Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reply to the ‘Comments on “Experimental and theoretical studies of the nanostructured {Fe3O4@SiO2@(CH2)3Im}C(CN)3 catalyst for 2-amino-3-cyanopyridine preparation via an anomeric based oxidation”, RSC Adv., 2016, 6, 50100–50111, and “The first computational study for the oxidative aromatization of pyrazolines and 1,4-dihydropyridines using 1,2,4-triazolinediones: an anomeric-based oxidation”, RSC Adv., 2016, 6, 102280–102291’ by S. Salehzadeh, RSC Adv., 2017, 7, 39704–39707, DOI: 10.1039/c6ra27033f†
Avat (Arman) Taherpour*ac and
Mohammad Ali Zolfigol *b
*b
aDepartment of Organic Chemistry, Faculty of Chemistry, Razi University, P.O.Box: 67149-67346, Kermanshah, Iran. E-mail: avatarman.taherpour@gmail.com
bFaculty of Chemistry, Bu-Ali Sina University, Hamedan, Iran. E-mail: zolfi@basu.ac.ir; mzolfigol@yahoo.com
cMedical Biology Research Center, Kermanshah University of Medical Sciences, Kermanshah, Iran
First published on 21st November 2017
Abstract
This response to Dr Salehzadeh’s comments on the papers mentioned in the title contains the comments where they have mentioned disagreement with basic chemistry concepts. The response to the comments include: (i) the experimental (X-ray) and theoretical reported results of the epimerism and the differences in the stereoisomer properties, as they are definitely not pair of enantiomers, and (ii) a discussion regarding internal molecular orbital (MO) electron transfer.
Introduction
Anomeric Based Oxidation (ABO) has opened up a new synthetic and theoretical branch of oxidation to make organic molecules where some of them have profound medicinal and biological activities.1–5 In this response to the comments of Dr Salehzadeh on the two recently published papers by Zolfigol et al.,1–3 we report on a study including a response to the main basis of the “Comments”6 problems to provide clarification for readers and to avoid confusion on the basic concepts. The items have been responded to one by one and some more advantages were added to the subject of our studies. Fortunately, the subject of the two papers and the other related published papers has interesting advantages relating to ABO phenomena, the MBO method and stereochemistry topics. These advantages were developed here in the response to the mentioned comment.1. The experimental (X-ray) and theoretical reported results of the epimerism and the differences in the stereoisomer properties
Amine molecules with different substituent groups on the pyramidal N atom (R1R2R3N; R1 ≠ R2 ≠ R3) show chiral properties.7 The barrier energy of the two enantiomers of this type of amine could be between low and high energies depending on the R1, R2 and R3 properties and the character of the molecular structures. The barrier energy of the two enantiomers would be high if there was a main steric restraint and strong hindering effect on the N atom due to the structure becoming planar with sp2 hybridization.7 The interconversion between the enantiomers would stop if the lone pair of the N atom became protonated and/or the sp2 N could not become planar because of the above mentioned reasons. See Fig. 1. The two forms will be diastereomers if another chiral centre (stereogenic centre) appears in the molecule.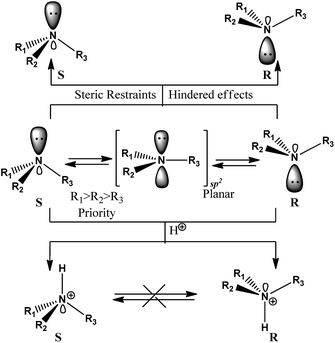 | ||
| Fig. 1 Interconversion of N-pyramidal atoms in enantiomeric amine derivatives and the restriction effects on the interconversion process. | ||
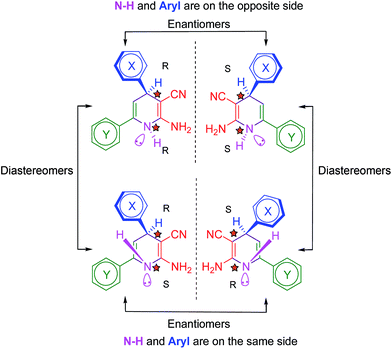
In this case, as there are two chiral centres, the two stereoisomers are epimeric diastereomers (and they are not enantiomers). This type of diastereomer has different chemical, energetic and structural properties such as differences in thermodynamic energy levels, kinetic properties, HOMO & LUMO energy levels, ΔEHOMO−LUMO, dipole moments and optical activities.8
The experimental (X-ray) and theoretical computational calculations have confirmed that the N atoms in the structures of 1,4-dihydro-4-phenylpyridine derivatives have pyramidal geometries. See the X-ray results for some 1,4-dihydro-4-phenylpyridine derivatives reported in 2016 by Prasad and Begum in the ESI.†9,10a,b In the X-ray results the N atoms are not planar (pyramidal form) and just one of the stereoisomers (diastereomer) was represented.
The two compounds that the author6 has pointed out are not enantiomers and they are essentially diastereomers (epimers). The main point is that in the structures of the epimer isomers the N atom shows pyramidality. So, the two structures are not mirror pictures of each other. As can be seen in Fig. 3 of the paper “RSC Adv., 2016, 6, 50100–50111”,2 there are different orientations of the N atoms in the two R and S isomers of intermediate 7. So, for “7” there are two epimers and not two enantiomers. Because of the different chemical properties of the “epimers” they have different kinetic and thermodynamic properties and this has been completely explained in the paper “RSC Adv., 2016, 6, 50100–50111”.2 Fig. 2 demonstrates the possible diastereomer structures (CRNR, CRNS, CSNS and CSNR). The pyramidality of the structures has been calculated in the optimized structure using the DFT-B3LYP/6-31G** method. The pyramidality differences between the sp2 N and sp3 N (and NH3 and *NHMeEt; * = chiral amine) were obtained to be about 57° and 40°, respectively. This topic, i.e. the conversion of the epimers to each other, is one of the new aspects in these studies.
Due to the existence of a nitrogen atom within the structure that possesses four different substituents, the molecule has two identified stereogenic centres so the existence of diastereomers is likely. It is clear that the diastereomers are quite different in terms of their chemical and physical properties. So it is not surprising that the investigated intermediates S and R are different from a chemical stability standpoint. Due to pyramidal inversion at the N chiral centres, we have considered only the C chiral centre. See Fig. 2.
Fig. 3 demonstrates the effect that a Lewis acid has on the chiral N atoms (and also –NH2 as an achiral functional group). It can be seen that the relationship between structures A′–D′ (after the addition of the Lewis acid on the N atoms) is the same as the relationship between structures A–D. Upon addition of a Lewis acid the chirality of the N1 atom would be rigid and the chirality of this atom would be clearer. In this case, the C4 carbon atoms retain their chirality states. The structures of A–D and A′–D′ are shown in Fig. 3 and the stereochemical relationships between the structures are shown in the box of Fig. 3.
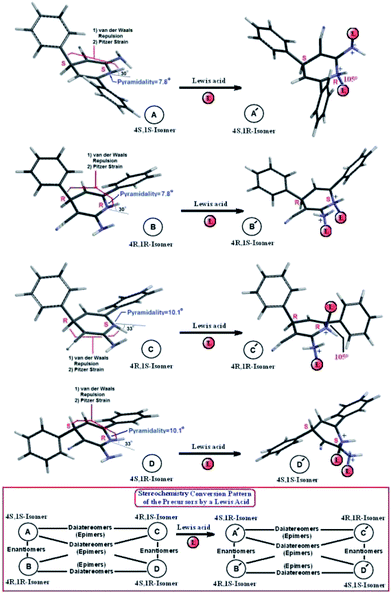 | ||
| Fig. 3 The structures of A–D (precursors) and A′–D′ (after Lewis acid addition). The stereochemical relationships between the structures A–D and A′–D′ are shown in the box. The possible epimer structures (CRNR; CRNS; CSNS; CSNR). The van der Waals repulsion and Pitzer strain effects.2 The pyramidality and angle deviations of the N chiral atoms from sp2 hybridization. | ||
Fig. 3 also shows the pyramidalities and the angle deviations of the chiral N atoms in the possible diastereomer structures (CRNR; CRNS; CSNS; CSNR). The pyramidalities for CSNS, CRNR, CRNS and CSNR were obtained as 7.8, 10.1, 10.1 and 7.9°, respectively. The angle deviations of the chiral N atoms from sp2 hybridization for CSNS, CRNR, CRNS and CSNR were obtained as: 30, 33, 33 and 30°, respectively. The van der Waals repulsions and Pitzer strains11 between N–H and C–H and/or C–Ph bonds are the main components, along with other substituent group effects, for the construction of the A–D forms of the epimers.
The calculated relative energy between CSNS and CSNR (A and D; as the most stable epimers) was zero. The calculated relative energies between CSNS and/or CSNR (A and D) with CRNR (B) and CRNS (C) were 0.31 and 3.61 kcal mol−1, respectively. The domain of the barrier energies for the N-inversion process are about 8–12 kcal mol−1. The N-inversion process in the interconversion process of the epimers were calculated about 10.5 kcal mol−1, respectively, by DFT-B3LYP/6-31G** method. The energy level differences, barrier to the N-inversion in the interconversion process of the epimers, pyramidality and other structural and thermodynamic properties will change by changing the calculation method. However, the concepts of the discussed properties will stay the same. So, overall, in contradiction to Dr Salehzadeh’s comments, the structures are not enantiomers and they are inherently structural diastereomers (epimers). The zero energy differences between the two CSNS and CSNR (A and D) epimers do not mean that they are structurally the same. They are different and they are diastereomers. In the 1,4-dihydro-4-phenylpyridine derivatives the N–H group achieves a planar and sp2 geometry (zwitterionic radical) after hν irradiation (as a useful dyad).10b To achieve the epimers in an experimental attempt one could apply a simple Lewis acid (for example D+ and not H+) to make the rigid structures and extract them. The X-ray results8,9 (as discussed) have shown just a pyramidal geometry for the N atom in the obtained crystals.
In the first paragraph of part 1 of the “Comments”6 the author has mentioned that: “Indeed, the energies of enantiomers can be different, in the femto-joule to pico-joule per mole range, only because of a parity violation.4 However, recently Zolfigol et al. during the study on the intermediate molecule shown in Fig. 1 have reported that the S isomer is, about 0.29 kcal mol−1 (≈1200 J mol−1), more stable than the R one”.6 In 2017, Juaristi et al. have reported an interesting study about stereoelectronic interactions as a probe for the existence of the intramolecular α-effect.12 In Table S4 (ESI)† of their investigation the calculated differences in ΔE and ΔG for the axial/equatorial conformational equilibria in some compounds with and without an anomeric effect were reported.12 In some cases the differences in ΔE and ΔG for the axial/equatorial conformations were trivial, so that in some conformers the reported calculated energy differences using the MP2/6-311+G(d,p) level of theory were between 0.1 and 0.3 kcal mol−1.12 In our study, the reported difference between the two diastereomers was 0.29 (≈0.3) kcal mol−1. We have introduced the data with two decimal numbers in kcal mol−1 (obviously, these data in our study were in kcal mol−1, and not in femto-joule to pico-joule per mole), so, the mentioned phrases do not have any relationship with our study and, secondly, as it was completely discussed the zero energy difference between the two diastereomers does not mean that they are same. The 0.29 kcal mol−1 value is another confirmation for their differences and by using another QM method (which calculates the steric restraint, van der Waals repulsions, Pitzer strains and other important effects with higher accuracy) we could see larger energy differences between these two structures (see the interpretations). It is obvious that the two diastereomers have different TS energies (different ΔG#) in reactions with chiral reagents. In the introduction section the author of the “Comments”6 also mentioned that “the authors have proved that the transition state (TS) for R isomer is, about 4.5 kcal mol−1 (≈19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 J mol−1), more stable than S one while we remember that these chiral molecules have reacted with an achiral ion.”6 The author of the “Comments”6 should note to this point that the difference energy (4.5 kcal mol−1) is related to the two different TS forms of the ABO reactions of the two diastereomers (with the special stereospecific structure of {Fe3O4@SiO2@(CH2)3Im}). In the next step of the reaction the intermediate reacts with the achiral −C(CN)3 anion. In addition to the other discussed reasons related to the small differences in the energy levels, there are some studies in the literature that show that the small amounts of energy (up to about 0.2–0.5 kcal mol−1) are determinative of the mechanism patterns, TS and/or the results of the studies.13
000 J mol−1), more stable than S one while we remember that these chiral molecules have reacted with an achiral ion.”6 The author of the “Comments”6 should note to this point that the difference energy (4.5 kcal mol−1) is related to the two different TS forms of the ABO reactions of the two diastereomers (with the special stereospecific structure of {Fe3O4@SiO2@(CH2)3Im}). In the next step of the reaction the intermediate reacts with the achiral −C(CN)3 anion. In addition to the other discussed reasons related to the small differences in the energy levels, there are some studies in the literature that show that the small amounts of energy (up to about 0.2–0.5 kcal mol−1) are determinative of the mechanism patterns, TS and/or the results of the studies.13
In the paper “RSC Adv., 2016, 6, 50100–50111”2 the R and S signs were applied to introduce just the chiral position of the anomeric chiral centre (to identify the structures) and we have noticed to this point that they are definitely diastereomers (epimers) which is in agreement with the discussion. In Fig. 1 of the “Comments” the author of the “Comments” showed the “schematic representation of chiral carbon in the compound studied by Zolfigol et al. (left)” and the molecular structures of the predicted R and S stereoisomers6 (right). The figures on the right side obviously have pyramidal and chiral N* atoms and they agree with the above interpretations (in contradiction to Dr Salehzadeh’s comments) that they are epimer diastereomers and not enantiomers.
All of the concepts and signs applied agree with standard texts and the IUPAC Gold book and are shown in Table S4 in the ESI† section.14–18 As shown in Table S4 in the ESI† section, arrows 1 and 2 represent electron transfer (in contradiction to Dr Salehzadeh’s comments). Fig. 3 in the text of the “Comments”6 was not in the main papers.1–4 Fig. 3 of Dr Salehzadeh’s comments has incorrectly presented tautomers interconversion as an “equilibrium between different structural isomers”.6 To the best of our knowledge, most of the structural isomers are detectable and/or separable from each other, whereas here the anionic tautomers are inseparable from each other (Fig. 4).
 | ||
| Fig. 4 Anionic tautomers interconversion is the correct phrase for this phenomenon and it is not an “equilibrium between different structural isomers”. | ||
2. Discussion regarding internal molecular orbital (MO) electron transfer
Our explanation is obviously related to the ET process between the MO of the discussed molecules (such as nN2 → π*C3C4 in the ET from nN2 and π*C3C4 and nN2 → π*C3C4 in the ET from nN2 to π*C3C4) and not between one atom/molecule/ion to another one. The final result of such an internal MO-ET process is the production of Hδ− and the separation of H− from the discussed molecules in the ABO reactions. So, in addition to the above explanation, as one can see, in agreement with Marcus theory,19 there is an outer sphere ET process to produce Hδ− and then the separation of H− from the discussed molecules occurs in the ABO reactions. Furthermore, an electron transfer phrase for the ET between MO orbitals was applied in the important primary and secondary sources. These concepts and our aims are too obvious so we do not need to do more interpretation about them.Conclusion
The “Comments”6 on the papers “RSC Adv., 2016, 6, 50100–50111” and “RSC Adv., 2016, 6, 102280–102291” were investigated. On the basis of the experimental (X-ray) and theoretical reported results, the epimerism and effect on the stereoisomer differences were discussed in part 1. The results have confirmed that the structures of the discussed 1,4-dihydro-4-phenylpyridine derivatives are epimer diastereomers (C and N pyramidal atoms; CRNR, CRNS, CSNS and CSNR). In part 2, we explained the internal molecular orbital (MO) electron transfer on the basis of Marcus theory and its classification for ET processes. In agreement with Marcus theory, there is an outer sphere MO-ET process to produce Hδ− and then the separation of H− from the discussed molecules occurs in the ABO reactions.20Conflicts of interest
There are no conflicts to declare.Notes and references
- M. A. Zolfigol, M. Kiafar, M. Yarie, A. Taherpour and M. Saeidi-Rad, RSC Adv., 2016, 6, 50100–50111 RSC.
- M. Kiafar, M. A. Zolfigol, M. Yarie and A. Taherpour, RSC Adv., 2016, 6, 102280–102291 RSC.
- M. Kiafar, M. A. Zolfigol, M. Yarie and A. Taherpour, RSC Adv., 2016, 6, 107295 RSC.
- M. A. Zolfigol, M. Kiafar, M. Yarie, A. A. Taherpour, T. Fellowes, A. N. Hancok and A. Yari, J. Mol. Struct., 2017, 1137, 674–680 CrossRef CAS.
- M. Yari, Iran. J. Catal., 2017, 7, 85–88 Search PubMed.
- S. Salehzadeh, RSC Adv., 2017, 7, 39704–39707 RSC.
- (a) Nitrogen Inversion in regards to Stereochemistry, http://web.chem.ucla.edu/%7Eharding/ec_tutorials/tutorial05.pdf; (b) J. M. Lehn, Nitrogen inversion; Experiment and theory, Dynamic Stereochemistry, Part of the Fortschritte der Chemischen Forschung book series (TOPCURRCHEM, volume 15/3) Conference paper, 2006, pp. 311–377 Search PubMed; (c) K. P. C. Vollhardt and N. E. Schore, Organic Chemistry, W. H. Freeman and Company, New York, USA, 6th edn, 2009 Search PubMed; (d) Illustrated Glossary of Organic Chemistry, Nitrogen Inversion part, http://web.chem.ucla.edu/%7Eharding/IGOC/N/nitrogen_inversion.html; (e) W. H. Brown, C. S. Foote, B. L. Iverson, E. V. Anslyn and B. M. Novak, Organic Chemistry, Brooks/Cole, Cengage Learning, Belmont, CA, USA, 6th edn, 2012, p. 119 Search PubMed.
- F. A. Carey and R. J. Sundberg, Advanced Organic Chemistry-Part A, Kluwer Academic/Plenum Publishers, New York, USA, 4th edn, 2000, pp. 103, and 131 (lines 2 and 3) Search PubMed.
- (a) E. V. Anslyn and D. A. Dougherty, Modern Physical Organic Chemistry, University Science Books, Sausalito, California, USA, 2006 Search PubMed; (b) M. Abdoli-Senejani, A. A. Taherpour, H. R. Memarian and M. Khosravani, Struct. Chem., 2013, 24, 191–200 CrossRef CAS.
- (a) N. L. Prasad and N. S. Begum, IUCrData, 2016, 1, x160722 Search PubMed; (b) N. A. Al-Awadi, M. R. Ibrahim, M. H. Elnagdi, E. John and Y. A. Ibrahim, Beilstein J. Org. Chem., 2012, 8, 441–447 CrossRef CAS PubMed.
- (a) IUPAC, Gold Book, Bond opposition strain, Pitzer strain, Torsional strain, https://goldbook.iupac.org/html/E/E01886.html; (b) G. Quinkert, E. Egert and C. Greisinger, Aspects of Organic Chemistry; Structure, VCH, Verlag, New York, USA, 1996, p. 95 Search PubMed.
- E. Juaristi, G. P. Gomes, A. O. Terent’ev, R. Notario and I. V. Alabugin, J. Am. Chem. Soc., 2017, 139, 10799–10813 CrossRef CAS PubMed.
- (a) V. I. Minkin, B. Y. Simkin and R. M. Minyaev, Quantum Chemistry of Organic Compounds, Mechanisms of Reactions, Chapter, Low-Energy Barrier Reactions. Structural Modeling, Springer, Berlin, Heidelberg, 1990, pp. 181–189 Search PubMed; (b) I. Yavari, A. A. Taherpour and M. Dadgar, J. Mol. Struct.: THEOCHEM, 1998, 422, 213–218 CrossRef CAS; (c) I. Yavari and A. A. Taherpour, J. Mol. Struct.: THEOCHEM, 1999, 488, 141–149 CrossRef CAS.
- IUPAC, Gold Book, mesomerism, https://goldbook.iupac.org/html/M/M03845.html.
- IUPAC, Gold Book, resonance, https://goldbook.iupac.org/html/R/R05326.html.
- IUPAC, Gold Book, canonical forms, https://goldbook.iupac.org/html/C/C01309.html.
- IUPAC, Gold Book, contributing structures, https://goldbook.iupac.org/plain/C01309-plain.html.
- P. J. Taylor, G. van der Zwan and L. Antonov, Tautomerism: Methods and Theories, Edition: First, Chapter: Tautomerism: Introduction, History, and Recent Developments in Experimental and Theoretical Methods, ed. L. Antonov, Wiley-VCH, 2014, pp. 1–24 Search PubMed.
- (a) R. A. Marcus, Rev. Mod. Phys., 1993, 65, 599–610 CrossRef CAS; (b) M. Andrea, Marcus Theory for Electron Transfer a short introduction, MPIP-Journal Club-Mainz, January 2008, p. 29 Search PubMed; (c) P. F. Barbara, J. Phys. Chem., 1996, 100, 13148–13161 CrossRef CAS; (d) M. D. Newton, Chem. Rev., 1991, 91, 350 CrossRef; (e) J. Jortner and K. F. Freed, J. Chem. Phys., 1970, 52, 6272–6291 CrossRef; (f) R. A. Marcus, J. Chem. Phys., 1965, 43, 679 CrossRef CAS; (g) R. A. Marcus and N. Sutin, Biochim. Biophys. Acta, 1985, 811, 265 CrossRef CAS; (h) M. G. Kuzmin, XVIIth IUPAC Symposium on Photochemistry, Book of Abstracts, Dresden, German, 22–27 July 2000, p. 372 Search PubMed.
- Note: The calculated data in ref. 2–4 were performed by licensed Spartan ’10, build 1.0.1, Wavefunction Inc., Irvine, 2011.
Footnote |
| † Electronic supplementary information (ESI) available: The X-ray crystals relating to the 1,4-dihydro-4-phenylpyridine derivatives and also some educational reported results and related figures are shown in the ESI (S1–S3). The standard definition of the main arrows, which were applied, are shown in the ESI (S4). See DOI: 10.1039/c7ra10812e |
| This journal is © The Royal Society of Chemistry 2017 |