. Find, U. Wild and R. Schlögl, Carbon, 2002, 40, 597–608 CrossRef.
- D. L. Jiang, L. L. Chen, J. M. Xie and M. Chen, Dalton Trans., 2014, 43, 4878–4885 RSC.
- J. W. Sun, Y. S. Fu, G. Y. He, X. Q. Sun and X. Wang, Catal. Sci. Technol., 2014, 4, 1742–1748 CAS.
- S.-H. Oh and G. B. Hoflund, J. Phys. Chem. A, 2006, 110, 7609–7613 CrossRef CAS PubMed.
- C. D. Wagner, W. M. Riggs, L. E. Davis, J. F. Moulder and G. E. Muilenberg, Handbook of X-ray Photoelectron Spectroscopy, 1979, p. 55 Search PubMed.
- A. Y. Klyushin, M. T. Greiner, X. Huang, T. Lunkenbein, X. Li, O. Timpe, M. Friedrich, M. Hävecker, A. Knop-Gericke and R. Schlögl, ACS Catal., 2016, 6, 3372–3380 CrossRef CAS.
- B. J. Hu, T. B. Wu, K. L. Ding, X. S. Zhou, T. Jiang and B. X. Han, J. Phys. Chem. C, 2010, 114, 3396–3400 CAS.
- V. B. Parambhath, R. Nagar and S. Ramaprabhu, Langmuir, 2012, 28, 7826–7833 CrossRef CAS PubMed.
- R. J. Madix, Science, 1986, 233, 1159–1166 CAS.
- M. Imran, A. B. Yousaf, X. Zhou, K. Liang, Y.-F. Jiang and A.-W. Xu, Langmuir, 2016, 32, 8980–8987 CrossRef CAS PubMed.
- Y. T. Gong, P. F. Zhang, X. Xu, Y. Li, H. R. Li and Y. Wang, J. Catal., 2013, 297, 272–280 CrossRef CAS.
- S. K. Konda and A. C. Chen, Mater. Today, 2016, 19, 100–108 CrossRef CAS.
- C. J. Heard, S. Siahrostami and H. Grönbeck, J. Phys. Chem. C, 2016, 120, 995–1003 CAS.
- E. Haque, J. W. Jun, S. N. Talapaneni, A. Vinu and S. H. Jhung, J. Mater. Chem., 2010, 20, 10801–10803 RSC.
- G. X. Chen, C. F. Xu, X. Q. Huang, J. Y. Ye, L. Gu, G. Li, Z. C. Tang, B. H. Wu, H. Y. Yang, Z. P. Zhao, Z. Y. Zhou, G. Fu and N. F. Zheng, Nat. Mater., 2016, 15, 564–569 CrossRef CAS PubMed.
- P. F. Zhang, Y. T. Gong, H. R. Li, Z. R. Chen and Y. Wang, Nat. Commun., 2013, 4, 1593 CrossRef PubMed.
- S. Syrenova, C. Wadell, F. A. A. Nugroho, T. A. Gschneidtner, Y. A. Diaz Fernandez, G. Nalin, D. Switlik, F. Westerlund, T. J. Antosiewicz, V. P. Zhdanov, K. Moth-Poulsen and C. Langhammer, Nat. Mater., 2015, 14, 1236–1244 CrossRef CAS PubMed.
- P. X. Liu, Y. Zhao, R. X. Qin, S. G. Mo, G. X. Chen, L. Gu, D. M. Chevrier, P. Zhang, Q. Guo, D. D. Zang, B. H. Wu, G. Fu and N. F. Zheng, Science, 2016, 352, 797–800 CrossRef CAS PubMed.
- Y. Bai, W. H. Zhang, Z. H. Zhang, J. Zhou, X. J. Wang, C. M. Wang, W. X. Huang, J. Jiang and Y. J. Xiong, J. Am. Chem. Soc., 2014, 136, 14650–14653 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, material characterizations and additional figures. See DOI: 10.1039/c7ra04498d |
| This journal is © The Royal Society of Chemistry 2017 |