Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Anion exchange coupled with the reduction and dimerisation of a copper(II) nitrate complex of tripyridyl dithioether via a single-crystal-to-single-crystal transformation†
Hyeong-Hwan
Lee
a,
In-Hyeok
Park
 *a,
Seulgi
Kim
a,
Eunji
Lee
a,
Huiyeong
Ju
a,
Jong Hwa
Jung
a,
Mari
Ikeda
b,
Yoichi
Habata
*a,
Seulgi
Kim
a,
Eunji
Lee
a,
Huiyeong
Ju
a,
Jong Hwa
Jung
a,
Mari
Ikeda
b,
Yoichi
Habata
 c and
Shim Sung
Lee
c and
Shim Sung
Lee
 *a
*a
aDepartment of Chemistry, Research Institute of Natural Science, Gyeongsang National University, Jinju 52828, South Korea. E-mail: pihghost@nate.com; sslee@gnu.ac.kr; Tel: +82 55-772-1483
bEducation Center, Faculty of Engineering, Chiba Institute of Technology, 2-1-1 Shibazono, Narashino, Chiba 275-0023, Japan
cDepartment of Chemistry, Toho University, 2-2-1 Miyama, Funabashi, Chiba 274-8510, Japan
First published on 3rd January 2017
Abstract
It is a challenge to develop methodologies involving multiple transformations for preparing new materials that cannot be obtained via direct synthesis. Herein, we report an anion exchange process accompanying cation reduction and dimerisation via a single-crystal-to-single-crystal transformation. First, a direct reaction of the flexible tripyridyl dithioether ligand L with CuI afforded a mixture of four bis(ligand) complexes (1a–1d). To avoid the formation of undesired mixed products, a copper(II) nitrate complex-mediated approach involving anion exchange and cation reduction was employed to generate a monomeric complex, [CuII(L)NO3]NO3·toluene (2). When the dark blue crystals of 2 were immersed in an aqueous NaI solution, the crystals were transformed to a pale yellow dimeric copper(I) iodide complex, [(μ-CuI2I2)(L)2] (3). The observed anion exchange promotes the reduction of copper(II) to copper(I) at the expense of I−/I3− oxidation as well as dimerisation via the formation of a Cu2I2 cluster. This result corresponds to the synthesis of a compound that otherwise was not able to be prepared via a direct synthetic procedure.
Introduction
Post-synthetic modification (PSM) or post-assembly modification (PAM) via a single-crystal-to-single-crystal (SCSC) transformation has proven to be a powerful tool, not only for creating new materials, but also for understanding the mechanistic details of their formation.1 Hence, the construction of extended solid materials via an external exchange process (such as an SCSC guest-exchange) has been of considerable interest because it can result in translocation/exchange of atoms and ions, in particular in solid matrices.2The PSM approach involving multiple molecular processes offers a relatively unexplored field for obtaining novel materials. However, in some cases anion exchange has been shown to be accompanied by bond breaking and/or bond making. The study of this behaviour involving metal-anion coordination has become an important emerging field due to its relevance to biological processes, environmental pollution, ionic liquids, catalysis, lithium batteries and health related areas.3 On the other hand, the SCSC transformations, involving the chemical reduction of a metal centre, provide a potentially important modification approach for generating new materials.4
In our recent study, direct reaction of tripyridyl dithioether [L, 2,6-bis(2-pyridylsulfanylmethyl)pyridine, Fig. 1] with copper(I) iodide was shown to form a mixture of four complexes that reflects the flexible nature of the ligand (see later). To avoid the formation of these mixed products, we have investigated a system that involves simultaneous anion exchange coupled with the reduction of a metal centre, as depicted in Fig. 1. The observed process appears to be the first example of the simultaneous exchange of an anion species and change of the metal oxidation state of the central metal in a coordination compound accompanying an SCSC transformation.
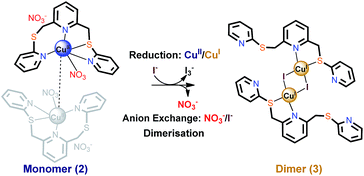 | ||
| Fig. 1 Anion exchange of copper(II) nitrate complex accompanying reduction and dimerisation via a SCSC transformation. | ||
Results and discussion
Reaction of the required precursor thiol and ditosylate in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio in the presence of potassium carbonate under reflux resulted in the formation of Lvia C–S bond formation (yield, 80%; Fig. S1†). The procedure employed in the present study showed improved yields over the related literature method for L.5 The reaction of L with copper(I) iodide to yield the mixture of four crystalline products (1a–1d in Fig. 2, S2 and S3†) was carried out in acetonitrile/dichloromethane. Single crystal X-ray diffraction (SC-XRD) analyses confirm that 1a–1d have similar structures, each adopting a bis(ligand) complex arrangement that is linked by a stepped cubane cluster (Cu4I4) to yield [(Cu4I4)(L)2] (1a), [(Cu4I4)(L)2] (1b), [(Cu4I4)(L)2]·CH2Cl2 (1c), and [(Cu4I4)(L)2]·2CH2Cl2 (1d). The metal complex units in 1a–1d are composed of identical building blocks; namely, two L ligands and one bridging Cu4I4 cluster with an ‘open’ cubane form. Thus, the overall conformational differences in 1a–1d mainly originate from the presence of solvent and changes in the coordinated ligand conformation (Fig. S3–S9†). Further, in 1a–1c, each L is tridentate, whereas it is tetradentate in 1d.
1 molar ratio in the presence of potassium carbonate under reflux resulted in the formation of Lvia C–S bond formation (yield, 80%; Fig. S1†). The procedure employed in the present study showed improved yields over the related literature method for L.5 The reaction of L with copper(I) iodide to yield the mixture of four crystalline products (1a–1d in Fig. 2, S2 and S3†) was carried out in acetonitrile/dichloromethane. Single crystal X-ray diffraction (SC-XRD) analyses confirm that 1a–1d have similar structures, each adopting a bis(ligand) complex arrangement that is linked by a stepped cubane cluster (Cu4I4) to yield [(Cu4I4)(L)2] (1a), [(Cu4I4)(L)2] (1b), [(Cu4I4)(L)2]·CH2Cl2 (1c), and [(Cu4I4)(L)2]·2CH2Cl2 (1d). The metal complex units in 1a–1d are composed of identical building blocks; namely, two L ligands and one bridging Cu4I4 cluster with an ‘open’ cubane form. Thus, the overall conformational differences in 1a–1d mainly originate from the presence of solvent and changes in the coordinated ligand conformation (Fig. S3–S9†). Further, in 1a–1c, each L is tridentate, whereas it is tetradentate in 1d.
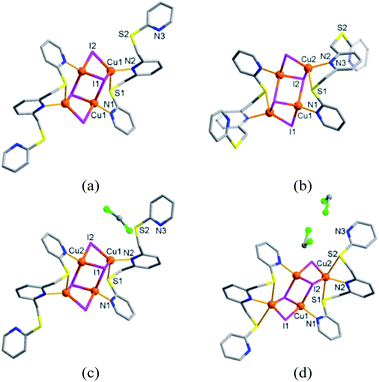 | ||
| Fig. 2 Crystal structures of (a) 1a, [(Cu4I4)(L)2], (b) 1b, [(Cu4I4)(L)2], (c) 1c, [(Cu4I4)(L)2]·CH2Cl2, and (d) 1d, [(Cu4I4)(L)2]·2CH2Cl2. | ||
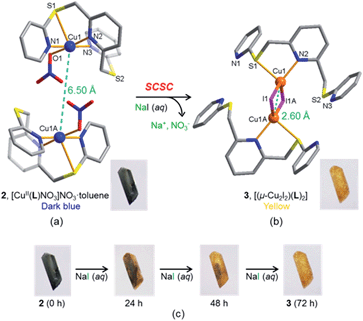
As an alternative, a Cu(II) complex-mediated two-step approach was employed. In the first step, copper(II) nitrate was reacted with L in acetonitrile/dichloromethane to yield the dark blue crystalline product 2 (Fig. 3a). SC-XRD analysis revealed that 2 is a typical mononuclear copper(II) complex of type [CuII(L)NO3]NO3·toluene, in which one nitrate ion coordinates, whereas the other resides in the lattice together with the toluene molecule. Complex 2 crystallised in the monoclinic space group C2/c with Z = 8. The copper(II) centre is in a pentacoordinated N3OS environment and can be best described as a square-pyramidal geometry (τ value: 0.02),6 with three pyridyl N atoms from L and one O atom from nitrate in the square plane, with the apical position occupied by a S atom from L. The Cu1–S1 distance is elongated at 2.690 Å (Fig. S10 and S11†). The experimental and simulated powder X-ray diffraction (PXRD) patterns for 2 confirmed that this product is homogeneous (Fig. S12 and S13†).
When the dark blue single crystals of 2 were immersed in a 3 M aqueous solution of NaI for four days, the size and shape of the daughter crystals 3 retained those of 2. While retention of single crystallinity was maintained, the colour changed from dark blue (2) to pale yellow (3, see Fig. 3c). Surprisingly, the SC-XRD analysis revealed that 3 is a bis(ligand) copper(I) iodide complex of type [(μ-CuI2I2)(L)2] in which two Cu(I) centres are bridged by two I− ions.
Unlike its precursor 2, a perspective view of 3 (Fig. 3b) shows that each copper(I) centre is four-coordinated, being bound to one pyridine N atom and one S atom from one L ligand. The two remaining sites are occupied by two iodide ions giving rise to a distorted tetrahedral geometry. The most striking structural feature of 3 is its dimeric form linked by a CuI2I2 square cluster. The associated change in the Cu1⋯Cu1A distance from 6.50 Å to 2.60 Å in going from 2 to 3 is remarkable in the solid state.
Note that the anion-induced SCSC transformation from 2 to 3 involves the rearrangement of the coordination sphere as well as framework distortion with, as mentioned already, a redox process associated with this change. Indeed, only a limited number of examples are reported where a complete structural change on anion exchange occurs in the solid state.7 In these previous cases, the structural conversion mainly involved bond breaking and/or bond making of the ligand/anion bond, without significant changes in the location of the metal centres and ligands.
When the solid copper(II) nitrate complex 2 is immersed in 3 M sodium iodide aqueous solution, it is proposed that the reaction presented in eqn (1) takes place. The nitrate ions in 2 are replaced by I− ions to generate both CuI and I2, with the reduced copper(I) iodide aggregating to afford a Cu2I2 unit. In the presence of surplus I−, I2 will be converted to I3−.8 The liberated I−, I2, and I3− were monitored by ESI-mass spectrometry (Fig. S14†).
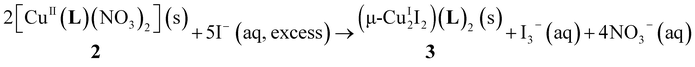 | (1) |
In 3, the individual monomeric complex units are connected through Cu–(I)2–Cu bonds, to form its dimeric structure. The structural change involves the conversion of monomer to dimer and the reduction of the coordination number from five to four at each copper centre. Note that the approach employed is also influenced by the match between the hardness or softness of the anion (Lewis base) and the metal centre (Lewis acid). That is, upon exposure to an aqueous solution of NaI, the relatively hard Cu2+ Lewis acid is reduced to soft Cu+, and then the hard base NO3− ion is easily exchanged by the soft I− ion.
The experimental and simulated PXRD patterns of 3 confirmed its homogeneous nature (Fig. S8†), indicating that complete SCSC transformation had occurred. PXRD patterns and IR spectra were obtained for the same samples before and after anion exchange in the solid state (Fig. 4). Both results are in agreement that the complete anion exchange has occurred. The elemental mapping by the energy dispersive spectroscopy (EDS) with SEM for both compounds also shows that 3 contains I atom as one component (Fig. S15†).
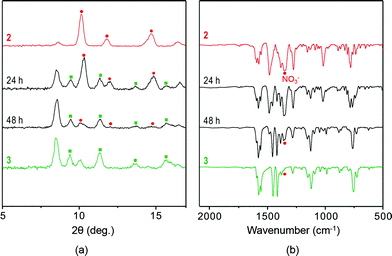 | ||
| Fig. 4 (a) PXRD patterns and (b) IR spectra of 2 (top) before and (bottom) after anion exchange employing 3 M NaI aqueous solution. | ||
To investigate any possible crystal morphology and other variation, single crystal samples before and after anion exchange were used for atomic force microscopy (AFM) investigations. The results indicate that the crystal surface profile undergoes significant alteration, implying a restructuring of the crystal surface (Fig. 5 and S16†). After 24 and 48 h, for example, the relatively homogenous and flat crystal surface of the original crystal (Fig. 5a) becomes rough, showing holes and clefts (Fig. 5b and c). However, after 72 h, the surface of the anion exchanged sample had become more regularly ordered and consisted of microcrystallites (Fig. 5d). These observations are typical for a solvent-mediated transformation,9 during which a new crystalline phase is generated on the surface.
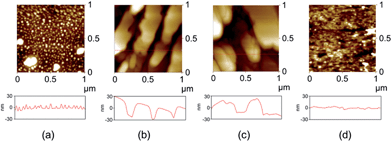 | ||
| Fig. 5 AFM images and height profiles of the surface of a single crystal of 2 before and after immersing in a 3 M NaI aqueous solution: (a) before anion exchange, (b) 24 h, (c) 48 h, and (d) 72 h. | ||
The anion exchange process was monitored by UV-vis spectroscopy at intervals based on the maximum adsorption peak of I3− at 352 nm.10 As shown in Fig. 6 and S17,† several single crystals of 2 plus 3 M NaI aqueous solution (3 mL) were added to a standard (1 cm) UV-vis quartz cell. Subsequently, the mother solution of the sample in the cell slowly changed from colourless to light yellow/brown (see the images in Fig. 6), implying the formation of I3−, as shown in eqn (1). The absorbance due to I3− increases with time until it reaches a maximum (36 h) where the time-dependent plot levels out.
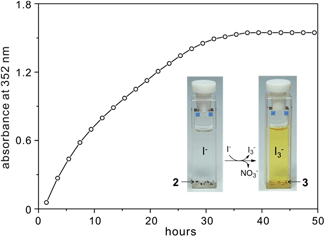 | ||
| Fig. 6 Time-dependent profile for NO3− release from 2 by detecting I3− in the supernatant NaI aqueous solution. | ||
In ‘control’ experiments, we found that no anion exchange or reduction occurred when we attempted the same procedure in aqueous NaCl or NaBr solution. Instead, when the dark blue single crystals of the mononuclear copper(II) nitrate complex 2 were exposed to 3 M NaCl aqueous solution or distilled water, on careful observation, the dark blue colour was seen to gradually disappear, finally turning colourless, leading to the recovery of single crystals of L (Fig. S18–S20†). In the TGA data, 3 shows a higher thermal stability than 2, which decomposes around 130 °C after the loss of the lattice solvent (Fig. S21†).
Conclusions
As far as we are aware, the present study is the first example of an anion exchange process coupled with the reduction and dimerisation of a metal centre of a complex occurring during an SCSC transformation. However, it is also clear that more work is required to understand the fundamental aspects governing the unique multiple transformation observed in this study. It is expected that this facile approach might provide a promising and efficient post-synthetic strategy for practical use in the future.Acknowledgements
This work was supported by the NRF (2012R1A4A1027750 and 2016R1A2A2A05918799), S. Korea.Notes and references
- (a) M. Meilikhov, S. Furukawa, K. Hirai, R. A. Fischer and S. Kitagawa, Angew. Chem., Int. Ed., 2013, 52, 341 CrossRef CAS PubMed; (b) H. Kitagawa, H. Ohtsu and M. Kawano, Angew. Chem., Int. Ed., 2013, 52, 12395 CrossRef CAS PubMed; (c) S. M. Cohen, Chem. Rev., 2012, 112, 970 CrossRef CAS PubMed; (d) E. D. Bloch, D. Britt, C. Lee, C. J. Doonan, F. J. Uribe-Romo, H. Furukawa, J. R. Long and O. M. Yaghi, J. Am. Chem. Soc., 2010, 132, 14382 CrossRef CAS PubMed; (e) T. K. Ronson, B. S. Pilgrim and J. R. Nitschke, J. Am. Chem. Soc., 2016, 138, 10417 CrossRef CAS PubMed; (f) D. A. Roberts, B. S. Pilgrim, J. D. Cooper, T. K. Ronson, S. Zarra and J. R. Nitschke, J. Am. Chem. Soc., 2015, 137, 10068 CrossRef CAS PubMed; (g) O. Oisaki, Q. Li, H. Furukawa, A. C. Czaja and O. M. Yaghi, J. Am. Chem. Soc., 2010, 132, 9262 CrossRef PubMed; (h) Z. Wang and S. M. Cohen, J. Am. Chem. Soc., 2007, 129, 12368 CrossRef CAS PubMed; (i) Z. Wang and S. M. Cohen, Chem. Soc. Rev., 2009, 38, 72 Search PubMed; (j) T. Gadzikwa, O. K. Farha, C. D. Malliakas, M. G. Kanatzidis, J. T. Hupp and S. T. Nguyen, J. Am. Chem. Soc., 2009, 131, 13613 CrossRef CAS PubMed; (k) A. D. Burrows, C. G. Frost, M. F. Mahon and C. Richardson, Angew. Chem., Int. Ed., 2008, 47, 8482 CrossRef CAS PubMed; (l) C. J. Doonan, W. Morris, H. Furukawa and O. M. Yaghi, J. Am. Chem. Soc., 2009, 131, 9492 CrossRef CAS PubMed; (m) K. M. L. Taylor-Pashow, J. D. Rocca, Z. Xie, S. Tran and W. Lin, J. Am. Chem. Soc., 2009, 131, 14261 CrossRef CAS PubMed; (n) I.-H. Park, R. Medishetty, H.-H. Lee, C. E. Mulijanto, H. S. Quah, S. S. Lee and J. J. Vittal, Angew. Chem., Int. Ed., 2015, 54, 7313 CrossRef CAS PubMed; (o) R. J. Marshall, S. L. Griffin, C. Wilson and R. S. Forgan, J. Am. Chem. Soc., 2015, 137, 9527 CrossRef CAS PubMed; (p) I.-H. Park, R. Medishetty, J.-Y. Kim, S. S. Lee and J. J. Vittal, Angew. Chem., Int. Ed., 2014, 53, 5591 CrossRef CAS PubMed; (q) I.-H. Park, A. Chanthapally, Z. Zhang, S. S. Lee, M. J. Zaworotko and J. J. Vittal, Angew. Chem., Int. Ed., 2014, 53, 414 CrossRef CAS PubMed; (r) R. Hota, K. Baek, G. Yun, Y. Kim, H. Jung, K. M. Park, E. Yoon, T. Joo, J. Kang, C. G. Park, S. M. Bae, W. S. Ahn and K. Kim, Chem. Sci., 2013, 4, 339 RSC; (s) Z. Zhang, W.-Y. Gao, L. Wojtas, S. Ma, M. Eddaoudi and M. J. Zaworotko, Angew. Chem., Int. Ed., 2012, 51, 9330 CrossRef CAS PubMed; (t) J. Y. Lee, H. J. Kim, J. H. Jung, W. Sim and S. S. Lee, J. Am. Chem. Soc., 2008, 130, 13838 CrossRef CAS PubMed; (u) T. H. Kim, Y. W. Shin, J. H. Jung, J. S. Kim and J. Kim, Angew. Chem., Int. Ed., 2008, 47, 685 CrossRef CAS PubMed; (v) J. Y. Lee, S. Y. Lee, W. Sim, K.-M. Park, J. Kim and S. S. Lee, J. Am. Chem. Soc., 2008, 130, 6902 CrossRef CAS PubMed; (w) B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1989, 111, 5962 CrossRef CAS; (x) Y.-H. Kiang, G. B. Gardner, S. Lee, Z. Xu and E. B. Lobkovsky, J. Am. Chem. Soc., 1999, 121, 8204 CrossRef CAS.
- (a) T.-Y. Ho, S.-M. Huang, J.-Y. Wu, K.-C. Hsu and K.-L. Lu, Cryst. Growth Des., 2015, 15, 4266 CrossRef CAS; (b) R. Podgajny, S. Choraży, W. Nitek, A. Budziak, M. Rams, C. J. Gomez-García, M. Oszajca, W. Łasocha and B. Sieklucka, Cryst. Growth Des., 2011, 11, 3866 CrossRef CAS; (c) J. Seo, R. Matsuda, H. Sakamoto, C. Bonneau and S. Kitagawa, J. Am. Chem. Soc., 2009, 131, 12792 CrossRef CAS PubMed; (d) T. Haneda, M. Kawano, T. Kawamichi and M. Fujita, J. Am. Chem. Soc., 2008, 130, 1578 CrossRef CAS PubMed; (e) S. Shimomura, R. Matsuda, T. Tsujino, T. Kawamura and S. Kitagawa, J. Am. Chem. Soc., 2006, 128, 16416 CrossRef CAS PubMed; (f) H. J. Choi and M. P. Suh, J. Am. Chem. Soc., 2004, 126, 15844 CrossRef CAS PubMed; (g) B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1990, 112, 1546 CrossRef CAS; (h) S. Muthu, J. H. K. Yip and J. J. Vittal, J. Chem. Soc., Dalton Trans., 2002, 4561 RSC; (i) B. Li, Y. Zhang, D. Ma, Z. Xing, T. Ma, Z. Shi, X. Ji and S. Ma, Chem. Sci., 2016, 7, 2138 RSC; (j) Y. Wang, J.-M. Yi, M.-Y. Zhang, P. Xu and X.-J. Zhao, Chem. Commun., 2016, 52, 3099 RSC; (k) Y. Wang, W. Jia, R. Chen, X.-J. Zhao and Z.-L. Wang, Chem. Commun., 2017, 53, 636 RSC.
- (a) G. Li, H. Yang, F. Li, F. Cheng, W. Shi, J. Chen and P. Cheng, Inorg. Chem., 2016, 55, 4935 CrossRef CAS PubMed; (b) J.-Y. Wu, Y.-C. Liu and T.-C. Chao, Inorg. Chem., 2014, 53, 5581 CrossRef CAS PubMed; (c) K. Chen, L. Huang, B. Yan, H. Li, H. Sun and J. Bi, Environ. Sci. Technol., 2014, 48, 12930 CrossRef CAS PubMed; (d) F. D. Monica, E. Luciano, G. Roviello, A. Grassi and S. Milione, Macromolecules, 2014, 47, 2830 CrossRef; (e) Q. Liu, L. Yu, Y. Wang, Y. Ji, J. Horvat, M.-L. Cheng, X. Jia and G. Wang, Inorg. Chem., 2013, 52, 2817 CrossRef CAS PubMed; (f) A. Noro, S. Matsushima, X. He, M. Hayashi and Y. Matsushita, Macromolecules, 2013, 46, 8304 CrossRef CAS; (g) D. Gelman and S. Musa, Catal., 2012, 2, 2456 CAS; (h) P. Nockemann, B. Thijs, S. Pittois, J. Thoen, C. Glorieux, K. V. Hecke, L. V. Meervelt, B. Kirchner and K. Binnemans, J. Phys. Chem. B, 2006, 110, 20978 CrossRef CAS PubMed; (i) J. Brunner, A. Mokhir and R. Kraemer, J. Am. Chem. Soc., 2003, 125, 12410 CrossRef CAS PubMed; (j) T. Dudev, J. A. Cowan and C. Lim, J. Am. Chem. Soc., 1999, 121, 7665 CrossRef CAS.
- P. B. Chatterjee, A. Audhya, S. Bhattacharya, S. M. T. Abtab, K. Bhattacharya and M. Chaudhury, J. Am. Chem. Soc., 2010, 132, 15842 CrossRef CAS PubMed.
- A. Rotondo, G. Bruno, F. Nicoló, G. Tresoldi and S. D. Pietro, Acta Crystallogr., 2010, C66, o15–o19 Search PubMed.
- A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn and G. C. Verschoor, J. Chem. Soc., Dalton Trans., 1984, 1349 RSC.
- (a) O.-S. Jung, Y. J. Kim, Y.-A. Lee, K.-M. Park and S. S. Lee, Inorg. Chem., 2003, 42, 844 CrossRef CAS PubMed; (b) C. Fang, Q.-K. Liu, J.-P. Ma and Y.-B. Dong, Inorg. Chem., 2012, 51, 3923 CrossRef CAS PubMed; (c) E. Lee, K.-M. Park, M. Ikeda, S. Kuwahara, Y. Habata and S. S. Lee, Inorg. Chem., 2015, 54, 5372 CrossRef CAS PubMed.
- Y. Liu, R. L. Sheaffer and J. R. Barker, J. Phys. Chem. A, 2003, 107, 10296 CrossRef CAS.
- (a) W. Wei, H. Yu, F. Jiang, B. Liu, J. Ma and M. Hong, CrystEngComm, 2012, 14, 1693 RSC; (b) H. Yang, L. Li, J. Wu, H. Hou, B. Xiao and Y. Fan, Chem.–Eur. J., 2009, 15, 4049 CrossRef CAS PubMed; (c) C. Thompson, N. R. Champness, A. N. Khlobystov, C. J. Roberts, M. Schröer, S. J. B. Tendler and M. J. Wilkinson, J. Microsc., 2004, 214, 261 CrossRef CAS PubMed; (d) A. N. Khlobystov, N. R. Champness, C. J. Roberts, S. J. B. Tendler, C. Thompson and M. Schröder, CrystEngComm, 2002, 4, 426 RSC; (e) J. Booth, R. G. Compton and J. H. Atherton, J. Phys. Chem. B, 1998, 102, 3980 CrossRef CAS; (f) J. Booth, Q. Hong, R. G. Compton, K. Prout and R. M. Payne, J. Colloid Interface Sci., 1997, 192, 207 CrossRef CAS PubMed.
- (a) A. E. Burgess and J. C. Davidson, J. Chem. Educ., 2012, 89, 814 CrossRef CAS; (b) R. L. G. N. P. Silva, A. F. de Oliveira and E. A. Neves, J. Braz. Chem. Soc., 1998, 9, 171 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: PXRD patterns, ESI-MS spectrum, SEM images, AFM images, UV spectra, crystal structures and TGA data. CCDC 1511571–1511577. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6sc05341f |
| This journal is © The Royal Society of Chemistry 2017 |