Open Access Article
Open Access ArticleSynthesis and photophysical studies of through-space conjugated [2.2]paracyclophane-based naphthalene fluorophores†
E. Benedetti *,
M.-L. Delcourt,
B. Gatin-Fraudet,
S. Turcaud and
L. Micouin*
*,
M.-L. Delcourt,
B. Gatin-Fraudet,
S. Turcaud and
L. Micouin*
Laboratoire de Chimie et Biochimie Pharmacologiques et Toxicologiques, UMR 8601 CNRS, Université Paris Descartes, Sorbonne Paris Cité, UFR Biomédicale, 45 rue des Saints Pères, 75006 Paris, France. E-mail: erica.benedetti@parisdescartes.fr
First published on 30th October 2017
Abstract
In this work we report a straightforward method for the synthesis of a new class of small organic fluorophores bearing both [2.2]paracyclophane and naphthalene subunits using an intramolecular dehydrogenative Diels–Alder reaction as a key step. These compounds are characterized by a compact three-dimensional structure as well as through-space conjugated push–pull systems, and possess interesting spectroscopic characteristics that may be useful for the development of innovative chemical probes and optical sensors.
Since the discovery of their parent compound in 1949,1 mono- and polysubstituted [2.2]paracyclophane derivatives (pCp) have attracted increasing attention within the scientific community and have become the object of many studies in the last few decades.
Initial investigations on the synthesis and derivatization of [2.2]paracyclophanes mainly aimed at disclosing the unusual reactivity of these molecules.2 More recently, functionalized pCps have emerged as powerful ligands or auxiliaries in asymmetric catalysis and stereoselective synthesis.3 Substituted paracyclophanes also found applications in material sciences as monomers for the synthesis of through-space conjugated polymers,4 or for the production of functionalized surfaces using chemical vapor deposition (CVD) methods.5
Due to their uncommon electronic structure, [2.2]paracyclophanes display intrinsic fluorescence and can be employed to access new organic dyes. Accordingly, a wide range of pCp-based stilbene fluorophores have been reported in the literature over the past few years.6 These molecules typically present a “branched” π-extended three-dimensional structure functionalized with differently substituted para-phenylene vinylene subunits (Fig. 1a). The pCp-based stilbene fluorophores, whose photophysical properties are generally tuned by introducing electron-donating or electron-withdrawing groups on their alkene moieties, revealed to be particularly useful in solid state application for the development of organic light-emitting diodes (OLEDs),7 and non-linear optical materials.8
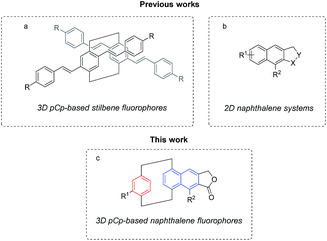 | ||
| Fig. 1 (a) Structure of pCp-based stilbene fluorophores. (b) General structure of two-dimensional naphthalene systems. (c) Structure of pCp-based naphthalene fluorophores. | ||
A different approach to modulate the spectroscopic characteristics of the pCp-based fluorophores consists in expanding the benzene rings of [2.2]paracyclophane to functionalized polycondensed aromatic hydrocarbons. This should lead to more compact dyes potentially useful for the design of innovative chemical probes and biosensors. Surprisingly, this approach has only been scarcely investigated up to now.9 In this context, our group recently became interested in the possibility to incorporate naphthalene π-conjugated frameworks (Fig. 1b) into one of the aromatic decks of pCp to tune their photophysical properties.10 We therefore decided to focus our attention on the elaboration of pCp-based naphthalene frameworks (Fig. 1c) by an intramolecular dehydrogenative-Diels–Alder (DDA) reaction. This strategy was reported to be particularly versatile for the synthesis of tunable solvatochromic fluorophores,11 but has never been described on pCp.
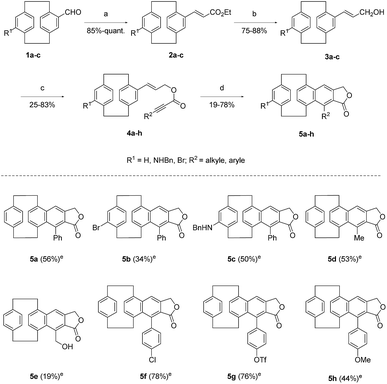
The DDA precursors 4a–h were prepared in three steps starting from differently substituted 4-formyl-[2.2]paracyclophanes12 (Scheme 1). The synthetic procedure involved a Horner–Wadsworth–Emmons reaction between the aldehydes 1a–c and triethyl phosphonoacetate, followed by a reduction of the esters 2a–c in the presence of diisobutylaluminium hydride (DIBAL-H), and the acylation of the resulting alcohols 3a–c with different propiolic acid derivatives (Scheme 1). The intramolecular dehydrogenative Diels–Alder reaction of styrenes 4a–h was then investigated under microwave irradiation at 180 °C. This transformation of substrates 4a–h into the corresponding fused aromatic systems 5a–h is not trivial due to their congested molecular structures and the known low thermal stability of [2.2]paracyclophanes.13 To our delight, sterically hindered paracyclophane 4a underwent the cycloaddition smoothly under microwave irradiation to deliver naphthalene 5a in 56% yield. Remarkably, both electron-poor and electron-rich cyclophane rings were compatible with the reaction conditions and compounds 5b-c could be isolated in acceptable yields (Scheme 1). The cyclization tolerated various alkyl- and aryl-substituted alkyne moieties as well, and naphthalenes 5d–h were obtained in moderate to good yields (Scheme 1). It is worth noting that better conversions were generally achieved when the triple bond terminal position was functionalized with electron-poor aromatic substituents (compounds 5f and 5g, Scheme 1).
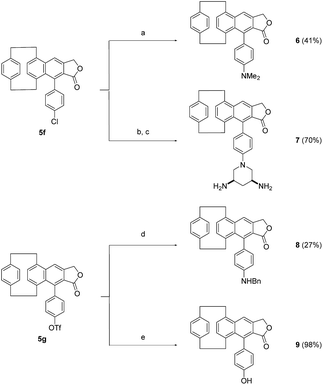
In an attempt to access fluorescent compounds bearing different electron-donating groups, the chlorinated paracyclophane 5f was submitted to a series of Buchwald–Hartwig cross-coupling reactions in the presence of commercially available RuPhos palladacycle and cesium carbonate (Scheme 2).
A first coupling of compound 5f with dimethylamine successfully afforded the amino-substituted paracyclophane 6 in 41% yield (Scheme 2). Substrate 5f was similarly converted into the more hydrophilic product 7 through a reaction with Boc-protected 3,5-diaminopiperidine,14 and subsequent removal of the carbamates under acidic conditions (Scheme 2). Triflate 5g underwent an analogous palladium-catalysed amination, in the presence of Pd2dba3, XantPhos and benzylamine, to form derivative 8 in 27% yield (Scheme 2). Compound 5g was finally transformed into the phenol derivative 9 following a different post-functionalization approach which involved the cleavage of the triflate ester with TBAF (Scheme 2).
With this series of pCp-based naphthalenes in hand, we next undertook a photophysical study in order to determine the influence of the “out-of-plane” paracyclophane deck (depicted in red in Fig. 1c) on the spectroscopic properties of the dyes. As a general trend, the pCp-based fluorophores showed red-shifted absorption and emission bands in comparison with unsubstituted [2.2]paracyclophane (Fig. 2). The aryl- and alkyl-substituted pCp-based naphthalenes 5a and 5d presented a weak absorption band around 375 nm that might be attributed to a cyclophane band when dissolved in CH2Cl2 (Table 1, entries 1 and 4). Moreover, these compounds were found to emit light around 440 and 420 nm respectively (Table 1, entries 1, and 4). Remarkably, the absorption and emission maxima of compounds 5a and 5d resulted to be significantly red-shifted in comparison to those of the paracyclophane-deprived naphthalene 10 previously described by Brummond and co-workers (Table 1, entry 13),11c,15 and for 5a, a good fluorescence quantum yield (QY) could be measured in dichloromethane (Table 1, entry 1). Compounds 5b, 5e, 5f, 5g and 5h, all lacking amino groups, displayed photophysical properties similar to those of dyes 5a and 5d (Table 1, entries 2 and 5–8). These results highlight that the “out-of-plane” ring of paracyclophanes can be considered as a non-conventional electron-donating substituent which, similarly to amine functionalities, induce a displacement of the absorption and emission maxima of the fluorophores towards longer wavelengths. Due to its aromatic nature, however, the “out-of-plane” ring of pCp is less affected by excited-state oxidation processes than classical electron-donating amino groups, leading to more photostable dyes.16
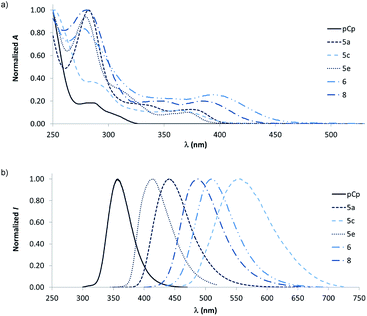 | ||
| Fig. 2 Normalized UV-vis absorption (a) and fluorescence emission spectra (b) of selected pCp-based naphthalene fluorophores in comparison with unsubstituted [2.2]paracyclophane. | ||
| Entry | Compound | R1, R2 | λabsmaxc (nm) | λemmaxd (nm) | QYe (%) |
|---|---|---|---|---|---|
| a 10−5M solutions of the dyes in CH2Cl2.b 10−5M solutions of the dyes in DMSO.c Absorption maximum of the most red-shifted band (putative cyclophane band).d Emission spectra do not change significantly upon excitation at different wavelengths.e Relative quantum yields (QY) were determined using anthracene and 9,10-diphenylanthracene as standards. See the ESI for more details. | |||||
| 1a | 5a | H, Ph | 376 | 440 | 62 |
| 2a | 5b | Br, Ph | 371 | 430 | 17 |
| 3a | 5c | NHBn, Ph | 365 | 555 | 6 |
| 4a | 5d | H, Me | 373 | 420 | 39 |
| 5a | 5e | H, CH2OH | 373 | 415 | 16 |
| 6a | 5f | H, p-Cl-Ph | 375 | 440 | 41 |
| 7a | 5g | H, p-OTf-Ph | 378 | 445 | 43 |
| 8a | 5h | H, p-OMe-Ph | 378 | 443 | 41 |
| 9a | 6 | H, p-NMe2-Ph | 400 | 510 | 23 |
| 10b | 7 | H, 3,5-diamino-N-phenylpiperidine | 384 | 520 | 10 |
| 11a | 8 | H, p-NHBn-Ph | 385 | 485 | 30 |
| 12b | 9 | H, p-OH-Ph | 374 | 454 | 16 |
| 13a | 10 | — | 345 | 380 | 22 |
The incorporation of amine moieties into the pCp-based fluorophores induced a further bathochromic displacement of their absorption and emission bands, yet with a detrimental effect on the photostability profiles of the dyes.17 The absorption maxima of the putative cyclophane band for compounds 6 and 8 were recorded at 400 and 385 nm respectively in dichloromethane, while the emission spectra of these molecules showed maxima at 510 and 485 nm (Table 1, entries 9 and 11). Naphthalene 5c, on its side, exhibited the most red-shifted emission maximum (555 nm in CH2Cl2, Table 1, entry 3) together with an exceedingly large Stokes-shift of 190 nm (absorption maximum of the cyclophane band around 365 nm, Table 1, entry 3). This large red-shifted emission clearly outlines the interest of playing with the two decks of the cyclophane to modulate the optical properties of these dyes. Unfortunately this through-space conjugated push–pull system was found to have a low fluorescence quantum yield possibly because of the non-optimal relative position of its electron-donating and electron-withdrawing groups (Table 1, entry 3). Compounds 7 and 9, containing primary amines or a free phenolic moiety, resulted to be poorly soluble in dichloromethane. The spectroscopic characterization of these dyes was therefore performed in DMSO. In this case, absorption and emission maxima were observed at 384 and 520 nm for the amino-substituted derivative 7; at 374 and 454 nm for hydroxylated fluorophore 9 (Table 1, entries 10 and 12).
Amine-containing pCp-based naphthalenes 5c, 6, and 8 displayed intriguing solvatochromic properties. Indeed, as the solvent polarity was increased from toluene to acetonitrile, intense bathochromic shifts were registered in the emission spectra of compounds 6 and 8 (61 and 58 nm respectively, Table 2, entries 2 and 3). Naphthalene 5c suffered from fluorescence quenching in acetonitrile due to its low quantum yield (Table 2, entry 1). Even so, this dye showed emission maxima at 510 nm in toluene and 595 nm in acetone, which resulted in a strong red-shift of its bands (up to 85 nm, Table 2, entry 1). This last observation indicates that the functionalization of the “out-of-plane” paracyclophane deck can strongly influence the solvatochromic behaviour of the pCp-based fluorophores. Further investigations are ongoing in order to better rationalize this effect and to design more efficient fluorescent dyes.
| Entry | Compound | λemmax in toluene (nm) | λemmax in CH2Cl2 (nm) | λemmax in acetone (nm) | λemmax in MeCN (nm) |
|---|---|---|---|---|---|
| a 1×10−5M solutions of the dyes were used to perform the analyses. Diminished fluorescence intensities were generally observed in polar solvents. | |||||
| 1 | 5c | 510 | 555 | 595 | n.d |
| 2 | 6 | 486 | 510 | 539 | 547 |
| 3 | 8 | 467 | 485 | 515 | 525 |
Conclusions
In conclusion, we have demonstrated that the intramolecular dehydrogenative Diels–Alder reaction is well suited to extend the benzene rings of [2.2]paracyclophane to functionalized polycondensed aromatic hydrocarbons. This synthetic approach enabled the synthesis of a new class of small organic fluorophores which incorporate both [2.2]paracyclophane and naphthalene subunits and exhibit unique photophysical properties. Our study demonstrated that the aromatic decks of paracyclophane could act as photostable electron donating groups capable of modulating the spectroscopic characteristics of the dyes. The synthesized molecules possess an unprecedented compact three-dimensional structure as well as through-space conjugated push–pull systems. These structural and electronic features are expected to widely extend the range of application of the pCp-based naphthalene probes. A further optimization of the fluorophores is currently under investigation in our laboratory in order to access tunable fluorescent markers for biological assays.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Ministère de l’Enseignement Supérieur et de la Recherche, the CNRS and the Paris Descartes University.Notes and references
- (a) C. J. Brown and A. C. Farthing, Nature, 1949, 164, 915 CrossRef CAS; (b) H. Hope, J. Bernstein and K. N. Trueblood, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1972, B28, 1733 CrossRef.
- For a review on the synthesis and derivatization of [2.2]paracyclophanes see, A. A. Aly and A. B. Brown, Tetrahedron, 2009, 65, 8055 CrossRef CAS.
- (a) V. I. Rozenberg, E. V. Sergeeva, and H. Hopf, in Modern Cyclophane Chemistry ed. R. Gleiter, H. Hopf, Wiley-VCH, Weinheim, 2004 Search PubMed. For selected reviews, see; (b) O. R. P. David, Tetrahedron, 2012, 68, 8977 CrossRef CAS; (c) S. E. Gibson and J. D. Knight, Org. Biomol. Chem., 2003, 1, 1256 RSC.
- (a) Y. Morusaki and Y. Chujo, Polym. Chem., 2011, 2, 1249 RSC; (b) Y. Morusaki, and Y. Chujo, Through-Space Conjugated Polymers, ed. Chujo R. Y., Wiley-VCH, Weinheim, Germany, 2010 Search PubMed; (c) H. Hopf, Angew. Chem., Int. Ed., 2008, 47, 9808 CrossRef CAS PubMed; (d) Y. Morusaki and Y. Chujo, Macromolecules, 2003, 36, 9319 CrossRef.
- H. Nandivada, H.-Y. Chen, L. Bondarenko and J. Lahann, Angew. Chem., Int. Ed., 2006, 45, 3360 CrossRef CAS PubMed.
- (a) J. L. Zafra, A. Molina, P. Ontoria, M. Mayorga Burrezo, M. Pena-Alvarez, J. Samoc, F. J. Szeremeta, M. D. Ramirez Lovander, C. J. Droske, T. M. Pappenfus, L. Echegoyen, J. T. Lopez Navarrete, N. Martin and J. Casado, J. Am. Chem. Soc., 2017, 139, 3095 CrossRef CAS PubMed; (b) J. W. Hong, H. Y. Woo, B. Liu and G. C. Bazan, J. Am. Chem. Soc., 2005, 127, 7435 CrossRef CAS PubMed; (c) H. Y. Woo, J. W. Hong, B. Liu, A. Mikhailovsky, D. Korystov and G. C. Bazan, J. Am. Chem. Soc., 2005, 127, 820 CrossRef CAS PubMed; (d) G. P. Bartholomew, M. Rumi, S. J. K. Pond, J. W. Perry, S. Tretiak and G. C. Bazan, J. Am. Chem. Soc., 2004, 126, 11529 CrossRef CAS PubMed; (e) J. W. Hong, H. Benmansour and G. C. Bazan, Chem.–Eur. J., 2003, 9, 3186 CrossRef CAS PubMed.
- J. P. Chen, K. Ueno, and K. Suzuki, Patent No: US 6869698, 2005.
- (a) H. Goromaru, N. Shigeiwa, M. Murata, and S. Maeda, Patent No: JP 4918803, 2012; (b) G. P. Bartholomew, I. Ledoux, S. Mukamel, G. C. Bazan and J. Zyss, J. Am. Chem. Soc., 2002, 124, 13480 CrossRef CAS PubMed; (c) J. Zyss, I. Ledoux, S. Volkov, V. Chernyak, S. Mukamel, G. P. Bartholomew and G. C. Bazan, J. Am. Chem. Soc., 2000, 122, 11956 CrossRef CAS.
- H. Hopf, J. Hucker and L. Ernst, Eur. J. Org. Chem., 2007, 1891 CrossRef CAS.
- Naphthalenes represent a well-known class of organic dyes that exhibit attractive photophysical properties such as strong solvatochromic behavior, high quantum yields, and good photostability, see: A. M. G. Silva, C. Queirós, and F. Monteiro-Silva, in Naphthalene: Structure, Properties and Application, ed. G. I. Antsyforov, and A. F. Ivanski, Nova Science Publishers, 2012 Search PubMed.
- (a) A. E. Bober, J. T. Proto and K. M. Brummond, Org. Lett., 2017, 19, 1500 CrossRef CAS PubMed; (b) L. S. Kocsis, H. N. Kagalwala, S. Mutto, B. Godugu, S. Bernhard, D. J. Tantillo and K. M. Brummond, J. Org. Chem., 2015, 80, 11686 CrossRef CAS PubMed; (c) L. S. Kocsis and K. M. Brummond, Org. Lett., 2014, 16, 4158 CrossRef CAS PubMed; (d) E. Benedetti, A. B. E. Veliz, M. Charpenay, L. S. Kocsis and K. M. Brummond, Org. Lett., 2013, 15, 2578 CrossRef CAS PubMed; (e) E. Benedetti, L. S. Kocsis and K. M. Brummond, J. Am. Chem. Soc., 2012, 134, 12418 CrossRef CAS PubMed; (f) L. S. Kocsis, E. Benedetti and K. M. Brummond, Org. Lett., 2012, 14, 4430 CrossRef CAS PubMed.
- For more details, see the ESI†. An efficient synthesis of enantiopure 4-formyl[2.2]paracyclophane was previously described by our group, see: M.-L. Delcourt, S. Turcaud, E. Benedetti and L. Micouin, Adv. Synth. Catal., 2016, 358, 1213 CrossRef CAS.
- W. R. Roth, H. Hopf, A. de Meijere, F. Hunold, S. Börner, M. Neumann, T. Wasser, J. Szurowski and C. Mlynek, Liebigs Ann., 1996, 2141 CrossRef CAS.
- This compound was previously synthetized by our group, see: (a) L. Micouin, A. Blond, V. Calvez, A.-G. Marcellin, C. Soulie, E. Corrot, Patent No: WO 2015181387, 2015; (b) A. Blond, P. Dockerty, R. Alvarez, S. Turcaud, T. Lecourt and L. Micouin, J. Org. Chem., 2013, 78, 12236 CrossRef CAS PubMed.
- For more details on the synthesis of naphthalene 10, see the ESI†.
- Photostability studies were performed for all the pCp-based naphthalenes. See the ESI† for more details.
- Amine-containing pCp-based naphthalenes 5c, 6 and 8 resulted to be less photostable in comparison with the other aryl- and alkyl-substituted pCp-based naphthalenes. See the ESI† for more details..
Footnote |
| † Electronic supplementary information (ESI) available: Details of experimental procedures, 1H NMR and 13C NMR spectra as well as absorption and emission spectra. See DOI: 10.1039/c7ra10038h |
| This journal is © The Royal Society of Chemistry 2017 |