Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photo-induced orientation behaviors of azobenzene liquid crystal copolymers for photonic crystals†
Sunnam Kim *a,
Shunsuke Ishiia,
Ryohei Yagia,
Yutaka Kuwaharaad,
Tomonari Ogatab and
Seiji Kurihara*acd
*a,
Shunsuke Ishiia,
Ryohei Yagia,
Yutaka Kuwaharaad,
Tomonari Ogatab and
Seiji Kurihara*acd
aGraduate School of Science and Technology, 2-39-1 Kurokami, Kumamoto 860-8555, Japan. E-mail: sn-kim@kumamoto-u.ac.jp; kurihara@gpo.kumamoto-u.ac.jp; Fax: +81-96-342-3679; Tel: +81-96-342-3677
bInnovative Collaboration Organic Kumamoto University, 2-39-1 Kurokami, Kumamoto 860-8555, Japan
cKumamoto Institute for PHOTO-Organics (PHOENICS), 3-11-38 Higashimachi, Higashi-ku, Kumamoto, 862-0901, Japan
dJST-CREST, Gobancho, Chiyoda-ku, Tokyo 102-0076, Japan
First published on 9th November 2017
Abstract
A certain wavelength of incident light is forbidden based on the optical periodicity related to the intervals and refractive indices of the comprising materials. For multi-bilayered films with periodicity, on–off switching of reflection is possible due to the presence or absence of a refractive index difference between two consisting films. Changes in the refractive index can be induced by stimuli-responsive orientation behaviors of liquid crystal (LC) molecules with birefringence. Azobenzene (Az) groups are employed for photo response and copolymerized with non-photo-responsive LC mesogen groups for cooperative orientation to improve light penetration by the dilution of Az concentration. LC mesogen groups are designed to have a high refractive index to improve the reflection contrast. The refractive indices and the orientation behaviors of the copolymer films are investigated. On–off switching of reflection for multi-bilayered films consisting of alternate Az-containing LC copolymers and poly vinyl alcohol is conducted using ultraviolet and visible light irradiation, and their reflection intensity and response speed are compared between the different LC mesogen types and molar ratios.
Introduction
In nature, such as in opal, neon tetra fish, peacock wings, and Morpho butterfly wings, structural coloration, that is, the reflection light by the optical periodic structure, can be found. The artificial periodic nanostructured-materials are called photonic crystals (PCs) and they have attracted considerable research interest for self-standing color reflective displays. It is a very suitable technology for paper-like displays attributed to low eye fatigue and low power consumption based on the lack of a necessity for a light source.1–7 Many researchers have studied the wavelength change based on responsible periodicity under external stimuli such as electrical or magnetic fields,8–10 pH,11–13 temperature,14,15 moisture and chemicals.15–17In addition, we recently reported the photochemical on–off switching of the reflection intensity for multi-bilayered films as one dimensional PCs.18,19 This could be achieved by controlling the difference in the refractive indices of the laminate of two films having different refractive indices overlapping one another. The reflectance, R, of the multi-bilayered films is given by the following equation for the normal incidence of light:20
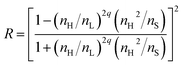 | (1) |
Changes in the refractive index of the film can be induced by changes in the orientation states when incorporating liquid crystal (LC) molecules with birefringence. The Az group is one of superior light-sensitive materials and can induce reversible and stable molecular orientation behaviors in the polymeric films. For the multi-bilayered films laminated with alternate PVA films (nPVA = 1.5) and Az-containing LC polymer films, on–off switching of the reflection was achieved by remote control of the orientation states of the LC films with a refractive index change between 1.5 and 1.6.18,19 However, since the absorbance of the azobenzene chromophore is high, there is the problem that light penetration is interrupted in the films and the response for the on–off switching of the reflection is delayed.
To improve the light response efficiency across the entire film, the non-photo-responsive LC mesogen groups are employed because it is possible to dilute the Az chromophore concentration and to cooperatively change the orientation states. For multi-bilayered films consisting of Az copolymers with biphenyl groups such as the LC mesogen groups, the light response speed was faster than that of the Az homopolymer.21 However, the reflection intensity deteriorated due to the low refractive index of the biphenyl group as reflection intensity relied on the refractive index difference of composite materials.
In order to improve the reflection intensity, herein we attempted to find LC groups exhibiting high refractive indices as well as good cooperative orientation behavior. Since the dielectric constant increases with the long-conjugated system, molecules with the longer conjugated system exhibit higher refractive indices. This is based on the relational expression of ε = n2, where ε and n represent the dielectric constant and refractive index, respectively. In addition, a Tolan group has been reported to increase the extraordinary refractive index with high conjugation along the molecular long axis, attributing to an increase of the average refractive index and birefringence.22–25,28–30 In this study, various LC mesogen groups with long conjugation on the molecular long axis such as Tolan, Schiff base, and stilbene groups were employed for Az-containing copolymers and their refractive indices, and the photo-induced orientation behaviors were compared. In addition, for the multi-bilayered films composed of the Az-containing copolymers and PVA, the photo-induced reflection behaviors were investigated in relation to their reflection intensity and light-response rate.
Experimental
Synthesis and polymerization
Az and LC mesogen monomers were synthesized according to the same method reported previously.26,27 The structures and purities of the monomers were identified by H-NMR (JEOL JNM-EX400) and the melting point by polarizing microscopic observation (POM; Olympus BHSP polarizing microscope; Mettler FP80 and FP82 hot stage and controller) and elemental analysis (Yanaco MICRO CORDER JM 10) is as follows.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.37–6.46, 5.78–5.84 (d, J = 10.8 Hz, 2H, CH2
CH), 6.37–6.46, 5.78–5.84 (d, J = 10.8 Hz, 2H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH), 6.95–7.90 (m, 8H, aromatic) C22H26N2O4 (Mw: 354.2); H; 6.85, C; 69.09, N; 7.32. Found (%); H; 6.91, C; 69.05, N; 7.30. Yield: 10.8 g (53%). Mp 95.5–96.7 °C.
CH), 6.95–7.90 (m, 8H, aromatic) C22H26N2O4 (Mw: 354.2); H; 6.85, C; 69.09, N; 7.32. Found (%); H; 6.91, C; 69.05, N; 7.30. Yield: 10.8 g (53%). Mp 95.5–96.7 °C.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 6.1 (m, 1H, CH2
CH); 6.1 (m, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 6.9 (m, 1H, CH
CH); 6.9 (m, 1H, CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 6.8, 7.4 (m, 8H, aromatic) C24H28O4 (Mw: 380.2); H; 7.42, C; 75.76, N; 0.00. Found (%); H; 7.28, C; 75.01, N; 0.14. Yield: 2.47 g (34.3%). Mp 124–126 °C.
CH); 6.8, 7.4 (m, 8H, aromatic) C24H28O4 (Mw: 380.2); H; 7.42, C; 75.76, N; 0.00. Found (%); H; 7.28, C; 75.01, N; 0.14. Yield: 2.47 g (34.3%). Mp 124–126 °C.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 6.1 (m, 1H, CH2
CH); 6.1 (m, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 6.8, 7.4 (m, 8H, aromatic) C24H26O4 (Mw: 378.2) (%), H; 6.92, C; 76.17, N; 0.00. Found (%), H; 6.94, C; 76.15, N; 0.00. Yield: 1.50 g (47.8%). Tm: 55.0–57.5 °C.
CH); 6.8, 7.4 (m, 8H, aromatic) C24H26O4 (Mw: 378.2) (%), H; 6.92, C; 76.17, N; 0.00. Found (%), H; 6.94, C; 76.15, N; 0.00. Yield: 1.50 g (47.8%). Tm: 55.0–57.5 °C.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 6.1 (m, 1H, CH2
CH); 6.1 (m, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) 6.7, (d, J = 8.8 Hz, 1H, aromatic) 6.8 (d, J = 8.8 Hz, 2H, aromatic); 7.3 (d, J = 8.8 Hz, 2H, aromatic); 7.4 (d, J = 8.8 Hz, 2H, aromatic) C25H28O4 (Mw: 392.2): H; 7.19, C; 76.50, N; 0.00. Found (%), H; 7.11, C; 76.40, N; 0.05. Yield: 3.97 g (65.6%). Tm: 58–61 °C.
CH) 6.7, (d, J = 8.8 Hz, 1H, aromatic) 6.8 (d, J = 8.8 Hz, 2H, aromatic); 7.3 (d, J = 8.8 Hz, 2H, aromatic); 7.4 (d, J = 8.8 Hz, 2H, aromatic) C25H28O4 (Mw: 392.2): H; 7.19, C; 76.50, N; 0.00. Found (%), H; 7.11, C; 76.40, N; 0.05. Yield: 3.97 g (65.6%). Tm: 58–61 °C.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH); 6.1 (m, 1H, CH2
CH); 6.1 (m, 1H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) 6.9 (d, J = 8.8 Hz 2H, aromatic); 7.0 (d, J = 8.8 Hz 2H, aromatic); 7.2 (d, J = 8.8 Hz 2H, aromatic); 7.8 (d, J = 8.8 Hz 2H, aromatic) 8.5 (s, 1H, CHN); C23H27NO4 (Mw: 381.2): H; 7.13, C; 72.42, N; 3.67. Found (%); H; 7.15, C; 76.35, N; 3.70. Yield: 3.0 g (42.7%). Tm: 82–85 °C.
CH) 6.9 (d, J = 8.8 Hz 2H, aromatic); 7.0 (d, J = 8.8 Hz 2H, aromatic); 7.2 (d, J = 8.8 Hz 2H, aromatic); 7.8 (d, J = 8.8 Hz 2H, aromatic) 8.5 (s, 1H, CHN); C23H27NO4 (Mw: 381.2): H; 7.13, C; 72.42, N; 3.67. Found (%); H; 7.15, C; 76.35, N; 3.70. Yield: 3.0 g (42.7%). Tm: 82–85 °C.Polymerization
Polymerizations were carried out as follows; first, 1.5 g of two monomers with various molar ratios (Az group![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LC mesogen group = 10
LC mesogen group = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0, 8
0, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 7
2, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 5
3, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, and 3
5, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) was dissolved in 15 ml of dimethyl formamide in a glass tube, and 15 mg (0.06 mmol) of 2,2′-azobisisobutylonitrile was also added to the solution. After purging with nitrogen, the tube was sealed and shaken in a water bath at 60 °C for 48 h. The reaction mixture was poured into excess methanol. Then the precipitated product was collected and dissolved in tetrahydrofuran (THF). After repeating this reprecipitation cycle several times, the produced copolymers were collected and dried under vacuum.
7) was dissolved in 15 ml of dimethyl formamide in a glass tube, and 15 mg (0.06 mmol) of 2,2′-azobisisobutylonitrile was also added to the solution. After purging with nitrogen, the tube was sealed and shaken in a water bath at 60 °C for 48 h. The reaction mixture was poured into excess methanol. Then the precipitated product was collected and dissolved in tetrahydrofuran (THF). After repeating this reprecipitation cycle several times, the produced copolymers were collected and dried under vacuum.
Preparation of multi-bilayered films
Multi-bilayered films were prepared according to previous reports.18,19,21 Az-containing copolymers were dissolved in cyclohexanone at 4.5 wt% and PVA was dissolved in water at 2.5 wt%. The solutions were spin coated at 3000 rpm on a glass substrate alternately and repeatedly, and 20-bilayered films were prepared. The thickness of Az100 and PVA was 95 nm and 90 nm, respectively, from a surface profiler (Dektak-150, ULVAC). The thickness of the 20-bilayered films was measured by atomic force microscopy (AFM, Agilent 5500) and field emission scanning electron microscopy (FE-SEM, Hitachi SU-8000) (Fig. S12†).Characterization
The thermal properties of the polymers were examined by means of differential scanning calorimetry (DSC; Seiko DSC-5020). The scan rate for the DSC measurements was 10 °C min−1. Molecular weight was determined by gel permeation chromatography (GPC; Jasco 870-UV detector at 254 nm, Shodex KF-804L column, THF as eluent). The copolymerization ratio was determined by UV-vis spectroscopy (Shimazu UV-1600PC). An ultrahigh pressure mercury lamp with a heat cut filter (NAF-50S-50H) was used as a light source (USHIO SX-UI 500H). UV light (λ = 365 nm, 8 mW cm−2) and visible light (λ = 436 nm, 60 mW cm−2) were used with filters Sigma UTVAF-35U and Sigma SCF-50s-42L, respectively. Refractive indices of the copolymer films were calculated by ellipsometry (Spectrum Ellipsometer FE-5000s, Otsuka Electronics Co., Ltd.) with the sodium D line at 589.3 nm.Results and discussion
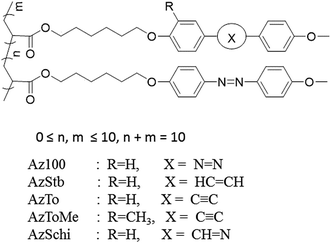
With the Az group and LC mesogen groups such as stilbene (Stb), Schiff base (Schi), Tolan (To), and Tolan methyl (ToMe) groups, copolymers were prepared at various molar ratios of the LC groups to the Az group, as described in Table 1. Az100 represents the Az homopolymer. The Az-containing copolymers are abbreviated as AzStb, AzSchi, AzTo, and AzToMe. The numbers in the abbreviations indicate the molar ratio of the Az to the LC groups. Their molecular structures are shown in Fig. 1.| LC mesogen | Az | LC mesogen | Az | LC mesogen | Sample name |
|---|---|---|---|---|---|
| (Loading ratio) | (Content ratio) | ||||
| Stilbene | 7 | 3 | 7 | 3 | AzStb73 |
| 5 | 5 | 5 | 5 | AzStb55 | |
| 3 | 7 | 2 | 8 | AzStb28 | |
| Schiff | 7 | 3 | 7 | 3 | AzSchi73 |
| 5 | 5 | 5 | 5 | AzSchi55 | |
| 3 | 7 | 3 | 7 | AzSchi37 | |
| Tolan | 7 | 3 | 8 | 2 | AzTo82 |
| 5 | 5 | 7 | 3 | AzTo73 | |
| 3 | 7 | 4 | 6 | AzTo46 | |
| Tolan methyl | 8 | 2 | 9 | 1 | AzToMe91 |
| 7 | 3 | 6 | 4 | AzToMe64 | |
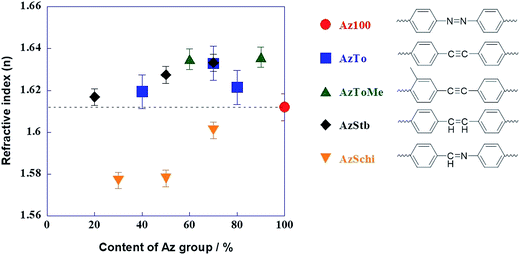
The average refractive indices of the copolymers in random orientation states were evaluated by ellipsometry measurements and the results are summarized in Fig. 2. The refractive indices of AzToMe, AzTo and AzStb were greater than or similar to that of Az100 (1.612). In contrast, the refractive indices of AzSchi were lower than that of Az100. The reason for the non-monotonous behavior of the refractive index is not clear at present.
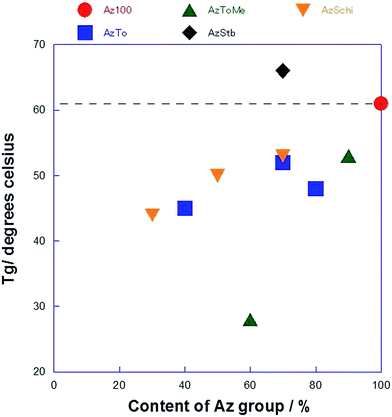
The phase transition temperatures of the copolymers were estimated by DSC measurements and the LC phases were confirmed by POM. The phase transition temperatures are summarized in Table 2. All the copolymers exhibited both smectic and nematic LC phases. However, for AzStb55 and AzStb28 with higher molar ratios of the Stb group, the phase transition temperatures were not clearly observed. As shown in Fig. 3, the glass transition temperatures (Tg) of the copolymers decreased compared to that of Az100, except for the Tg of AzStb. The Tg of AzStb73 was estimated to be 66 °C, which was slightly higher than that of Az100. Tg is considered to be an important factor for orientation behaviors of polymers.
| Abbreviation | Mn | Mw/Mn | Phase transition temperature [°C] |
|---|---|---|---|
| a *Mn and Mw/Mn are measured by GPC. **G, S, N and I respectively present glass transition temperature, smectic phase, nematic phase and isotropic phase. | |||
| Az100 | 7500 | 1.23 | G 61 S 99 N 139 I |
| AzTo82 | 6800 | 1.57 | G 48 S 84 N 127 I |
| AzTo73 | 6700 | 1.35 | G 52 S 78 N 127 I |
| AzTo46 | 7600 | 1.55 | G 45 S 74 N 123 I |
| AzToMe91 | 7000 | 1.43 | G 53 S 69 N 124 I |
| AzToMe64 | 7800 | 1.41 | G 28 S 47 N 113 I |
| AzStb73 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
2.32 | G 66 S 81 N 105 I |
| AzStb55 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
2.26 | — |
| AzStb28 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.71 | — |
| AzSchi73 | 9200 | 1.41 | G 53 S 102 N 139 I |
| AzSchi55 | 4900 | 1.22 | G 50 S 69, 103 N 130 I |
| AzSchi37 | 4700 | 1.21 | G 44 S 65, 102 N 125 I |
Photo-response isomerization behaviors were investigated in THF solution (Fig. S1†). For the UV-vis absorption spectra, the absorption bands at 360 nm and 450 nm corresponded to the π–π* transition and the n–π* transition, respectively, of the Az group. The changes in the absorption spectra based on trans–cis isomerization behavior were observed by irradiation of UV light (365 nm, 8 mW cm−2) and visible light (436 nm, 60 mW cm−2). It was observed that using UV light, the absorbance decreased at 365 nm and increased at 450 nm, whereas using 436 nm visible light, reverse behaviors were observed. These changes were similar and reversible for all the samples, although the absorption bands of the Stb and Schi groups were partially overlapped with the π–π* transition absorption bands of the Az groups. Therefore, it was demonstrated that the photo-responsive orientation behavior of the Az group occurred stably even when the mesogen groups coexisted.
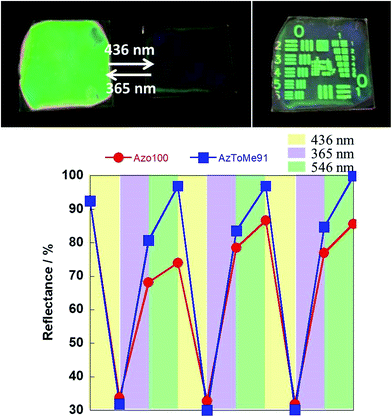
For the thin films prepared using the spin-coating method, the initial states were in random orientation states. The orientation states could be controlled by irradiation of 365 nm, 436 nm, and 546 nm UV-vis light, as shown in Fig. 4. Molar fractions of cis-isomers in each orientation state were respectively estimated to be 94%, 15%, and 1% (supported by Fig. S8 and S9†). At 436 nm visible light, the Az units have absorption bands of both the cis and trans isomers and thus they can undergo repeated cis–trans isomerization. Then, the direction of alignment depends on the incident light direction due to the photo-orientation process, which is induced by anisotropic light absorption of trans-azobenzene fragments and subsequent re-arrangement of the orientation structure of the material.31–38 Therefore, an out-of-plane orientation can be induced by normal incident light of 436 nm for the thin films. For 365 nm UV light, cis isomers are generated and the orientation state can be destroyed. Consequently, a random state is induced. Using the subsequent 546 nm visible light, the isomerization behavior from the cis to trans isomers is conducted and thus the random orientation state with the trans isomers can be induced.
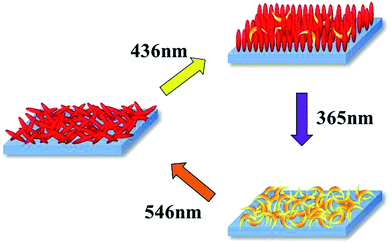 | ||
| Fig. 4 Schematic of the orientation states of the films after irradiation by UV-vis light at 365 nm, 436 nm, and 546 nm. | ||
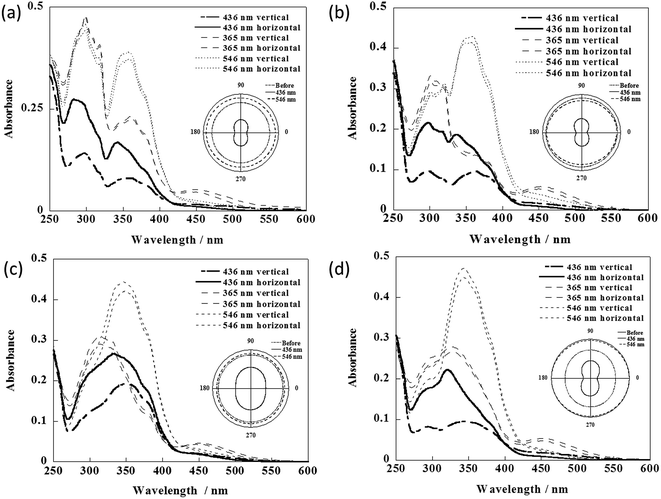
The orientation states of the films induced by irradiation of light were evaluated by the changes in the absorption spectra (Fig. S3 and S4†). For light at 436 nm, π–π* transition absorption bands of the Az groups and LC groups at 360 nm and 300 nm decreased. When the films were horizontally tilted 40° to the plane normal to the monitoring light, anisotropic absorption spectra were observed for the vertically and horizontally polarized directions on the film, as shown in Fig. 5. In addition, eight patterns were observed in their polar graphs after irradiation by 436 nm visible light. Therefore it was demonstrated that out-of-plane orientation was induced. By irradiation by 365 nm UV light, absorbance at 300 nm and 450 nm increased. It was demonstrated that cis isomers of Az groups were generated and alignment of LC mesogen groups was destroyed, which led to a random orientation state. Subsequently, by irradiation by 546 nm visible light, absorbance at 360 nm increased and absorbance at 450 nm decreased, indicating cis to trans isomerization of the Az groups. From the isotropic absorbance, it was assumed that trans isomers of the Az group were randomly aligned.
The out-of-plane orientation states were evaluated using the order parameter (S) of the Az groups.39 S was calculated with absorbance using the following eqn (2), when the films were horizontally tilted 40° to the plane normal to the monitoring light:
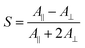 | (2) |
For the thin films, the S values with light of 365 nm, 436 nm, and 546 nm were compared, as shown in Fig. 6. For the random orientation states before irradiation of light, S = 0. With 436 nm visible light, S of Az100 increased to 0.6 in the out-of-plane orientation state. S of the copolymers was lower than that of Az100. For AzStb, especially, the out-of-plane orientation state was hardly induced by light irradiation, presumably due to a high Tg, as mentioned above. Finally, using UV light of 365 nm following visible light of 546 nm, random orientation states were induced and S approached 0.
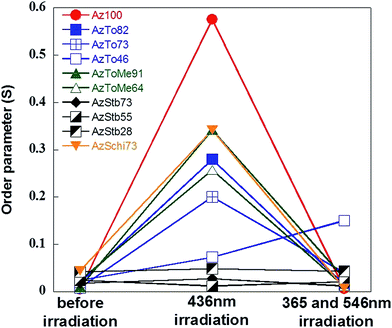 | ||
| Fig. 6 The changes in S for the films before and after light irradiation by 436 nm visible light, and 365 nm UV light following 546 nm visible light. | ||
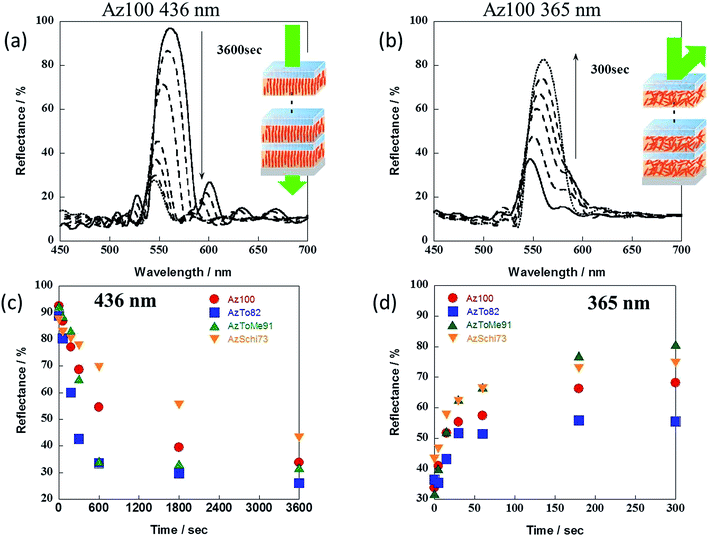
For the multi-bilayered films comprising alternate Az-containing films and PVA films, the photo-responsive speeds for on–off switching of the reflection intensity were compared, as shown in Fig. 7. For 436 nm visible light, the reflection for all films decreased. For copolymers of AzTo82 and AzToMe91, the response speed of reflection was faster than that of Az100. It was assumed that out-of-plane orientation behaviors in the multi-bilayered films were improved by copolymerization with To and ToMe groups, possibly due to their lower Tg than that of Az100. On the other hand, the photo-response speed of reflection of AzSchi73 was slower than that of Az100 even though it has a lower Tg. This is attributed to the surface energy depending on the hydrophobicity of LC mesogen groups when sandwiched by hydrophilic PVA films.
In order to estimate the hydrophobicity of LC molecules, dipole moments and the partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) were calculated using Spartan’14 (Wavefunction, Inc.) with the B3LYP/6-31G* method. For Az, Schi, and To groups they were respectively estimated to be 0 Debye, 1.24 Debye, and 0 Debye for dipole moments; and 4.22, 3.65, and 3.72 for log
P) were calculated using Spartan’14 (Wavefunction, Inc.) with the B3LYP/6-31G* method. For Az, Schi, and To groups they were respectively estimated to be 0 Debye, 1.24 Debye, and 0 Debye for dipole moments; and 4.22, 3.65, and 3.72 for log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P. Consequently, a Schi group exhibiting higher polarity is relatively less hydrophobic. Therefore, AzSchi films sandwiched by PVA films are less preferable for out-of-plane orientation compared with the other copolymers, based on higher surface energy. This is supported by the experimental results of thermally induced out-of-plane orientation behaviors. When AzTo73 and AzSchi73 were annealed at 70 °C, their S values for out-of-plane orientation were respectively estimated to be 0.73 and 0.34. S of AzSchi73 was lower than that of AzTo73, as expected.
P. Consequently, a Schi group exhibiting higher polarity is relatively less hydrophobic. Therefore, AzSchi films sandwiched by PVA films are less preferable for out-of-plane orientation compared with the other copolymers, based on higher surface energy. This is supported by the experimental results of thermally induced out-of-plane orientation behaviors. When AzTo73 and AzSchi73 were annealed at 70 °C, their S values for out-of-plane orientation were respectively estimated to be 0.73 and 0.34. S of AzSchi73 was lower than that of AzTo73, as expected.
On the other hand, for 365 nm UV light, reflection for all films increased. The light response of AzToMe91 was faster than that of Az100 and other copolymers, indicating improvement of random orientation behavior attributed to light penetration deep inside. On the other hand, AzTo82 had apparently poor response to UV light. Namely, random orientation was not sufficiently induced by accumulation of cis isomers of Az groups. This is due to strong intermolecular interactions between To groups as described in our previous reports.28,29 It is also supported by the fact that the Tg of AzTo decreased less with an increase in the molar ratio of the mesogen group than that of AzToMe (Fig. 3). In contrast, the ToMe group is considered to be advantageous for random orientation because of the steric hindrance of the methyl group.
Reversible on–off switching of reflection was examined by the sequence of light irradiation by 436 nm visible light (for the out-of-plane orientation state; reflection = OFF) and 365 nm UV light following 546 nm visible light (for the random orientation state; reflection = ON), as shown in Fig. 8. For photo-induced on–off switching, the reflection contrast of AzToMe91 was improved owing to a better orientation behavior and higher refractive index.
Conclusion
LC mesogen groups with long-conjugated systems were synthesized and the copolymers with the Az group and the LC groups showed higher refractive indices compared with that of Az100, except for the copolymers with the Schi group. For the thin films, the orientation states were controlled by irradiation of 365 nm, 436 nm and 546 nm light, except for the copolymers with the Stb group. For the multi-bilayered films comprising Az-containing copolymers and PVA, photo-induced on–off switching of reflection was conducted. Reflectance and photo responses were improved compared to those of Az100 and the best performance was obtained for AzToMe91. The methyl group of ToMe relaxed the strong intermolecular interaction between To groups. For the copolymers with the Schi and Stb groups, the photo-responses were poor, possibly due to the higher surface energy and molecular rigidity, respectively.Conflicts of interest
There are no conflicts to declare.References
- B. Comiskey, J. D. Albert, H. Yoshizawa and J. Jacobson, Nature, 1998, 16, 253–255 CrossRef.
- Y. Chen, J. Au, P. Kazlas, A. Ritenour, H. Gates and M. McCreary, Nature, 2003, 8, 136 CrossRef PubMed.
- P. Mach, P. Wiltzius, M. Megens, D. A. Weitz, K. Lin and T. C. Lubensky, et al., Europhys. Lett., 2002, 58, 679 CrossRef CAS.
- S. Kuai, G. Bader and P. V. Ashrit, Appl. Phys. Lett., 2005, 86, 221110 CrossRef.
- S. W. Leonard, J. P. Mondia, H. M. van Driel, O. Toader, S. John and K. Busch, et al., Phys. Rev. B, 2000, 61, R2389–R2392 CrossRef CAS.
- C. I. Aguirre, E. Reguera and A. Stein, Adv. Funct. Mater., 2010, 20, 2565–2578 CrossRef CAS.
- A. C. Arsenault, D. P. Puzzo, I. Manners and G. A. Ozin, Nat. Photonics, 2007, 1, 468–472 CrossRef CAS.
- K. Busch and S. John, Phys. Rev. Lett., 1999, 83, 967–970 CrossRef CAS.
- S. O’Brien and J. B. Pendry, J. Phys.: Condens. Matter, 2002, 14, 4035 CrossRef.
- J. Zhou, C. Q. Sun, K. Pita, Y. L. LAm, Y. Zhou, S. L. NAg, C. H. Kam, L. T. Li and Z. L. Gui, Appl. Phys. Lett., 2001, 78, 661–663 CrossRef CAS.
- K. Lee and S. A. Asher, J. Am. Chem. Soc., 2000, 122, 9534–9537 CrossRef CAS.
- K. Ueno, K. Matsubara, M. Watanabe and Y. Takeoka, Adv. Mater., 2007, 19, 2807–2812 CrossRef CAS.
- Y. J. Lee and P. Braun, Adv. Mater., 2003, 15, 563–566 CrossRef CAS.
- J. M. Weissman, H. B. Sunkara, A. S. Tse and S. A. Asher, Science, 1996, 274, 959–963 CrossRef CAS PubMed.
- J. H. Holtz and S. A. Asher, Nature, 1997, 389, 829–832 CrossRef CAS PubMed.
- R. A. Barry and P. Wiltzius, Langmuir, 2006, 22, 1369–1374 CrossRef CAS PubMed.
- Y. Takeoka and M. Watanabe, Langmuir, 2003, 19, 9554–9557 CrossRef CAS.
- R. Yagi, H. Katae, Y. Kuwahara, S. Kim, T. Ogata and S. Kurihara, Polymer, 2014, 55, 1120–1127 CrossRef CAS.
- M. Moritsugu, T. Ishikawa, T. Kawata, T. Ogata, Y. Kuwahara and S. Kurihara, Macromol. Rapid Commun., 2011, 32, 1546–1550 CrossRef CAS PubMed.
- T. Komikado, A. Inoue, K. Masuda, T. Ando and S. Umegaki, Thin Solid Films, 2007, 515, 3887–3892 CrossRef CAS.
- R. Yagi, H. Iwamoto, Y. Kuwahara, S. Kim, T. Ogata and S. Kurihara, RSC Adv., 2015, 5, 84762–84769 RSC.
- S. Yoneyama, T. Yamamoto, O. Tsutsumi, A. Kanazawa, T. Shiono and T. Ikeda, Macromolecules, 2002, 35, 8751–8758 CrossRef CAS.
- S. D. Sarkar and B. Choudhury, Acta Phys. Pol., A, 2010, 118, 665–669 CrossRef CAS.
- F. Ye, A. Orita, J. Yaruva, T. Hamada and J. Otera, Chem. Lett., 2004, 33, 528–529 CrossRef CAS.
- M. S. Zakerhamidi, Z. Ebrahimi, H. Tajalli, A. Ghanadzadeh, M. Moghadam and A. Ranjkesh, J. Mol. Liq., 2010, 157, 119–124 CrossRef CAS.
- K. Okano, A. Shishido and T. Ikeda, Adv. Mater., 2006, 18, 523–527 CrossRef CAS.
- T. Ikeda, S. Horiuchi, D. B. Karanjit, S. Kurihara and S. Tazuke, Macromolecules, 1990, 23, 36–42 CrossRef CAS.
- M. Moritsugu, T. Shirota, S. Kubo, T. Ogata, O. Sato and S. Kurihara, J. Polym. Sci., Part B: Polym. Phys., 2009, 47, 1981–1990 CrossRef CAS.
- T. Shirota, M. Moritsugu, S. Kubo, T. Ogata, T. Nonaka and O. Sato, et al., Mol. Cryst. Liq. Cryst., 2009, 513, 79–88 CrossRef CAS.
- N. Tanio and M. Irie, Jpn. J. Appl. Phys., Part 1, 1994, 33, 3942 CrossRef CAS.
- I. Zebger, M. Rutloh, U. Hoffmann, J. Stumpe, H. W. Siesler and S. Hvilsted, J. Phys. Chem. A, 2002, 106, 3454–3462 CrossRef CAS.
- M. Han, S. Morino and K. Ichimura, Macromolecules, 2000, 33, 6360–6371 CrossRef CAS.
- O. V. Yaroshchuk, A. D. Kiselev, Y. Zakrevskyy, T. Bidna, J. Kelly and L. Chien, et al., Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2003, 68, 011803 CrossRef CAS PubMed.
- K. Ichimura, M. Han and S. Morino, Chem. Lett., 1999, 28, 85–86 CrossRef.
- T. Ikeda, J. Mater. Chem., 2003, 13, 2037–2057 RSC.
- A. Shishido, Polym. J., 2010, 42, 525–533 CrossRef CAS.
- A. V. Bogdanov and A. K. Vorobiev, J. Phys. Chem. B, 2013, 117, 13936–13945 CrossRef CAS PubMed.
- S. P. Palto and G. Durand, J. Phys. II, 1995, 5, 963–978 CrossRef CAS.
- Y. Wu, Y. Demachi, O. Tsutsumi, A. Kanazawa, T. Shiono and T. Ikeda, Macromolecules, 1998, 31, 349–354 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7ra07160d |
| This journal is © The Royal Society of Chemistry 2017 |