Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The structure and properties of Co substituted Bi7Ti4NbO21 with intergrowth phases
Changhui Liua,
Zezhi Chena,
Ranran Peng *ab,
Zhengping Fuab,
Xiaofang Zhaibc and
Yalin Lu*abc
*ab,
Zhengping Fuab,
Xiaofang Zhaibc and
Yalin Lu*abc
aCAS Key Laboratory of Materials for Energy Conversion, Department of Materials Science and Engineering, University of Science and Technology of China, Hefei 230026, P. R. China. E-mail: yllu@ustc.edu.cn; pengrr@ustc.edu.cn
bSynergetic Innovation Center of Quantum Information & Quantum Physics, University of Science and Technology of China, Hefei, Anhui 230026, China
cNational Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei 230026, PR China
First published on 30th October 2017
Abstract
Multiferroic complex oxides with intergrowth aurivillius phases are gaining more and more attention due to the potential to greatly adjust their ferroelectricity (FE) and ferromagnetism (FM) using non-integer layer numbers. In this work, the 2 + 3 aurivillius intergrowth phases of Bi7Ti4−2xCoxNb1+xO21 were successfully synthesized via a solid reaction method. X-ray diffraction (XRD) and high angle annular dark field scanning transmission electron microscopy (HAADF-STEM) analyses clearly demonstrated that Co substituted Bi7Ti4−2xCoxNb1+xO21 keeps an intergrowth phase structure when x ≤ 0.3. A new analysis method that maps the linear brightness in HAADF images was used to give the clear Bi atom position, and this revealed that the lattice shrinkage in the c direction caused by Co substitution mainly occurred at the (BiTiNbO7)2− block in the (Bi3TiNbO9) layer, which was also confirmed by an investigation using Raman spectroscopy. Polarization–electric field (P–E) investigations and pulsed polarization positive-up negative-down (PUND) measurements indicated that Bi7Ti4−2xCoxNb1+xO21 (x = 0.1, 0.2, and 0.3) presents much enhanced properties compared with non-substituted Bi7Ti4NbO21. For example, 2Ec = 135.23 kV cm−1 and 2Pr = 9.33 μC cm−2 can be achieved when x = 0.3. Also with Co substitution, Bi7Ti4NbO21 changed from diamagnetic (χ < 0) to paramagnetic (χ ≈ 7 × 10−5). The calculated effective magnetic moments in the Bi7Ti4−2xCoxNb1+xO21 samples have similar values, suggesting that the cobalt atoms in the materials have almost the same efficient moment.
Introduction
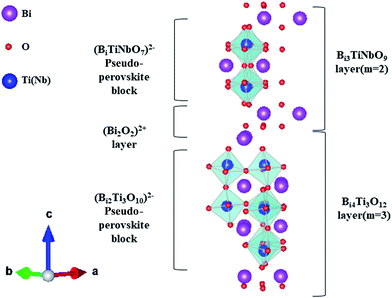
Ferroelectric and/or ferromagnetic co-existing layered bismuth complex oxides with an aurivillius structure (LBOA) have recently been attracting considerable interest because of their amazing layered crystalline structures, valuable coupled physical properties, and potential applications in multiple-state storage, transducers, amplitude modulators, and optical wave devices.1–9 Normally, the crystalline structures of LBOAs can be generally formulated as (Bi2O2)2+ (Am−1BmO3m+1)2− in which the (Bi2O2)2+ fluorite-type layers alternate with (Am−1BmO3m+1)2− perovskite slabs, where A is a mono-, di-, or tri-valent ion, such as Na+, K+, Pb2+, Ba2+, Sr2+, Bi3+, or a mixture of them; B represents a small sized cation with tetra-, penta-, or hexa-valence, such as Ti4+, Nb5+, Ta5+, or W6+; and m, usually an integer, is the number of mono-octahedral layers between the (Bi2O2)2+ layers.10 As their crystalline structures, chemical stability, as well as dielectric and ferroelectric properties can be affected by m, the intergrowth of LBOA compounds with two constituent structures (generally m and m + 1) alternating along the common c-axis, such as Bi2WO6–Bi3TiNbO9, Bi3TiNbO9–Bi4Ti3O12, and Bi4Ti3O12–Bi5Ti3FeO15,10–19 was designed and synthesized to enable further tailoring of their properties. It is generally believed that these compounds with intergrowth aurivillius phases show better properties compared with non-intergrowth LBOAs. For example, the remanent polarization (2Pr) of Bi3TiNbO9–Bi4Ti3O12 is ∼20.1 μC cm−2 at 423 K, much larger than the values of Bi3TiNbO9 (∼11 μC cm−2) and Bi4Ti3O12 (∼16 μC cm−2).10 In general, the majority of such materials investigated before were ferroelectric.In the ferroelectric Bi3TiNbO9–Bi4Ti3O12 (Bi7Ti4NbO21) system shown in Fig. 1, for example, 3-layered (Bi2Ti3O10)2− and 2-layered (BiTiNbO7)2− arrange alternately between the neighbouring (Bi2O2)2+ layers. The theoretical and property studies of these amazing structures of Bi7Ti4NbO21 have attracted much attention.10,12 Moreover, to intensively modulate the properties of Bi7Ti4NbO21, many types of A-site substitution, such as La, W, Er and Na, were adapted, and the properties of some of these were obviously improved.12,14,16,18,19 Unfortunately, although it may trigger another regulating factor in the properties, substituting the magnetic ions in the B-sites to generate a multiferroic has rarely been studied in the intergrowth of Bi7Ti4NbO21.
In this work, Bi7Ti4−2xCoxNb1+xO21 (x = 0, 0.1, 0.2, 0.3, and 0.4) specimens were successfully synthesized via a solid state reaction. The amounts of the Ti and Nb ions changed simultaneously with Co substitution to keep the charge balance. The effect of Co substitution on the structure of Bi7Ti4−2xCoxNb1+xO21 was investigated in detail, and the ferroelectric and ferromagnetic properties of Bi7Ti4−2xCoxNb1+xO21 were also studied as functions of the Co content.
Experimental section
Polycrystalline Co-substituted Bi7Ti4NbO21 powders were prepared via a conventional solid-state reaction. Appropriate amounts of Bi2O3 (99.99% purity, Jiangsu Gold Wall Reagent Co., China), TiO2 (99.71% purity, Sinopharm Chemical Reagent Co., China), Nb2O5 (99.5% purity, Sinapharm Chemical Reagent Co., China), and Co2O3 (99.98% purity, Jiangsu Gold Wall Reagent Co., China) were mixed in an agate jar, blending with agate balls for more than 24 hours in ethanol. An excess of 5 mol% Bi2O3 was used to compensate for the volatilization of bismuth oxide during the calcination process. After drying at 70 °C for 24 h, the milled powders were heated at 900 °C for 7 h. To fabricate ceramic pellets, the Bi7Ti4NbO21 powders were cold-pressed into disks that were 12 mm in diameter and 0.9 mm thick at a pressure of 150 MPa, and then sintered at 1180 °C for 4 h in air to prepare dense Bi7Ti4−2xCoxNb1+xO21 ceramics.The crystalline structures of the Bi7Ti4−2xCoxNb1+xO21 samples were investigated using powder X-ray diffraction (XRD) with Cu-Kα radiation (TTR-III, Japan). The atomic structures of the samples were visualized using high angle annular dark field aberration corrected scanning transmission electron microscopy (HAADF-STEM, JEM-ARM200F, JEOL, Japan). The microstructures of the fractured samples were observed using scanning electron microscopy (SEM) (JEOL, JSM-6400). Raman spectra were obtained with a SPEX-1403 Laser Raman spectrometer using a 514.5 nm Ar+ laser as an excitation source (LabRamHR, France). For the ferroelectric and dielectric measurements, the pellets were polished to about 0.3 mm in thickness, and then Ag was evaporated on both of the surfaces, which would act as electrodes. Ferroelectric measurements were conducted using a Precision LC ferroelectric analyzer at an applied frequency of 50 Hz (Radiant Technology, USA). The magnetic properties of the samples were characterized by a vibrating sample magnetometer option of the Quantum Design physical property measurement system (PPMS-VSM, Quantum Design, USA).
Results and discussion
XRD and HAADF-STEM
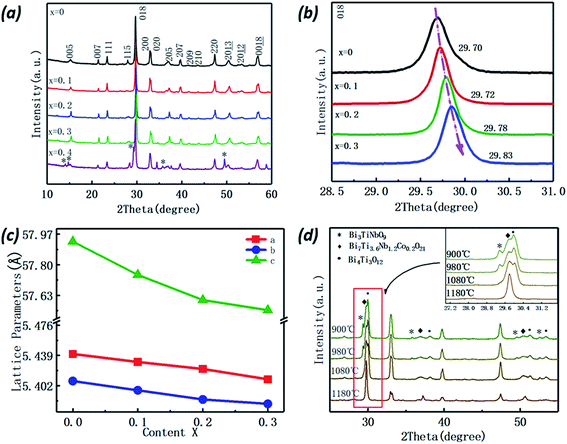
Fig. 2(a) shows the room-temperature XRD patterns of the Bi7Ti4−2xNb1+xCoxO21 (x = 0, 0.1, 0.2, 0.3, and 0.4) pellets sintered at 1180 °C. When x ≤ 0.3, all the XRD diffraction peaks match well to those of Bi7Ti4NbO21with a space group symmetry of I2cm,10 indicating that no secondary phase was detected, whereas the Bi3TiNbO9 phase precipitated when x was set as 0.4. Therefore, in this work, to eliminate the effects from impurities, the properties of Bi7Ti4−2xNb1+xCoxO21 (BTNC) were studied when x ≤ 0.3.Enlarged views of the XRD spectra near the main peak (018) are shown in Fig. 2(b) for the BTNC samples (x ≤ 0.3). It can be clearly seen that the main peak shifts toward the higher angle when increasing the Co content, indicating the reduced lattice parameter c. Rietveld refinements of the XRD patterns were performed with the Material Studio 6.0 program and the refined cell parameters are listed in Table 1 and also shown in Fig. 2(c) as a function of the Co content. It can be clearly seen that all the lattice parameters reduce with an increase of the Co content. Considering the ionic radii of Co3+ (54.5 pm), Nb5+ (64.0 pm) and Ti4+ (60.5 pm), the shrinkage of the lattice parameters should result from the smaller average radius of Co3+ and Nb5+ (59.25 pm), which are substituted as a pair to keep the charge balance.
| x | Lattice parameter (Å) | Unit cell volume (Å3) | Rwp | Rp | ||
|---|---|---|---|---|---|---|
| a | b | c | ||||
| 0 | 5.4408 (3) | 5.4076 (7) | 57.9292 (31) | 1704.40 (71) | 8.26% | 15.35% |
| 0.1 | 5.4309 (8) | 5.3964 (1) | 57.7439 (70) | 1692.34 (91) | 8.78% | 18.44% |
| 0.2 | 5.4224 (7) | 5.3852 (1) | 57.6039 (00) | 1682.10 (20) | 9.64% | 19.91% |
| 0.3 | 5.4098 (7) | 5.3798 (9) | 57.5475 (79) | 1680.71 (60) | 9.43% | 18.37% |
| Bi3TiNbO9 (ref. 20) | 5.4394 | 5.3985 | 25.13 | |||
| Bi4Ti3O12 (ref. 16) | 5.45 | 5.41 | 32.84 | |||
To illustrate the formation process for its 2 + 3 intergrowth structure, the crystalline structures of Bi7Ti3.6Co0.2Nb1.2O21 powders calcined at different temperatures were also investigated. As shown in Fig. 2(d), when calcined at 900 °C, the spectra corresponding to Bi3TiNbO9, Bi4Ti3O12 and Bi7Ti4NbO21 based oxides can be clearly identified. With the enhancement of the calcination temperatures, the peaks corresponding to Bi3TiNbO9 and Bi4Ti3O12 recede and become invisible when calcined at 1180 °C, suggesting that Bi3TiNbO9 and Bi4Ti3O12 based oxides react to form Bi7Ti4NbO21 based complex oxides. It should be noted that the detailed formation procedure for how Bi3TiNbO9 inserts into the Bi4Ti3O12 lattice (or inverse) to form Co substituted Bi7Ti4NbO21 is still unclear and needs intensive investigation.
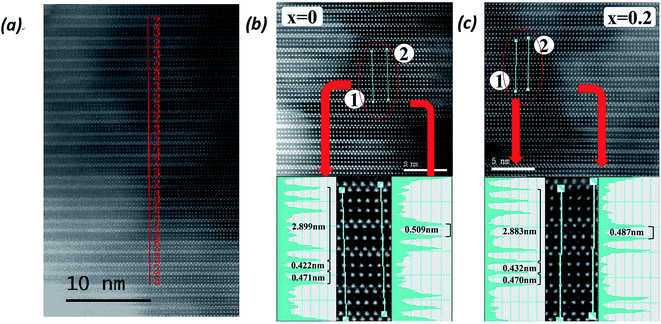
The HAADF-STEM images of the Bi7Ti4−2xCoxNb1+xO21 powders (x = 0 and 0.2) are shown in Fig. 3. It is well known that the brightness of the atoms in the HAADF-STEM images depends on the atomic number. Therefore, the spots representing the Bi atoms are much brighter than those representing all the other atoms. As shown in Fig. 3(a), 2 and 3 layers of the Bi atoms (bright spots) are sandwiched by two closely stacked Bi layers, which present in an orderly and alternating manner, indicating the intergrowth of the two pseudo-perovskite blocks (as indicated in Fig. 1).
To indicate the substitution effect on the atomic structure, enlarged views of the HAADF-STEM images for the x = 0 and x = 0.2 samples are shown in Fig. 3(b) and (c), respectively.33 To this end, a new method to map the brightness scanned along two lines (indicated in the images) is applied to give the clear Bi atom positions. Shown in Fig. 3(b), line 1 (along the c direction) in the Bi7Ti4NbO21 sample starts from the Bi atom in the (Bi2O2)2+ layer and passes through the Bi atoms in the (Bi2Ti3O10)2− block (indicated in Fig. 1); yet, when entering the neighbouring (Bi2O2)2+ layer, line 1 goes between the two adjacent Bi ions caused by the shifted position of Bi in the [Bi2O2]2+ layer, which makes line 1 pass through the dark spots corresponding to the B site ions, such as Ti, Nb, and Co atoms, in the following (BiTiNbO7)2− block, and therefore, the brightness corresponding to line 1 presents 4 strong peaks and then two weak peaks. It should be noted that the two Bi ions in [Bi2O2]2+ only present one peak with a small tail caused by the shifted position of the neighbouring Bi ions. From the peak position along the line, the layer distance in each block can be calculated as c/2 ≈ 2.899 nm, very close to the simulated value of c (c/2 = 5.79292/2 ≈ 2.8965 nm). Importantly, the Bi–Bi distance in the Bi4Ti3O12 layer is found to show great dependence on the positions, suggesting different lengths of the oxygen octahedral along the c direction. As shown in Fig. 3(b), the lengths of the oxygen octahedral are about 0.471 when located close to the (Bi2O2)2+ layer and 0.422 nm when located in the centre of the (Bi2Ti3O10)2− block.
Similarly, line 2 starts from the (Bi2O2)2+ layer and goes between the Bi atoms in the (Bi2Ti3O10)2− block and passing through the Bi atoms in the neighbouring (Bi2Ti3O10)2− block caused position shifts of Bi3+ in the (Bi2O2)2+ layer. Accordingly, the brightness of line 2 presents four weak peaks at first and then three strong peaks. Therefore, the Bi–Bi distance in the c axis can be measured as 0.509 nm in the (BiTiNbO7)2− block, indicating a much longer oxygen octahedral along the c direction than that in the (Bi2Ti3O10)2− block.
While for Bi7Ti3.6Co0.2Nb1.2O21, the Bi–Bi distances in the c axis can be measured as 0.487 nm in the (BiTiNbO7)2− based block and 0.470 and 0.432 nm in the (Bi2Ti3O10)2− based block, respectively, illustrated in Fig. 3(c). Compared to Bi7Ti4NbO21, the main change in the Bi–Bi distance occurs in the (BiTiNbO7)2− block, shrinking from 0.509 to 0.487 nm. This result seems to imply that Co with an ionic radius of 54.5 pm mainly incorporates into the (BiTiNbO7)2− block. One thing to be noted is that the value of c/2 measured in Fig. 3(c) is 2.883 nm for Bi7Ti3.6Co0.2Nb1.2O21, very close to that simulated from the XRD spectra (c/2 ≈ 2.8802 nm). This result further indicates the credibility of this brightness map method.
Raman analysis
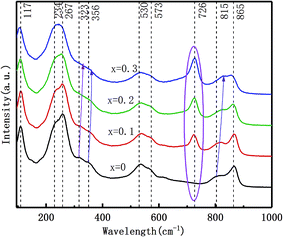
Raman spectroscopy was used to investigate the Co substitution effect on the structure of Bi7Ti4NbO21. As shown in Fig. 4, for Bi7Ti4NbO21 (x = 0), the Raman modes at 117, 234, 267, 323, 356, 530, 573, 815 and 865 cm−1 can be clearly observed. Previous studies11,21,31,32 indicated that the bands below 200 cm−1 should originate from the vibration of the heavy mass Bi3+ ions, and the bands above 200 cm−1 are ascribed to the TiO6 and NbO6 octahedrals.23,29 Therefore, the 117 cm−1 bands should result from the vibration of the Bi3+ ions at the A-sites in the perovskite-like layer;23the modes at 234 and 267 cm−1 represent the twisting vibrations of the O–Ti–O or O–(Ti, Nb)–O structures; the 323 and 356 cm−1 modes correspond to the different directions of the O–Ti–O or O–(Ti, Nb)–O bending in the a–b plane;24,28 the modes located at 530 and 573 cm−1 21 are attributed to the relative vibration of the O atoms at the top of the TiO6 octahedral; and the bands at 815 and 865 cm−1 represent the symmetric stretching of the (Ti, Nb)O6 and TiO6 octahedrals, respectively.11,24,28–30For the Co-doped specimens, the Raman spectra have similar shapes to that of Bi7Ti4NbO21, of which the original peaks still exist in spite of the little peak shifts or intensity changes. A new mode at 726 cm−1 appears and increases substantially with the increase of the Co content in the Bi7Ti4−2xCoxNb1+xO21 samples. This mode may be caused by the vibration of the CoO6 octahedra, which can be observed in the Raman spectra of LaCoO3 and La0.8Sr0.2CoO3. This result clearly indicates that the Co ions have entered the perovskite slab.22 It should also be noted that the two modes above 800 cm−1 show different dependence on the Co substitution: the mode at 815 cm−1 shifts to higher frequency, while the mode at 865 cm−1 does not shift obviously. This result may suggest that cobalt substitution in the specimens mainly impacts the (Ti, Nb)O6 octahedral in the [Bi3TiNbO9] layer instead of the TiO6 octahedral in the Bi4Ti3O12 lattice. This result is in good agreement with the HAADF-STEM image analysis.
The SEM results of Bi7Ti4−2xNb1+xCoxO21 (x = 0, 0.1, 0.2, and 0.3) are shown in Fig. 5. It can clearly be seen that all the samples are dense and suitable for later ferroelectric and ferromagnetic study.
 | ||
| Fig. 5 The fractured microstructure of Bi7Ti4−2xNb1+xCoxO21 with (a) x = 0, (b) x = 0.1, (c) x = 0.2, and (d) x = 0.3. | ||
Ferroelectric properties
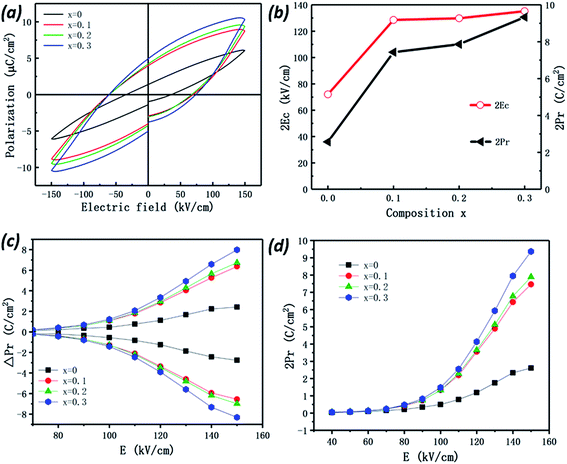
The room-temperature polarization–electric field (P–E) hysteresis loops of all the Bi7Ti4−2xCoxNb1+xO21 samples are shown in Fig. 6(a), and the resulting remanent polarization (2Pr) and coercive field (2Ec) measured under a field of 150 kV cm−1 are shown in Fig. 6(b) as a function of the Co content (x) in the samples.Measured at 150 kV cm−1, the remanent polarization (2Pr) of x = 0 is ∼2.57 μC cm−2, which has good consistency with the previous report.34 As shown in Fig. 6(b), with the increase of the Co content, both 2Pr and the coercive fields (2Ec) increase sharply first, then slowly, and reach about 9.33 μC cm−2 and 135.23 kV cm−1 for x = 0.3, larger than those of Bi5.5Nd1.5Ti4NbO21 (2Pr ∼ 8 μC cm−2 and 2Ec ∼ 90 kV cm−1).34
To exclude a possible artificial polarization from the contribution of leakage current, pulsed polarization positive-up negative-down (PUND) measurements were performed and the results are shown in Fig. 6(c). The pulsed remanent polarizations ΔP (switched polarization − non-switched polarization) of the samples have similar Co-content (x) dependence with 2Pr determined from the P–E loops (shown in Fig. 6(d)). Measured at 150 kV cm−1, the values of ΔP are 2.42 and 6.98 μC cm−2 for x = 0 and x = 0.2, respectively, in good accordance with those values of 2Pr. This result indicates that the polarization of the samples arises mainly from the intrinsic materials and rarely from leakage current contributions.25,26 As a result of this, the increase of the 2Pr value with cobalt incorporation should be attributed to the structural evolution (lattice shrinkage). As indicated in Table 1, with Co substitution in Bi3TiNbO9–Bi4Ti3O12, the lattice of Bi7Ti4−2xNb1+xCoxO21 shrinks greatly, to have much lower lattice parameters than those of pure Bi3TiNbO9 (ref. 20) and Bi4Ti3O12.16 Considering that Co is mainly incorporated into the (Bi3TiNbO9) layer, a large amount of tension will be applied to the (Bi4Ti3O12) layer to make the lattice shrink accordingly. This will introduce large lattice distortion and should be the main reason for the observed enlarged values of Pr and Ec in the ferroelectric measurements.
Magnetic properties
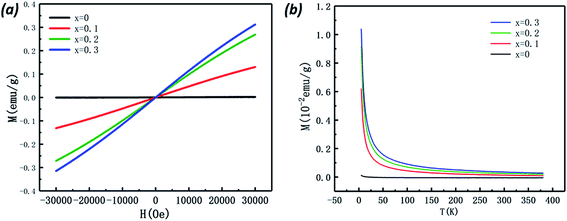
Fig. 7(a) shows the magnetic hysteresis loops of the Bi7Ti4−2xCoxNb1+xO21 (x = 0, 0.1, 0.2, 0.3) ceramics measured at 10 K. When x = 0, a diamagnetism characteristic is observed; while in the case of the Co-doped specimens, the loops show magnetic nature, which increases with the Co content. The temperature dependence of magnetization for the Bi7Ti4−2xCoxNb1+xO21 (x = 0, 0.1, 0.2, and 0.3) samples is shown in Fig. 7(b). It can be clearly seen that the magnetic moment increased with the heavily Co doped samples. There was no phase transition or glass state appearing within the measured temperature range.To get more information about the magnetic properties of the Co-doped specimens, we use the Curie–Weiss law to explore the magnetic interaction and effective magnetic moment (Meff) of the samples.25–27 According to the Curie–Weiss law, the magnetic susceptibility (χ) of a paramagnetic substance complies with the following formula (1):
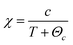 | (1) |
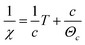 | (2) |
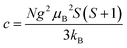 | (3) |
The effective magnetic moment is the function of the spin angular momentum and therefore can be related to the Curie–Weiss constant, as shown in eqn (4):
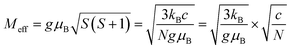 | (4) |
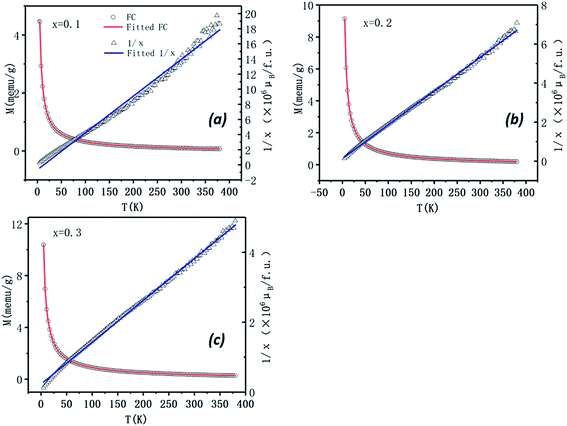
According to Fig. 8 the effective magnetic moment can be calculated as 1.12 μB, 1.12 μB, and 1.14 μB in the x = 0.1, 0.2, and 0.3 specimens, respectively. The similar Meff values suggest that the cobalt atoms in the materials have almost the same efficient moment in those samples.
Conclusions
(1) Co atoms can be a substitution for Ti atoms in the Bi7Ti4NbO21 lattice without destroying the structure.(2) Because of the different radii of the Co, Nb and Ti ions, the lattice of the 2 + 3 intergrowth aurivillius complex oxide shrinks after Co insertion, which mainly occurs in the (BiTiNbO7)2− slab.
(3) The ferroelectric properties of Co substituted Bi7Ti4NbO21 improve dramatically, with 2Pr increasing from 2.57 μC cm−2 (x = 0) to more than 9.33 μC cm−2 (x = 0.3).
(4) The original Bi7TI4NbO21 was a diamagnetic compound. With Co substitution, Bi7Ti4−2xCoxNb1+xO21 (x = 0.1, 0.2, and 0.3) presents paramagnetic property.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Key R&D Program of China (2016YFA0401004), the Chinese Universities Scientific Fund (CUSF, WK2310000055), the Anhui Provincial Natural Science Foundation (P. R. China, No. 1608085QE91), and the External Cooperation Program of BIC (Chinese Academy of Sciences, No. 211134KYSB20130017).References
- Y. Moritomo, A. Asamitsu, H. Kuwahara and Y. Tokura, Nature, 1996, 380, 141–144 CrossRef CAS.
- M. Fiebig, J. Phys. D: Appl. Phys., 2005, 38, R123–R152 CrossRef CAS.
- K. F. Wang, J. M. Liu and Z. F. Ren, Adv. Phys., 2009, 58, 321–448 CrossRef CAS.
- N. A. Spaldin, S.-W. Cheong and R. Ramesh, Phys. Today, 2010, 63, 38–43 CrossRef.
- S. Ikegami and I. Ueda, Jpn. J. Appl. Phys., 1974, 13, 1572–1577 CrossRef CAS.
- B. Park, B. Kang, S. Bu, T. Noh, J. Lee and W. Jo, Nature, 1999, 401, 682–684 CrossRef CAS.
- M. Maeder, D. Damjanovic and N. Setter, J. Electroceram., 2004, 13, 385–392 CrossRef CAS.
- T. Takenaka and H. Nagata, J. Eur. Ceram. Soc., 2005, 25, 2693–2700 CrossRef CAS.
- S. Zhang, F. Yu and D. J. Green, J. Am. Ceram. Soc., 2011, 94, 3153–3170 CrossRef CAS.
- Z. G. Yi, Y. Wang, Y. X. Li and Q. R. Yin, J. Appl. Phys., 2006, 99, 114101 CrossRef.
- W. Wang, S.-P. Gu, X.-Y. Mao and X.-B. Chen, J. Appl. Phys., 2007, 102, 024102 CrossRef.
- X. Gao, H. Gu, Y. X. Li, Z. G. Yi, M. Čeh and K. Žagar, J. Mater. Sci., 2011, 46, 5423–5431 CrossRef CAS.
- Z. G. Yi, Y. X. Li, Q. B. Yang and Q. R. Yin, Ceram. Int., 2008, 34, 735–739 CrossRef CAS.
- H. Ogawa, D. Iida, S. Takahashi, T. Moriyama and A. Kan, Ferroelectrics, 2016, 498, 1–11 CrossRef CAS.
- L. Fei, Z. Zhou, S. Hui and X. Dong, Ceram. Int., 2015, 41, 9729–9733 CrossRef CAS.
- X. Tian, S. Qu, B. Wang, H. Du and Z. Xu, J. Inorg. Organomet. Polym. Mater., 2013, 24, 355–359 CrossRef.
- S. Nakashima, H. Fujisawa, S. Ichikawa, J. M. Park, T. Kanashima, M. Okuyama and M. Shimizu, J. Appl. Phys., 2010, 108, 074106 CrossRef.
- H. Zhang, H. Yan and M. J. Reece, J. Appl. Phys., 2010, 107, 104111 CrossRef.
- H. Zou, J. Li, Q. Cao, X. Wang, X. Hui, Y. Li, Y. Yu and X. Yao, J. Adv. Dielectr., 2014, 04, 1450028 CrossRef CAS.
- Z. Zhang, H. Yan, X. Dong and Y. Wang, Mater. Res. Bull., 2003, 38, 241–248 CrossRef CAS.
- Y. L. Du, M. S. Zhang, Q. Chen, Z. R. Yuan, Z. Yin and Q. A. Zhang, Solid State Commun., 2002, 124, 113–118 CrossRef CAS.
- X. Li, Z. Y. Peng, W. Fan, K. Guo, J. M. Gu, M. Y. Zhao and J. F. Meng, Mater. Chem. Phys., 1996, 46, 50–54 CrossRef CAS.
- J. Zhu, X.-Y. Mao and X.-B. Chen, Acta Phys. Sin., 2004, 53, 3929–3933 CAS.
- J. Zhu, X.-B. Chen, J.-h. He and J.-C. Shen, Phys. Lett. A, 2007, 362, 471–475 CrossRef CAS.
- Y. Huang, S. Sun, G. Wang, J. Wang, R. Peng and Y. Lu, RSC Adv., 2014, 4, 29264 RSC.
- J. Wang, L. Li, R. Peng, Z. Fu, M. Liu, Y. Lu and X. Tan, J. Am. Ceram. Soc., 2015, 98, 1528–1535 CrossRef CAS.
- D. L. Zhang, W. C. Huang, Z. W. Chen, W. B. Zhao, L. Feng, M. Li, Y. W. Yin, S. N. Dong and X. G. Li, Sci. Rep., 2017, 7, 43540 CrossRef CAS PubMed.
- P. J. Klar and T. Rentschler, Solid State Commun., 1997, 103, 341–345 CrossRef CAS.
- X. Hu, W. Wang, X. Mao and X. Chen, Magnetic and electric properties of co-doped Bi5Ti3FeO15 multiferroic ceramics, Chinese Physical Society, 2010, vol. 59, pp. 8160–8107 Search PubMed.
- Q. Li, J. Wang, M. Li, S. Guo, J. Zhang, Z. Hu, Z. Zhou, G. Wang, X. Dong and J. Chu, Phys. Rev. B, 2017, 96, 024101–024110 CrossRef.
- I. Saitoh and S. Kojima, Ferroelectrics, 1998, 217, 83–89 CrossRef CAS.
- R. E. Melgarejo, M. S. Tomar, A. Hidalgo and R. S. Katiyar, Ferroelectrics, 2010, 269, 297–302 CrossRef.
- Y. M. Kim, J. He, M. D. Biegalski, H. Ambaye, V. Lauter, H. M. Christen, S. T. Pantelides, S. J. Pennycook, S. V. Kalinin and A. Y. Borisevich, Nat. Mater., 2012, 11, 888–894 CrossRef CAS PubMed.
- C. Shao, Y. Lu, D. Wang and Y. Li, J. Eur. Ceram. Soc., 2012, 32, 3781–3789 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2017 |