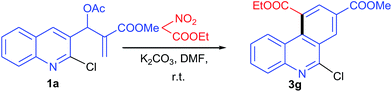Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Active methylene compounds (AMCs) controlled facile synthesis of acridine and phenanthridine from morita Baylis–Hillman acetate†
Tanu Gupta,
Jay Bahadur Singh,
Kalpana Mishra and
Radhey M. Singh *
*
Department of Chemistry, Centre of Advanced Study, Institute of Science, Banaras Hindu University, Varanasi, India. E-mail: rmohan@bhu.ac.in
First published on 28th November 2017
Abstract
We carried out simple and facile syntheses of acridines and phenanthridines from MBH acetates of 2-chloro-quinoline-3-carbaldehydes with active methylene compounds (AMCs). Formation of products was found to be dependent on the functional group of the AMC. For example, ethylcyanoacetate and malononitrile favoured the formation of acridines and cyanoacetamide, and ethyl nitroacetate and malonic esters favoured formation of angularly-fused phenanthridines. The reactions leading to the formation of phenanthridines proceeded through single bond rotation of SN2′ intermediate which was attributed to electronic/steric repulsion between the functional groups of AMCs and the nitrogen of quinoline.
Introduction
Acridines and phenanthridines constitute an important class of linearly and angularly benzo-fused quinolines.1 Derivatives of these quinolines have garnered great interest from synthetic chemists due to their significant biological activities,2 such as their antitumor, antimalarial, antituberculosis, antibacterial, antiprotozoal, antileukemic, anticancer, and anti-HIV activities, and due to their ability to intercalate between base pairs of double-stranded DNA and hence alter the cellular machinery.3 Some derivatives are used as pigments and dyes in industry4 and are also used as biological fluorescent probes to monitor polymerization processes.5 In addition, conjugated derivatives showing electronic and photophysical properties are used as organic semiconductor materials.6Owing to the great medicinal and industrial importance of acridine derivatives, several methods for synthesizing them have been reported in the past few decades, with these methods including dehydrogenation,7 metal-catalyzed coupling,8 C–H functionalization,9 and inter-10 and intramolecular11 cyclization. Similarly, radical reactions,12 metal-catalyzed coupling reactions,13 cycloaddition reactions14 and other synthetic methods,15 mostly involving ortho-functionalized biaryl derivatives as substrates, have been reported for the synthesis of phenanthridine derivatives. However, these reactions constructed the aza ring of the acridine and phenanthridine moieties. Substrates, in particular quinoline derivatives, affording either acridines or phenanthridines via benzannulation have, in contrast, been less explored.16 However, these methods have certain limitations such as requiring a high temperature, a strongly basic or acidic medium, expensive reagents, starting materials that are difficult to obtain, and moderate yields. Therefore, developing simple routes from easily accessible precursors, under relatively mild reaction conditions, is highly desirable for the synthesis of acridines and phenanthridines.
In recent years, Morita Baylis Hillman (MBH) reaction, a three-component, atom-economical, carbon–carbon bond-forming reactions of aldehydes, activated alkenes and catalysts led to the formation of MBH adduct which is an attractive precursors for various organic synthetic transformations17 with various functionalities.
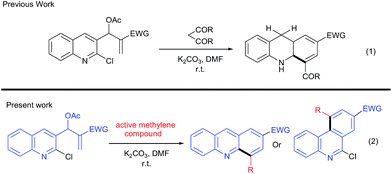
Recently, we prepared MBH acetates of 2-chloro-quinoline-3-carbaldehydes,18 which afforded the synthesis of benzo[b][1,8]naphthyridines.18i We have also reported a new route for the synthesis of dihydroacridines18d (instead of the synthesis of acridine) by reacting these MBH acetates with acetylacetone/acetoacetic esters (Fig. 1, eqn (1)).
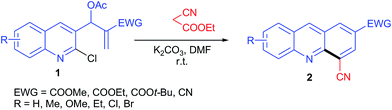
These unusual results have inspired us to systematically investigate using other active methylene compounds (AMCs) such as ethylcyanoacetate, malononitrile, cyanoacetamide, ethylnitroacetate, dimethyl/diethylmalonate. We herein report the reaction of AMCs with MBH acetates of 2-chloro-quinoline-3-carbaldehydes, which provided facile routes to acridines and phenanthridines, respectively.
Results and discussion
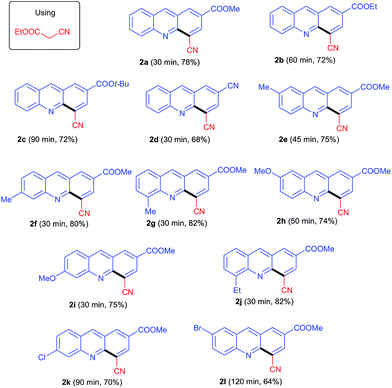
Initially we focused our studies on the reaction of the ethylcyanoacetate reagent (1.5 equiv.) with MBH acetate 1a (0.5 mmol) and K2CO3 (1.5 equiv.) under our previously reported conditions18d in DMF at room temperature. The reaction was completed in 30 min and afforded the single product 2a in 78% yield. The structure of 2a was determined to be 4-cyanoacridine-2-carboxylic acid methyl ester on the basis of its spectral and analytical data. This result suggested the decarboxylation of the ester followed by dehydrogenation to be faster than the elimination of the cyano group and decarboxylation could be attributed due to the formation of carboxylate anion (I) (Scheme 1).The scope of ethylcyanoacetate was further investigated with other MBH acetates. All reactions proceeded smoothly to afford acridines 2a–l in good to excellent yields. The results are listed in Table 1. Lengthening or branching of ester alkyl in the MBH acetate lowered the yields (2b & 2c). Replacing the ester with a cyano group in the MBH acetate resulted in product 2d, but with a decreased yield of 68%. Similar reactions were further investigated with MBH acetates of substituted quinolines. The results are given in Table 1 (2e–l). MBH acetates with electron-donating groups (EDGs) were more reactive than those with electron-withdrawing groups (EWGs), and hence afforded the product in higher yields (2e–j).
| a All reactions were performed with 1 (0.5 mmol) by using ethylcyanoacetate (1.5 equiv.) and K2CO3 (1.5 equiv.) in DMF (2 mL). The reaction time and yields of isolated products are given. |
|---|
 |
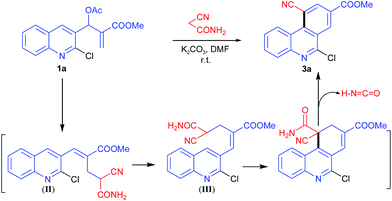
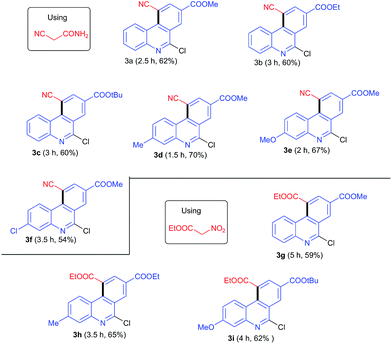
The malononitrile reagent was also examined with 1a under similar conditions and afforded the same product 2a, in 5 min with 92% yield, as described above. Next, we reacted the cyanoacetamide reagent with 1a for the purpose of synthesizing an acridine derivative. When 1a was treated with cyanoacetamide (1.5 equiv.) and K2CO3 (1.5 equiv.) under similar conditions as described above, the reaction took a longer time (2.5 h) to complete, and the isolated product 3a was obtained, instead of acridine 2a, in 62% yield. The structure of 3a was determined to be 6-chloro-10-cyano-phenanthridine-8-carboxylic acid methyl ester from its spectral and analytical data. The formation of 3a suggested that SN2′ product (II) underwent rotation about the single bond connecting two sp2 carbons to form conformation (III) followed by cyclization, deamidation and dehydrogenation to form product 3a (Scheme 2). The loss of the amide group may have been due to isocyanic acid (H–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)18d (Scheme 2).
O)18d (Scheme 2).
The dependence of the synthesis of planar/angular tricyclic benzo-fused quinolines such as dihydroacridines, acridines and phenanthridines on the functional group of the AMC inspired us to test the reactions of additional AMCs with MBH acetate. We tested the reaction with ethylnitroacetate and found that MBH acetate provided phenanthridine template as did cyanoacetamide. When MBH acetate 1a was treated with ethylnitroacetate (1.5 equiv.) and K2CO3 under reaction conditions similar to those described above, the reaction completed in 5 h and a single product was isolated in 59% yield and characterized as angularly fused phenanthridine derivative 3g. The corresponding linearly fused cyclized acridine was not isolated from the reaction mixture (Scheme 3). However, the aromatization step proceeded via elimination of the nitro group. Rotation about the carbon–carbon single bond of the SN2′ product (II) in nitrogen-containing functional groups of AMCs could be presumed to be due to electronic repulsion between the nitrogen functional group and the nitrogen of the quinoline, and to be a source of instability of SN2′ product (II).
The scopes of both the cyanoacetamide and ethylnitroacetate reagents were further investigated with other MBH acetates under the optimized conditions. All reactions proceeded and afforded phenanthridine derivatives 3a–f with cyanoacetamide in 54–70% yields and 3g–i with ethylnitroacetate in 59–66% yields. Lengthening and branching of the ester group of MBH acetate gave products 3a–c in nearly the same yields, specifically 60–62%. Similarly, MBH acetates bearing EDG were more reactive than those bearing EWG, and hence gave products 3d–e and 3h–i in better yields than 3f. The results are summarized in Table 2.
| a All reactions were performed with 1 (0.5 mmol) by using cyanoacetamide/nitroethylacetate (1.5 equiv.) and K2CO3 (1.5 equiv.) in DMF (2 mL). The reaction time and yield of isolated product are given. |
|---|
 |
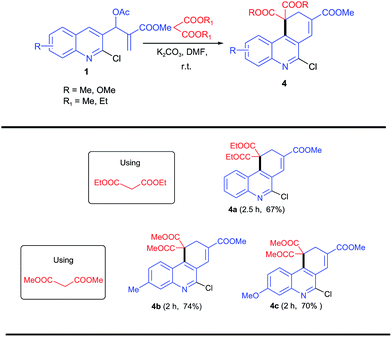
Next, we explored the reaction of malonic ester reagents with MBH acetate 1a for the purpose of synthesizing either acridines or 1,4-dihydroacridines. For this purpose, MBH acetate 1a was employed with malonic esters under similar reaction conditions. The reactions were completed in 2–3 h and afforded products 4 in 67–74% yields and the products 4 were determined to be 1,4-dihydro-phenanthridines. The reaction mixture was further heated at 70 °C for 24 hours for the purpose of effecting a complete aromatization of the product but failed to proceed and the dihydro product was recovered. The reagent applicability was further evaluated with other MBH acetates, and corresponding dihydro products 4a–c were afforded in 67–74% yields (Scheme 4).
Conclusions
In conclusion, we have developed an efficient method for synthesis of acridines and phenanthridines from reactions of MBH acetates of 2-chloro-quinoline-3-carbaldehydes with various AMCs in one pot at room temperature under aerobic conditions. Formation of products was found to be dependent on the functional group of the AMC. Our synthetic route is advantageous with respect to reaction conditions, and the availability of substrates from easily accessible starting materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
TG is thankful to UGC, New Delhi, for JRF. JBS is thankful to CSIR, New Delhi for SRF. KM is thankful to DRDO ERIP/ER/1203055/M/01/1471 for a fellowship. RMS is thankful to the director IISER Bhopal for HRMS spectra and DST-SERB, New Delhi (EMR/2016/000706) and CSIR for funding (02(0073)/12EMR-II).Notes and references
- (a) A. Albert, The Acridines, Edward Arnold Ltd., London, 2nd edn, 1996 Search PubMed; (b) S. Bonse, C. Santelli-Rouvier, J. Barbe and R. L. Krauth-Siegel, J. Med. Chem., 1999, 42, 5448–5454 CrossRef CAS PubMed; (c) M. A. P. Martins, C. P. Frizzo, D. N. Moreira, L. Buriol and P. Machado, Chem. Rev., 2009, 109, 4140–4182 CrossRef CAS PubMed; (d) V. Nadaraj, S. T. Selvi and S. Mohan, Eur. J. Med. Chem., 2009, 44, 976–980 CrossRef CAS PubMed; (e) Z. Jin, Nat. Prod. Rep., 2011, 28, 1126–1142 RSC.
- (a) Y. L. Janin, A. Croisy, J. F. Riou and E. Bisagni, J. Med. Chem., 1993, 36, 3686–3692 CrossRef CAS PubMed; (b) S. A. Gamage, D. P. Figgitt, S. J. Wojcik, R. K. Ralph, A. Ransijn, J. Mauel, V. Yardley, D. Snowdon, S. L. Croft and W. A. Denny, J. Med. Chem., 1997, 40, 2634–2642 CrossRef CAS PubMed; (c) F. Hamy, V. Brondani, A. Flörsheimer, W. Stark, M. J. J. Blommers and T. Klimkait, Biochemistry, 1998, 37, 5086–5095 CrossRef CAS PubMed; (d) M. J. Wainwright, J. Antimicrob. Chemother., 2001, 47, 1 CrossRef CAS PubMed; (e) C. Santelli-Rouvier, J.-M. Barret, C. M. Farrell, D. Sharples, B. T. Hill and J. Barbe, Eur. J. Med. Chem., 2004, 39, 1029–1038 CrossRef CAS PubMed; (f) M. O. Anderson, J. Sherrill, P. B. Madrid, A. P. Liou, J. L. Weisman and J. L. DeRisi, Bioorg. Med. Chem., 2006, 14, 334–343 CrossRef CAS PubMed; (g) G. Y. Park, J. J. Wilson, Y. Song and S. J. Lippard, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 11987–11992 CrossRef CAS PubMed; (h) N. Nagesh, K. M. Naidu, D. H. Rao, J. P. Sridevi, D. Sriram, P. Yogeeswari and K. V. Chandrasekhar, Bioorg. Med. Chem. Lett., 2013, 23, 6805–6810 CrossRef PubMed; (i) B. Zhang, X. Li, B. Li, C. Gao and Y. Jiang, Expert Opin. Ther. Pat., 2014, 24, 647–664 CrossRef CAS PubMed; (j) M. Rivaud and V. Jullian, Planta Med., 2014, 80, 902–906 CrossRef PubMed.
- (a) J. Joseph, E. Kuruvilla, A. T. Achuthan, D. Ramaiah and G. B. Schuster, Recent Res. Dev. Bioconjugate Chem., 2004, 15, 1230–1235 CrossRef CAS PubMed; (b) M. E. Budiman, U. Bierbach and R. W. Alexander, Biochemistry, 2005, 44, 11262–11268 CrossRef CAS PubMed; (c) S. Dollinger, S. Löber, R. Klingenstein, C. Korth and P. Gmeiner, J. Med. Chem., 2006, 49, 6591–6595 CrossRef CAS PubMed; (d) M.-K. Cheng, C. Modi, J. C. Cookson, I. Hutchinson, R. A. Heald, A. J. McCarroll, S. Missailidis, F. Tanious, W. D. Wilson, J.-L. Mergny, C. A. Laughton and M. F. G. Stevens, J. Med. Chem., 2008, 51, 963–975 CrossRef CAS PubMed; (e) N. H. Campbell, G. N. Parkinson, A. P. Reszka and S. Neidle, J. Am. Chem. Soc., 2008, 130, 6722–6724 CrossRef CAS PubMed; (f) J. R. Goodell, A. V. Ougolkov, H. Hiasa, H. Kaur, R. Remmel, D. D. Billadeau and D. M. Ferguson, J. Med. Chem., 2008, 51, 179–182 CrossRef CAS PubMed; (g) Z. Ma, L. Rao and U. Bierbach, J. Med. Chem., 2009, 52, 3424–3427 CrossRef CAS PubMed.
- C. D. Geddes, Dyes Pigm., 2000, 45, 243–251 CrossRef CAS.
- (a) M. Sameiro and T. Gonçalves, Chem. Rev., 2009, 109, 190–212 CrossRef PubMed; (b) C. Chen, G. Shang, J. Zhou, Y. Yu, B. Li and J. Peng, Org. Lett., 2014, 16, 1872–1875 CrossRef CAS PubMed; (c) M. T. Bakić, D. Jadreško, T. Hrenar, G. Horvat, J. Požar, N. Galić, V. Sokol, R. Tomaš, S. Alihodžić, M. Žinić, L. Frkanec and V. Tomišić, RSC Adv., 2015, 5, 23900–23914 RSC.
- (a) J. L. Vivero-Escoto, I. I. Slowing and V. S.-Y. Lin, Biomaterials, 2010, 31, 1325–1333 CrossRef CAS PubMed; (b) A. Goel, V. Kumar, S. P. Singh, A. Sharma, S. Prakash, C. Singh and R. S. Anand, J. Mater. Chem., 2012, 22, 14880–14888 RSC; (c) D. Zhang, X. Jiang, H. Yang, A. Martinez, M. Feng, Z. Donga and G. Gao, Org. Biomol. Chem., 2013, 11, 3375–3381 RSC; (d) H. Ma, B. Liu, B. Li, L. Zhang, Y.-G. Li, H.-Q. Tan, H.-Y. Zang and G. Zhu, J. Am. Chem. Soc., 2016, 138, 5897–5903 CrossRef CAS PubMed.
- J. Wu, D. Talwar, S. Johnston, M. Yan and J. Xiao, Angew. Chem., Int. Ed., 2013, 52, 6983–6987 CrossRef CAS PubMed.
- (a) Y. Lian, J. R. Hummel, R. G. Bergman and J. A. Ellman, J. Am. Chem. Soc., 2013, 135, 12548–12551 CrossRef CAS PubMed; (b) X. Pang, C. Chen, X. Su, M. Li and L. Wen, Org. Lett., 2014, 16, 6228–6231 CrossRef CAS PubMed; (c) T.-J. Wang, W.-W. Chen, Y. Lia and M.-H. Xu, Org. Biomol. Chem., 2015, 13, 6580–6586 RSC; (d) X. Pang, Z. Lou, M. Li, L. Wen and C. Chen, Eur. J. Org. Chem., 2015, 3361–3369 CrossRef CAS.
- (a) D. Tsvelikhovsky and S. L. Buchwald, J. Am. Chem. Soc., 2010, 132, 14048–14051 CrossRef CAS PubMed; (b) Y. Zagranyarski, A. Skabeev, Y. Ma, K. Müllen and C. Li, Org. Chem. Front., 2016, 3, 1520–1523 RSC; (c) W. Hu, Q. Zheng, S. Sun and J. Cheng, Chem. Commun., 2017, 53, 6263–6266 RSC.
- (a) D. G. Pintori and M. F. Greaney, Org. Lett., 2010, 12, 168–171 CrossRef CAS PubMed; (b) C. Donald, D. C. Rogness and R. C. Larock, J. Org. Chem., 2010, 75, 2289–2295 CrossRef PubMed; (c) X. Huang and T. Zhang, J. Org. Chem., 2010, 75, 506–509 CrossRef CAS PubMed; (d) A. V. Dubrovskiy and R. C. Larock, J. Org. Chem., 2012, 77, 11232–11256 CrossRef CAS PubMed; (e) H.-M. Guo, R.-Z. Mao, Q.-T. Wang, H.-Y. Niu, M.-S. Xie and G.-R. Qu, Org. Lett., 2013, 15, 5460–5463 CrossRef CAS PubMed.
- (a) Y. Kuninobu, T. Tatsuzaki, T. Matsuki and K. Takai, J. Org. Chem., 2011, 76, 7005–7009 CrossRef CAS PubMed; (b) Q. Su, P. Li, M. He, Q. Wu, L. Ye and Y. Mu, Org. Lett., 2014, 16, 18–21 CrossRef CAS PubMed.
- (a) Y. Zhou, C. Wu, X. Dong and J. Qu, J. Org. Chem., 2016, 81, 5202–5208 CrossRef CAS PubMed; (b) W. Wan, G. Ma, J. Li, Y. Chen, Q. Hu, M. Li, H. Jiang, H. Deng and J. Hao, Chem. Commun., 2016, 52, 1598–1601 RSC; (c) Y. Xu, Y. Chen, W. Li, Q. Xie and L. Shao, J. Org. Chem., 2016, 81, 8426–8435 CrossRef CAS PubMed; (d) J. Rong, L. Deng, P. Tan, C. Ni, Y. Gu and J. Hu, Angew. Chem., Int. Ed., 2016, 55, 2743–2747 CrossRef CAS PubMed; (e) L. Noël-Duchesneau, E. Lagadic, F. Morlet-Savary, J.-F. Lohier, I. Chataigner, M. Breugst, J. Lalevée, A.-C. Gaumont and S. Lakhdar, Org. Lett., 2016, 18, 5900–5903 CrossRef PubMed; (f) P. Xiao, J. Rong, C. Ni, J. Guo, X. Li, D. Chen and J. Hu, Org. Lett., 2016, 18, 5912–5915 CrossRef CAS PubMed; (g) Y. Jin, H. Yang and H. Fu, Org. Lett., 2016, 18, 6400–6403 CrossRef CAS PubMed.
- (a) R. K. Chinnagolla and M. Jeganmohan, Chem. Commun., 2014, 50, 2442–2444 RSC; (b) X. Bao, W. Yao, Q. Zhu and Y. Xu, Eur. J. Org. Chem., 2014, 7443–7450 CrossRef CAS; (c) W. Han, X. Zhou, S. Yang, G. Xiang, B. Cui and Y. Chen, J. Org. Chem., 2015, 80, 11580–11587 CrossRef CAS PubMed; (d) B. Wang, Y. Dai, W. Tong and H. Gong, Org. Biomol. Chem., 2015, 13, 11418–11421 RSC; (e) M. Ghosh, A. Ahmed, R. Singha and J. K. Ray, Tetrahedron Lett., 2015, 56, 353–355 CrossRef CAS; (f) S.-Y. Yang, W.-Y. Han, D.-L. Zhang, X.-J. Zhou, M. Bai, B.-D. Cui, N.-W. Wan, W.-C. Yuan and Y.-Z. Chen, Eur. J. Org. Chem., 2017, 996–1003 CrossRef CAS; (g) Z. Xiong, J. Wang, Y. Wang, S. Luo and Q. Zhu, Org. Chem. Front., 2017, 4, 1768–1771 RSC.
- (a) B. K. Mehta, K. Yanagisawa, M. Shiro and H. Kotsuki, Org. Lett., 2003, 5, 1605–1608 CrossRef CAS PubMed; (b) W.-G. Shou, Y.-Y. Yang and Y.-G. Wang, J. Org. Chem., 2006, 71, 9241–9243 CrossRef CAS PubMed; (c) R. Yanada, K. Hashimoto, R. Tokizane, Y. Miwa, H. Minami, K. Yanada, M. Ishikura and Y. Takemoto, J. Org. Chem., 2008, 73, 5135–5138 CrossRef CAS PubMed; (d) L. Sripada, J. A. Teske and A. Deiters, Org. Biomol. Chem., 2008, 6, 263–26510 RSC.
- (a) L.-M. Tumir, M. R. Stojković and I. Piantanida, Beilstein J. Org. Chem., 2014, 10, 2930–2954 CrossRef PubMed; (b) Y.-F. Chen, Y.-S. Wu, Y.-H. Jhan and J.-C. Hsieh, Org. Chem. Front., 2014, 1, 253–257 RSC; (c) B. Zhang and A. Studer, Chem. Soc. Rev., 2015, 44, 3505–3521 RSC; (d) W.-L. Chen, C.-Y. Chen, Y.-F. Chen and J.-C. Hsieh, Org. Lett., 2015, 17, 1613–1616 CrossRef CAS PubMed; (e) X.-D. An and S. Yu, Org. Lett., 2015, 17, 2692–2695 CrossRef CAS PubMed; (f) S. Battula, A. Kumar, A. P. Gupta and Q. N. Ahmed, Org. Lett., 2015, 17, 5562–5565 CrossRef CAS PubMed; (g) W. Shen, J. Li, C. Zhang, M. Shi and J. Zhang, Chem.–Asian J., 2016, 11, 1883–1886 CrossRef CAS PubMed; (h) P. Natarajan, N. Kumar and M. Sharma, Org. Chem. Front., 2016, 3, 1265–1270 RSC; (i) A. Borah and P. Gogoi, Eur. J. Org. Chem., 2016, 2200–2206 CrossRef CAS; (j) Y.-Y. Jhang, T.-T. Fan-Chiang, J.-M. Huan and J.-C. Hsieh, Org. Lett., 2016, 18, 1154–1157 CrossRef CAS PubMed; (k) J. Ge, X. Wang, T. Liu, Z. Shi, Q. Xiao and D. Yin, RSC Adv., 2016, 6, 19571–19575 RSC; (l) W.-S. Guo, Q. Dou, J. Hou, L.-R. Wen and M. Li, J. Org. Chem., 2017, 82, 7015–7022 CrossRef CAS PubMed; (m) C. Zhang, T. Li, L. Wang and Y. Rao, Org. Chem. Front., 2017, 4, 386–391 RSC; (n) J. Fang, W.-G. Shen, G.-Z. Ao and F. Liu, Org. Chem. Front., 2017, 4, 2049–2053 RSC.
- (a) P. Belmont, J.-C. Andrez and C. S. M. Allan, Tetrahedron Lett., 2004, 45, 2783–2786 CrossRef CAS; (b) P. Belmont and T. Belhadj, Org. Lett., 2005, 7, 1793–1795 CrossRef CAS PubMed; (c) I. Cikotiene, Tetrahedron Lett., 2009, 50, 2570–2572 CrossRef CAS; (d) I. Cikotiene, R. Buksnaitiene and R. Sazinas, Tetrahedron, 2011, 67, 706–717 CrossRef CAS; (e) S. P. Shukla, R. Tiwari and A. K. Verma, Tetrahedron, 2012, 68, 9035–9044 CrossRef CAS; (f) D. Waghray, J. Zhang, J. Jacobs, W. Nulens, N. Basarić, L. V. Meervelt and W. Dehaen, J. Org. Chem., 2012, 77, 10176–10183 CrossRef CAS PubMed.
- (a) D. Basavaiah, A. J. Rao and T. Satyanarayana, Chem. Rev., 2003, 103, 811–891 CrossRef CAS PubMed; (b) P. Narender, U. Srinivas, M. Ravinder, A. Rao, C. Ramesh, K. Harakishore, B. Gangadasu, U. S. N. Murthy and V. J. Rao, Bioorg. Med. Chem., 2006, 14, 4600–4609 CrossRef CAS PubMed; (c) V. Singh and S. Batra, Tetrahedron, 2008, 64, 4511–4574 CrossRef CAS; (d) S. Gowrisankar, K. H. Kim and J. N. Kim, Bull. Korean Chem. Soc., 2008, 29, 2537–2539 CrossRef CAS; (e) D. Basavaiah, B. S. Reddy and S. S. Badsara, Chem. Rev., 2010, 110, 5447–5674 CrossRef CAS PubMed; (f) D. Basavaiah and G. Veeraraghavaiah, Chem. Soc. Rev., 2012, 41, 68–78 RSC; (g) K. C. Bharadwaj, RSC Adv., 2015, 5, 75923–75946 RSC; (h) W.-Y. Huang, S. Anwar and K. Chen, Chem. Rec., 2017, 17, 1–20 CrossRef PubMed; (i) D. K. Nair, T. Kumar and I. N. N. Namboothiri, Synlett, 2016, 27, 2425–2442 CrossRef CAS.
- (a) K. Mishra, J. B. Singh, T. Gupta and R. M. Singh, Org. Chem. Front., 2017, 4, 1926–1930 RSC; (b) K. Mishra, J. B. Singh, T. Gupta and R. M. Singh, Org. Chem. Front., 2017, 4, 1794–1798 RSC; (c) J. B. Singh, K. Mishra, T. Gupta and R. M. Singh, ChemistrySelect, 2017, 2, 1207–1210 CrossRef CAS; (d) T. Gupta, K. C. Bharadwaj and R. M. Singh, Eur. J. Org. Chem., 2016, 4981–4984 CrossRef CAS; (e) R. M Singh, R. Kumar, K. C Bharadwaj and T. Gupta, Org. Chem. Front., 2016, 3, 1100–1104 RSC; (f) J. B. Singh, K. C. Bharadwaj, T. Gupta and R. M. Singh, RSC Adv., 2016, 6, 26993–26999 RSC; (g) M. Asthana, R. Kumar, T. Gupta and R. M. Singh, Tetrahedron Lett., 2015, 56, 907–912 CrossRef CAS; (h) D. Nandini, M. Asthana, T. Gupta, R. P. Singh and R. M. Singh, Tetrahedron, 2014, 70, 8592–8599 CrossRef CAS; (i) B. Singh, A. Chandra and R. M. Singh, Tetrahedron, 2011, 67, 2441–2446 CrossRef CAS; (j) A. Srivastava and R. M. Singh, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2005, 44, 1868–1875 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedure, data and 1H & 13C NMR spectra. See DOI: 10.1039/c7ra09447g |
| This journal is © The Royal Society of Chemistry 2017 |