Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A matrix of heterobimetallic complexes for interrogation of hydrogen evolution reaction electrocatalysts†
Pokhraj
Ghosh
a,
Shengda
Ding
a,
Rachel B.
Chupik
a,
Manuel
Quiroz
a,
Chung-Hung
Hsieh
b,
Nattami
Bhuvanesh
a,
Michael B.
Hall
 a and
Marcetta Y.
Darensbourg
a and
Marcetta Y.
Darensbourg
 *a
*a
aDepartment of Chemistry, Texas A & M University, College Station, TX 77843, USA. E-mail: marcetta@chem.tamu.edu
bDepartment of Chemistry, Tamkang University, New Taipei City, Taiwan 25157
First published on 12th October 2017
Abstract
Experimental and computational studies address key questions in a structure–function analysis of bioinspired electrocatalysts for the HER. Combinations of NiN2S2 or [(NO)Fe]N2S2 as donors to (η5-C5H5)Fe(CO)+ or [Fe(NO)2]+/0 generate a series of four bimetallics, gradually “softened” by increasing nitrosylation, from 0 to 3, by the non-innocent NO ligands. The nitrosylated NiFe complexes are isolated and structurally characterized in two redox levels, demonstrating required features of electrocatalysis. Computational modeling of experimental structures and likely transient intermediates that connect the electrochemical events find roles for electron delocalization by NO, as well as Fe–S bond dissociation that produce a terminal thiolate as pendant base well positioned to facilitate proton uptake and transfer. Dihydrogen formation is via proton/hydride coupling by internal S–H+⋯−H–Fe units of the “harder” bimetallic arrangements with more localized electron density, while softer units convert H−⋯H−via reductive elimination from two Fe–H deriving from the highly delocalized, doubly reduced [Fe2(NO)3]− derivative. Computational studies also account for the inactivity of a Ni2Fe complex resulting from entanglement of added H+ in a pinched –Sδ−⋯H+⋯δ−S− arrangement.
Introduction
From protein crystallography the bimetallic active site structures in enzymes such as [NiFe]-, [FeFe]-hydrogenases (H2ase), CO-dehydrogenases and acetyl coA synthase (ACS) have been convincingly interpreted in terms of characteristics needed for their organometallic-like functions.1,2 Whereas most major homogeneous catalytic applications involving redox processes use precious metals that can operate as single site catalysts, the intricate molecular arrangements in nature's biocatalysts harness combinations of at least two first row transition metals, connected by sulfides or thiolates, along with Lewis acid/base sites.3–5 Over the past two decades a rich area in synthetic chemistry inspired by such natural products has developed, yielding biomimetics for insight into enzyme mechanisms. In addition the link between the [NiFe]- and [FeFe]-H2ase active sites and base metal, sustainable catalysts for the Hydrogen Evolution Reaction (HER) holds promise for the production of H2 from “solar” (photovoltaic) electrons via electrocatalysis.6 Specific efforts have been directed towards the use of metallodithiolates from MN2S2 complexes as bidentate donor ligands (readily deduced from the structure of the ACS enzyme active site), that bind to receiver metal units via bridging dithiolates.7–25 The electronic requirements of the thiolate sulfurs have a steric consequence in the butterfly M(μ-SR)2M′ cores that are seen in the H2ase active sites, placing M and M′ within close proximity.26The advancement of chemistry via structure/function analysis of sets of compounds with well-known differences in composition and structure is a challenge in the complicated area of HER electrocatalysis. Nonetheless the metallodithiolate-as-synthon approach, inspired from the ACS active site, permits modular design that includes some features of the bimetallic [NiFe]- and [FeFe]-H2ase active sites beyond the obvious dithiolate core structures. An initial foray explored the properties of the diiron, trinitrosyl complex shown in Fig. 1.8,27 With it we intended to exploit the redox-activity of 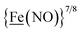 in the
in the 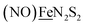 metalloligand bound to a redox-active {Fe(NO)2}9/10, iron dinitrosyl unit. Electrochemical studies of
metalloligand bound to a redox-active {Fe(NO)2}9/10, iron dinitrosyl unit. Electrochemical studies of 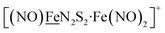 ,
, 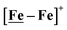 , Fig. 1, found two single-electron, reversible reduction events, −0.78 V and −1.33 V, assigned to {Fe(NO)2}9/10 and
, Fig. 1, found two single-electron, reversible reduction events, −0.78 V and −1.33 V, assigned to {Fe(NO)2}9/10 and 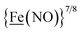 couples, respectively.8 The Fe of the
couples, respectively.8 The Fe of the 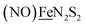 metalloligand is herein distinguished as
metalloligand is herein distinguished as 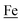 ; the electron count of the iron nitrosyl units uses the Enemark–Feltham approach.28 Consistent with the stoichiometric reaction shown in Fig. 1, the {Fe(NO)2}9/10 couple, at −0.78 V, was the catalytically active center for electrochemical proton reduction in the presence of strong acid, HBF4·Et2O. Although modest in overpotential and TOF, electrocatalysis for H2 production was observed at this potential; preliminary computational studies indicated that a hydride-bound {Fe(NO)2}8 could likely be a transient intermediate, however the overall H2 releasing mechanism was at that stage incomplete.8 Interestingly, the second reduction process, related to the more negative
; the electron count of the iron nitrosyl units uses the Enemark–Feltham approach.28 Consistent with the stoichiometric reaction shown in Fig. 1, the {Fe(NO)2}9/10 couple, at −0.78 V, was the catalytically active center for electrochemical proton reduction in the presence of strong acid, HBF4·Et2O. Although modest in overpotential and TOF, electrocatalysis for H2 production was observed at this potential; preliminary computational studies indicated that a hydride-bound {Fe(NO)2}8 could likely be a transient intermediate, however the overall H2 releasing mechanism was at that stage incomplete.8 Interestingly, the second reduction process, related to the more negative 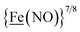 couple, showed a current response to added weak acid, however H2 was not detected. Computational study attributed this to a non-productive reduction event with protonation on the nitrosyl, which terminates the catalytic cycle.8
couple, showed a current response to added weak acid, however H2 was not detected. Computational study attributed this to a non-productive reduction event with protonation on the nitrosyl, which terminates the catalytic cycle.8
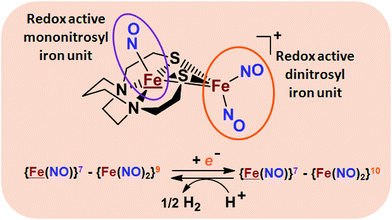 | ||
Fig. 1 Structure and redox activity of 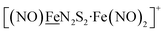 , , 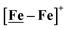 ; protonation of the one-electron reduced diiron complex yields H2.8 ; protonation of the one-electron reduced diiron complex yields H2.8 | ||
We have made analogues of the diirion trinitrosyl complex making use of NiN2S2 and 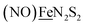 metallodithiolates in combination with η5-C5R5 derivatives (R = H, CH3),9,10 of FeII shown in Fig. 2. The large differences in reduction potential of the MN2S2 ligands, with the d8-NiII being more negative because of a more rigid, less polarizable electronic structure as compared to the delocalized
metallodithiolates in combination with η5-C5R5 derivatives (R = H, CH3),9,10 of FeII shown in Fig. 2. The large differences in reduction potential of the MN2S2 ligands, with the d8-NiII being more negative because of a more rigid, less polarizable electronic structure as compared to the delocalized 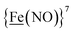 unit, of greater electronic flexibility, inspired the labels of “hard” for the former MN2S2 unit, and “soft” S-donor unit for the latter. The Fe-receivers also differ in electronic flexibility and their ease of electron uptake, the term “soft” describing the highly delocalized {Fe(NO)2}9 unit, and the indefinite oxidation state of the iron, as compared to the definite FeII of the η5-C5R5, “hard” receiver derivatives.8,9 The hard receiver unit, (η5-C5H5)Fe(CO)+, is herein distinguished from the soft Fe(NO)2 unit by Fe′ and Fe, respectively.
unit, of greater electronic flexibility, inspired the labels of “hard” for the former MN2S2 unit, and “soft” S-donor unit for the latter. The Fe-receivers also differ in electronic flexibility and their ease of electron uptake, the term “soft” describing the highly delocalized {Fe(NO)2}9 unit, and the indefinite oxidation state of the iron, as compared to the definite FeII of the η5-C5R5, “hard” receiver derivatives.8,9 The hard receiver unit, (η5-C5H5)Fe(CO)+, is herein distinguished from the soft Fe(NO)2 unit by Fe′ and Fe, respectively.
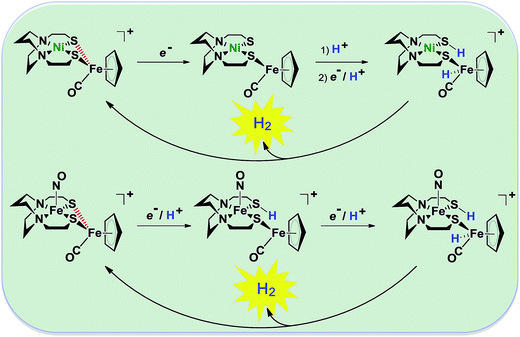 | ||
| Fig. 2 Abbreviated computational mechanisms for electrocatalysis of H2 production by the [Fe–Fe′]+ and [Ni–Fe′]+ electrocatalysts.9 Shown in red is the Fe–S bond that undergoes reductive bond cleavage. | ||
Notable from the computational approach that guided the interpretation of electrochemical events of the [Ni–Fe′]+ and 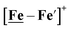 complexes in the presence of acid was the indication of a reductive iron–sulfur bond cleavage (shown in red, Fig. 2) that converted the bidentate dithiolate into a monodentate S-donor, thus creating a transient frustrated Lewis pair, i.e., yielding reactive sites for proton and electron uptake on the free thiolate and the open site on iron, respectively, see Fig. 2.9 In this way, complexes, that do not have an amine pendant base for proton uptake and storage, or open sites on iron for a hydride, as in the [FeFe]-H2ase active site,29–33 adjust their structures to accommodate coupled electron/proton uptake. While the mild potential for the first EC process for the
complexes in the presence of acid was the indication of a reductive iron–sulfur bond cleavage (shown in red, Fig. 2) that converted the bidentate dithiolate into a monodentate S-donor, thus creating a transient frustrated Lewis pair, i.e., yielding reactive sites for proton and electron uptake on the free thiolate and the open site on iron, respectively, see Fig. 2.9 In this way, complexes, that do not have an amine pendant base for proton uptake and storage, or open sites on iron for a hydride, as in the [FeFe]-H2ase active site,29–33 adjust their structures to accommodate coupled electron/proton uptake. While the mild potential for the first EC process for the 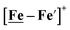 complex required both proton/electron uptake for genesis of the pendant base, the more negative potential that reduces the [Ni–Fe′]+ labilizes the sulfur and creates an FeIII–H at the first reduction, Fig. 2.8,9
complex required both proton/electron uptake for genesis of the pendant base, the more negative potential that reduces the [Ni–Fe′]+ labilizes the sulfur and creates an FeIII–H at the first reduction, Fig. 2.8,9
In this report we provide a more complete matrix of MN2S2–Fe complexes of electrocatalytic potential for experimental and computational analysis. Specifically a redox innocent (“hard”) metalloligand, NiN2S2, of more negative reduction potential, is incorporated in place of “soft” 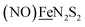 . The thus generated [NiIIN2S2·Fe(NO)2]0/+, a “hard”/“soft” complex may be compared to the other members of the matrix. The solid state structures of [NiIIN2S2·Fe(NO)2]0/+ in two redox levels and characteristics as an electrocatalyst (robustness and turnover frequency), for proton reduction are also described. Computational study, addresses the diversity of geometries of di- and poly-metallic compounds containing N2S2 metalloligands by inspecting the versatile bonding orbitals of the metalloligands. The computational mechanisms contrast the working electrocatalysts against a non-working analogue by exploring possible intermediates in the proposed catalytic cycles. Here important roles for hemi-labile and redox active ligands are revealed.
. The thus generated [NiIIN2S2·Fe(NO)2]0/+, a “hard”/“soft” complex may be compared to the other members of the matrix. The solid state structures of [NiIIN2S2·Fe(NO)2]0/+ in two redox levels and characteristics as an electrocatalyst (robustness and turnover frequency), for proton reduction are also described. Computational study, addresses the diversity of geometries of di- and poly-metallic compounds containing N2S2 metalloligands by inspecting the versatile bonding orbitals of the metalloligands. The computational mechanisms contrast the working electrocatalysts against a non-working analogue by exploring possible intermediates in the proposed catalytic cycles. Here important roles for hemi-labile and redox active ligands are revealed.
Results and discussion
Synthesis and characterization
Shown in Scheme 1A are the synthetic routes to NiN2S2·Fe(NO)2, [Ni–Fe]0, and its one-electron oxidized analogue, [Ni–Fe]+, isolated and crystallographically characterized as a dimer, [NiN2S2·Fe(NO)2]22+ or [Ni2–Fe2]2+, (N2S2 = N,N-bis(2-mercaptoethyl)-1,5-diazacyclooctane or bme-daco). Infrared values listed for the diatomic ligands were recorded in CH2Cl2 or THF solution. Freshly prepared Fe(CO)2(NO)2 in THF readily reacts with NiN2S2 at 22 °C, with replacement of one CO, releasing the second CO under photolysis, or within 20 min at 40 °C, thus converting the NiN2S2 from mono- to bidentate ligand.34–37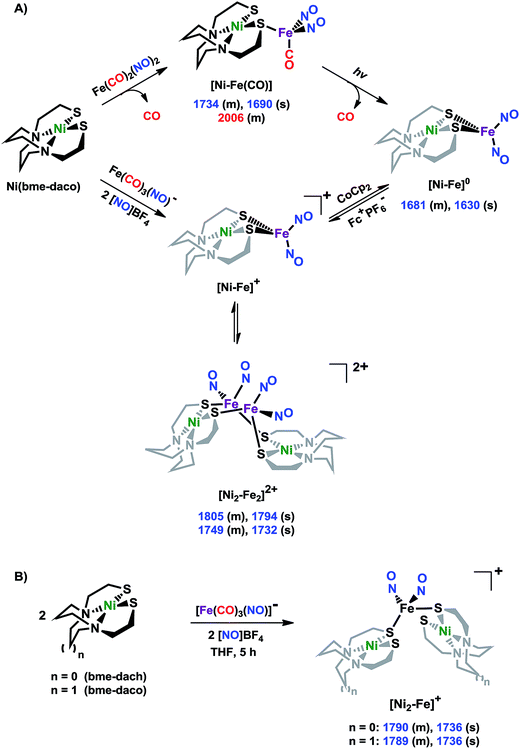 | ||
| Scheme 1 (A) The syntheses of [Ni–Fe]0 and [NiFe(CO)]0 as neutral complexes, and [Ni2–Fe2]2+ and (B) [Ni2–Fe]+ as BF4− salt. The IR values (in cm−1) of CO and NO are in red and blue, respectively. | ||
From this approach the [Ni–Fe]0 complex was isolated as a brown solid that is stable at ambient temperature under Ar. Oxidation of [Ni–Fe]0 by Fc+PF6− at 0 °C resulted in a color change of the THF solution from brown to dark purple with concomitant shifts of the ν(NO) values by an average of ca. 110 cm−1 to higher wave numbers. The reversibility of this oxidation was confirmed by reaction with cobaltocene and return to the reduced [Ni–Fe]0. The ν(NO) bands listed under [Ni2–Fe2]2+, Scheme 1A, reflect the presence of overlapping components which were resolved into two sets of absorbances, interpreted as a likely mixture of monomeric cation and dicationic dimer, with the set at lower values slightly less in intensity (Fig. S24†). As other experimental data, vide infra, as well as computational studies, indicate the predominance of monomeric [Ni–Fe]+, we postulate that the set of absorbances at slightly lower wavenumbers (as shoulders on the major bands) are due to the dimeric [Ni2–Fe2]2+. We note that the electron-spray ionization mass spectrum of [Ni–Fe]+ has a parent ion with isotopic bundle distribution at m/z that is consistent with a monomeric [Ni–Fe]+ rather than a dimeric [Ni2–Fe2]2+, Fig. S28.† The difference between two consecutive isotopic mass units is ∼1, rather than 0.5, which indicates the predominance of the monomer, [Ni–Fe]+, in the polar solvents in which they are soluble.
The magnetic moments of [Ni–Fe]+ and [Ni2–Fe]+ are 1.54 B.M. and 1.76 B.M., respectively, measured by Evans' method at room temperature in CD2Cl2. This is consistent with the presence of a single unpaired electron, Fig. S1 and S2.† The EPR spectra for both complexes display the isotropic g = 2.03 signal that is prototypical of the {Fe(NO)2}9 unit, Fig. S21 and S22,† respectively. The 77 K EPR spectrum of the [Ni–Fe]+ displayed fine structure requiring two signals for simulation: A major isotropic signal of g = 2.035 showed coupling with nitrogen of A(14N) = 32.74 MHz and a minor anisotropic signal had parameters of gxyz = 2.183, 2.012, 1.908 and no observable hyperfine coupling, Fig. S21.†
X-ray diffraction quality crystals of the oxidized NiFe compound were obtained from the one-pot reaction of equimolar NiN2S2 and (putative) [Fe(CO)2(NO)2]+ (prepared in situ by reacting [Fe(CO)3(NO)]− with two equivalents of [NO]BF4)38 in CH2Cl2 at ambient temperature, Scheme 1. A third Ni–Fe complex, [Ni2–Fe]+, was obtained on combining NiN2S2 with [Fe(CO)2(NO)2]+ in 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in THF solution, Scheme 1B. X-ray quality crystals of this trimetallic as its BF4− salt were obtained from hexane/THF layering at −28 °C. Its v(NO) IR spectral features are typical of monomeric DNICs in the {Fe(NO)2}9 redox level.
1 ratio in THF solution, Scheme 1B. X-ray quality crystals of this trimetallic as its BF4− salt were obtained from hexane/THF layering at −28 °C. Its v(NO) IR spectral features are typical of monomeric DNICs in the {Fe(NO)2}9 redox level.
X-ray diffraction studies
The molecular structures of the heterometallic complexes [Ni–Fe]0, [Ni2–Fe2]2+ and [Ni2–Fe]+ are shown in Fig. 3. The bimetallic complex [Ni–Fe]0, exhibits an overall butterfly-like Ni(μ-SR)2Fe core, analogous to the report of Pohl et al., where an open chain N2S2 ligand chelated the NiII.36 The converging lone pairs (see below) on the cis-dithiolates engage in bidentate binding and impose a hinge angle (the intersection of the best N2S2 plane with the S2Fe plane) of ca. 117°, vis-à-vis constricting the ∠S–Ni–S angle by ca. 4° compared to the free metalloligand.39 The two nitrosyl units bound to the pseudo tetrahedral iron center are slightly bent towards each other, in an “attracto” orientation;40 the average ∠Fe–N–O angle is ∼163.8°. The Ni⋯Fe distance of 3.001 (2) Å, is slightly longer (by 0.022 Å) than that obtained in the Pohl, et al. structure.36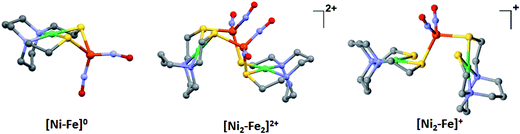 | ||
| Fig. 3 Molecular structures of (a) [Ni–Fe]0, (b) [Ni2–Fe2]2+ and (c) [Ni2–Fe]+ from X-ray diffraction. The BF4− counter ions of [Ni2–Fe2]2+ and [Ni2–Fe]+ are omitted for clarity. | ||
The oxidized NiFe complex crystallizes as dimeric [Ni2–Fe2]2+ with two BF4− anions; two dinitrosyl iron units are bridged by two NiN2S2 metalloligands. The tetrahedral geometry about each Fe(NO)2 unit is thus completed by two thiolates from different NiN2S2 metalloligands, thus generating an abbreviated paddlewheel, or propeller type, complex seen in examples of nickel–gold tetrametallic complexes.41 Likewise, the molecular structure of [Ni2–Fe]+ demonstrates that one Fe(NO)2 unit bridges two NiN2S2 metalloligands, each acting as a monodentate ligand. As shown in the [Ni2–Fe]+ structure, Fig. 3, the free unbound thiolates of two NiN2S2 units are transoid to each other. The addition of a second Fe(NO)2+ unit to generate the dication, [Ni2–Fe2]2+, would require rotation about one Fe–S bond in order to align the two metalloligands.
The average Ni⋯Fe distances in [Ni2–Fe2]2+ and [Ni2–Fe]+, are 3.680 (2) Å and 3.521 (2) Å, respectively, and are longer than in the [Ni–Fe]0 reduced complex by ca. 0.5 Å. The NiII maintains a square planar geometry in the reduced and oxidized complexes with a displacement of no more than 0.1 Å from the N2S2 best plane. Overall these structures demonstrate the impressive adaptability of the NiN2S2 metalloligands, and their potential to template clusters through S-based aggregation.7
Computational structural study
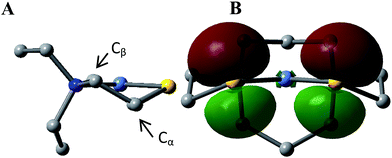
This computational section uses density functional theory (DFT) analysis to address the structural question in particular that was raised by the X-ray diffraction report: is there an electronic factor that governs the different μ2-SR binding modes found in the three forms of NiFe heterometallic aggregates? The functional/basis set combination, TPSS/6-311++G(d,p), and natural bond orbital (NBO) analysis were applied to the computational structural modeling of the free metalloligand NiN2S2 and its derivatives [Ni–Fe]0, [Ni2–Fe2]2+; more details of the computational methodology is available in ESI.†The orientation of this remaining p lone pair in the NiN2S2 metalloligand is determined by the NiN2S2 metalloligand's Ni–S–Cα–Cβ–N five-membered rings that adopt a non-planar envelope conformation like cyclopentane. The Cα carbon, the “flap” of the envelope conformation, puckers towards one side or the other of the N2S2 plane, Fig. 3. Fig. 4 shows how this puckering tilts the remaining 3p-lone pair on each sulfur from the normal to the N2S2 plane. This tilt causes two p-orbital lobes (green lobes) to converge on the side to which the flap puckers, while the red lobes diverge on the opposite side. The orientation of the added Fe(NO)2 receiver unit(s), will be thus determined by such directional property of the donor p lone pairs. The structure of the reduced monomer [Ni–Fe]0 shows the Fe(NO)2 fragment is on the same side as the flap; while in the oxidized dimer [Ni2–Fe2]2+ the flap and the Fe(NO)2 fragment(s) appear on different sides of each N2S2 plane, thus, binding to the other end of the p lone pair. Based on the analysis above, the converging lobes of the p donor lone pairs maximize contact to the Fe(NO)2 unit in the monomer [Ni–Fe]0, while the diverging lobes are preferred by two bridging Fe(NO)2 units between two metalloligands in the dimer [Ni2–Fe2]2+. The utilization of the divergent lobes apparently lessens the steric repulsion between Fe(NO)2 units. In summary, the binding position of the Fe(NO)2 unit with respect to the flap in the Ni–S–Cα–Cβ–N five-membered rings are correlated by the competition between chemical bond directionality of the binding sulfurs and steric repulsion of the receiver units.
Electrochemistry
The cyclic voltammograms of [Ni–Fe]+ (in CH2Cl2), [Ni2–Fe]+ (in CH3CN), as BF4− salts, and [Ni–Fe]0 (in CH2Cl2), were recorded under Ar at 22 °C, and referenced to Fc0/+ (E1/2 = 0.0 V) as an internal standard. Both the neutral complex [Ni–Fe]0 and the cationic analogue, [Ni–Fe]+, used in the CV studies as its BF4− salt, displayed reversible reduction events at ca. −0.73 V (CH2Cl2), assigned to the {Fe(NO)2}9/10 couple; see Fig. S13–S15† for CV scans. The NiFe complexes also present two irreversible oxidation events at ca. −0.10 V and ca. +0.45 V, with minor differences in intensities according to the neutral or cationic sources. Both of these events are assumed to be S-based.In CH3CN, the trimetallic complex [Ni2–Fe]+, showed a reversible event at, E1/2 = −0.75 V, assigned to the {Fe(NO)2}9/10 couple and an irreversible oxidation event at E1/2 = −0.05 V, see Fig. S16.† The E1/2 value for the {Fe(NO)2}9/10 couple, is anodically shifted by ca. 30 mV relative to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, NiFe complexes, resulting from the greater electron donation of two NiN2S2 centers to the Fe(NO)2 redox marker. The scan rate dependences of the {Fe(NO)2}9/10 couple for all three complexes support the assignments to reversible or quasi-reversible as described above, see Fig. S17–S19.†
1, NiFe complexes, resulting from the greater electron donation of two NiN2S2 centers to the Fe(NO)2 redox marker. The scan rate dependences of the {Fe(NO)2}9/10 couple for all three complexes support the assignments to reversible or quasi-reversible as described above, see Fig. S17–S19.†
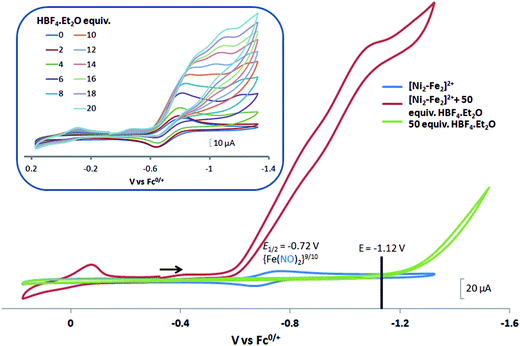
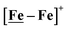 complex was examined under similar experimental conditions. Sequential addition of HBF4·Et2O to a CH2Cl2 solution of [Ni–Fe]+ (2 mM [Ni2–Fe2]2+) showed an increase in the cathodic current at the {Fe(NO)2}9/10 redox event at −0.73 V. The initial cathodic current response at −0.8 V saturates with ∼20 equivalents of the acid, Fig. 5 (inset). A second rise in cathodic current at −1.10 V, commences upon addition of >12 equivalents of the acid, which continues to rise as the catalytic current response, Fig. 5. The first response is attributed to the reduction of [Ni–Fe]+ followed by a protonation. The second response is assigned to the up-take of another electron by the reduced and protonated counterpart of [Ni–Fe]+. The mechanism below connects the successive protonation to the production of H2, thus closing the catalytic cycle. Overlays of this response of the NiFe complex in presence of 50 equivalents of HBF4·Et2O (0.1 M), as well as the CV of the free acid, are shown in Fig. 5. The catalytic H2 produced was confirmed by applying a constant potential at −1.12 V for 60 min (black bold line in Fig. 5), and analysis of the headspace by gas chromatography. The H2 was quantified by an average of two consistent constant potential coulometry experiments with subtraction of the H2 produced from the free acid.9,10 The nitrosylated compounds [Ni–Fe]+ and
complex was examined under similar experimental conditions. Sequential addition of HBF4·Et2O to a CH2Cl2 solution of [Ni–Fe]+ (2 mM [Ni2–Fe2]2+) showed an increase in the cathodic current at the {Fe(NO)2}9/10 redox event at −0.73 V. The initial cathodic current response at −0.8 V saturates with ∼20 equivalents of the acid, Fig. 5 (inset). A second rise in cathodic current at −1.10 V, commences upon addition of >12 equivalents of the acid, which continues to rise as the catalytic current response, Fig. 5. The first response is attributed to the reduction of [Ni–Fe]+ followed by a protonation. The second response is assigned to the up-take of another electron by the reduced and protonated counterpart of [Ni–Fe]+. The mechanism below connects the successive protonation to the production of H2, thus closing the catalytic cycle. Overlays of this response of the NiFe complex in presence of 50 equivalents of HBF4·Et2O (0.1 M), as well as the CV of the free acid, are shown in Fig. 5. The catalytic H2 produced was confirmed by applying a constant potential at −1.12 V for 60 min (black bold line in Fig. 5), and analysis of the headspace by gas chromatography. The H2 was quantified by an average of two consistent constant potential coulometry experiments with subtraction of the H2 produced from the free acid.9,10 The nitrosylated compounds [Ni–Fe]+ and 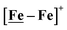 were found to have low turnover numbers and faradaic efficiencies, 68 ± 2% and 58 ± 1%, respectively, for H2 production. In contrast the [Ni–Fe′]+ gave a faradeic efficiency of ca. 96%. We assume that the former involves alternate protonation pathways, particularly at NO, that lead to degradation and hence low F.E. In addition the TON for the robust Ni–Fe′ complex in 50 equiv. of TFA, measured at −1.73 V and over a period of 8 h was found to be 6.7, assuring catalytic proton reduction. The electrocatalytic response of the reduced complex, [Ni–Fe]0 in the presence of HBF4·Et2O, is, as expected, the same as [Ni–Fe]+ and is shown in Fig. S20-B.†
were found to have low turnover numbers and faradaic efficiencies, 68 ± 2% and 58 ± 1%, respectively, for H2 production. In contrast the [Ni–Fe′]+ gave a faradeic efficiency of ca. 96%. We assume that the former involves alternate protonation pathways, particularly at NO, that lead to degradation and hence low F.E. In addition the TON for the robust Ni–Fe′ complex in 50 equiv. of TFA, measured at −1.73 V and over a period of 8 h was found to be 6.7, assuring catalytic proton reduction. The electrocatalytic response of the reduced complex, [Ni–Fe]0 in the presence of HBF4·Et2O, is, as expected, the same as [Ni–Fe]+ and is shown in Fig. S20-B.†
Following the approach of Helm and Appel,45 and Wiese,46 the turnover frequency (TOF) as calculated from the CV experiment for [Ni–Fe]+, was 39.7 s−1, which is slightly better than the 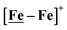 complex, 26.7 s−1, calculated under similar experimental conditions. The [Ni–Fe]+ shows a saturation of the more negative catalytic current upon addition of 80 equivalents of HBF4·Et2O, i.e., ∼0.16 M CH2Cl2 solution. Notably, the behavior of the
complex, 26.7 s−1, calculated under similar experimental conditions. The [Ni–Fe]+ shows a saturation of the more negative catalytic current upon addition of 80 equivalents of HBF4·Et2O, i.e., ∼0.16 M CH2Cl2 solution. Notably, the behavior of the 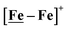 complex is similar, and further addition of acid leads to decomposition of both catalysts. The precise calculation of overpotential is indeterminable as the thermodynamic potential (EHBF4/H2,BF4−) of 0.1 M HBF4·Et2O in CH2Cl2 is unavailable.47 Using the thermodynamic potential of HBF4·Et2O in acetonitrile (−0.26 V),48,49 an estimate of the overpotential of [Ni–Fe]+ and
complex is similar, and further addition of acid leads to decomposition of both catalysts. The precise calculation of overpotential is indeterminable as the thermodynamic potential (EHBF4/H2,BF4−) of 0.1 M HBF4·Et2O in CH2Cl2 is unavailable.47 Using the thermodynamic potential of HBF4·Et2O in acetonitrile (−0.26 V),48,49 an estimate of the overpotential of [Ni–Fe]+ and 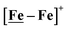 were 711 mV and 660 mV, respectively, which are lower than those of the [Ni–Fe′]+ and
were 711 mV and 660 mV, respectively, which are lower than those of the [Ni–Fe′]+ and 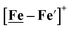 electrocatalysts by over 220 mV.
electrocatalysts by over 220 mV.
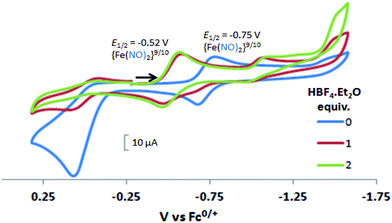
In contrast to the NiFe complexes, addition of HBF4·Et2O to a 2.0 mM CH3CN solution of [Ni2–Fe]+, (the N2S2 ligand used in this electrochemical study is bme-dach) did not show an increase in the cathodic current at −0.75 V, the reversible {Fe(NO)2}9/10 redox event. Instead, a new reversible redox event at E1/2 = −0.52 V, appeared upon addition of two equivalents of HBF4·Et2O with a concomitant disappearance of the original redox process, Fig. 6. Further addition of acid resulted in electrode fouling, Fig. S20-A.† A possible explanation, from computational chemistry, vide infra, for the positive 230 mV shift is that [Ni2–Fe]+ can be protonated on its exposed thiolate sulfur by HBF4·Et2O, vide infra. Such would account for the greater ease of reduction for the {Fe(NO)2}9/10 couple, compared to the [Ni2–Fe]+ complex. Supporting this conclusion is that addition of 1 equivalent of HBF4·Et2O to a CH3CN solution of [Ni2–Fe]+ produced a small but definite shift of the ν(NO) in the IR spectrum from 1787 and 1734 cm−1 to 1793 and 1737 cm−1, Fig. S27.† In addition, the irreversible oxidation event at 0.07 V, which is assumed to be sulfur-based oxidation, shows a decrease in the anodic current upon addition of acid, indicating disulfide formation is inhibited under acidic conditions.
Computational mechanistic study
The electrochemical study points to additional questions for computational study: (A) how do the calculated electrocatalytic mechanisms compare for the hard–soft vs. soft–soft donor/receiver adducts? (B) Can computational analysis clarify those cases of non-catalytic electrochemical responses to added protons? Modeling is extended to [Ni2–Fe]+, along with [Ni–Fe]0, [Ni–Fe]+, in various oxidation states and with multiple added protons to answer these questions.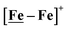 .
An earlier computational analysis of a HER electrocatalysis mechanism proposed for the
.
An earlier computational analysis of a HER electrocatalysis mechanism proposed for the 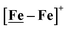 complex, Fig. 1, focused on the first reduction event with a strong acid proton source.8 The successive reduction event ultimately allowed for double proton addition to the Fe(NO)2 unit and formation of a dihydride, panel A of Fig. 7.8,50 Making use of electron transfer from the reduced {Fe(NO)}8, reductive elimination from the {Fe(NO)}6–{Fe(NO)2}8 morphed into an η2-H2–Fe(NO)2, restoring {Fe(NO)}7–{Fe(NO)}9, with H2 formation and loss. Note that no hemi-lability of the metallodithiolate ligand9 is necessary here as the mechanism does not entail hydride/proton coupling to H2, but rather reductive elimination from two hydrides.7
complex, Fig. 1, focused on the first reduction event with a strong acid proton source.8 The successive reduction event ultimately allowed for double proton addition to the Fe(NO)2 unit and formation of a dihydride, panel A of Fig. 7.8,50 Making use of electron transfer from the reduced {Fe(NO)}8, reductive elimination from the {Fe(NO)}6–{Fe(NO)2}8 morphed into an η2-H2–Fe(NO)2, restoring {Fe(NO)}7–{Fe(NO)}9, with H2 formation and loss. Note that no hemi-lability of the metallodithiolate ligand9 is necessary here as the mechanism does not entail hydride/proton coupling to H2, but rather reductive elimination from two hydrides.7
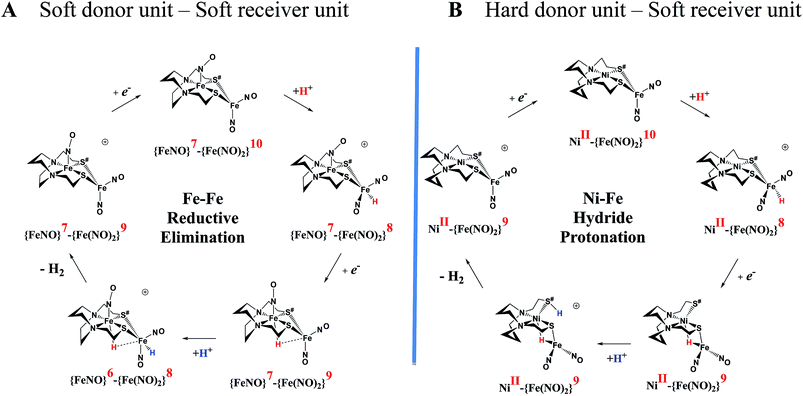 | ||
Fig. 7 Comparative catalytic cycles for H2 production catalyzed by 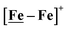 and [Ni–Fe]+. All pKa, thermodynamic, and metric data for the two mechanisms are available in a separate report.50 and [Ni–Fe]+. All pKa, thermodynamic, and metric data for the two mechanisms are available in a separate report.50 | ||
The [Ni–Fe]+ and its reduced counterpart [Ni–Fe]0 are determined to be electrocatalysts at −0.73 V for H2 production with HBF4·Et2O, Fig. 5. [Note: The computational study finds that the [Ni2–Fe2]2+, whose dimeric structure was established in the solid state by crystallography, finds greater stability in solution as the monomeric form, [Ni–Fe]+. Experimental evidences including ESI-MS and determination of μeff support this thesis, vide supra.] The catalytic cycle is thus initiated with the monomer [Ni–Fe]+ (Fig. 7B). As indicated in panel B of Fig. 7, the {Fe(NO)2}9 in the [Ni–Fe]+ unit accepts the first incoming electron, followed by the first proton, to create a hydride on the now {Fe(NO)2}8 unit. Addition of a second electron activates the hemi-lability of the bridging thiolate to break one S–Fe bond, while the terminal hydride becomes bridging between Fe and Ni. The cleavage of the S–Fe dative bond essentially releases one p lone pair of the thiolate so that S can act as a pendant base to accept the second proton and guide it to a coupling position with the hydride and produce H2. Details of the full catalytic cycle with energetics and analysis of electronic structure evolution for both 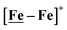 and [Ni–Fe]+ are presented in a separate report.50
and [Ni–Fe]+ are presented in a separate report.50
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex [Ni2–Fe]+ to act as a pendant base, in fact this complex does not show any catalytic activity in the presence of strong acid, HBF4·Et2O, within the solvent potential window. A computational study, summarized in Fig. 8, reveals that while reduction still occurs on the Fe(NO)2 unit, the protonation process is diverted from the Fe(NO)2 unit. In this 2
1 complex [Ni2–Fe]+ to act as a pendant base, in fact this complex does not show any catalytic activity in the presence of strong acid, HBF4·Et2O, within the solvent potential window. A computational study, summarized in Fig. 8, reveals that while reduction still occurs on the Fe(NO)2 unit, the protonation process is diverted from the Fe(NO)2 unit. In this 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex, the computations show that only one thiolate from each NiN2S2 binds to Fe(NO)2, while the other thiolate, is “free” to interact with other electron acceptors; thus it may also be protonated, even before the reduction of the {Fe(NO)2}9 unit occurs, see Table S9,† and shifts the its potential, which is supported by experiment, vide supra.
1 complex, the computations show that only one thiolate from each NiN2S2 binds to Fe(NO)2, while the other thiolate, is “free” to interact with other electron acceptors; thus it may also be protonated, even before the reduction of the {Fe(NO)2}9 unit occurs, see Table S9,† and shifts the its potential, which is supported by experiment, vide supra.
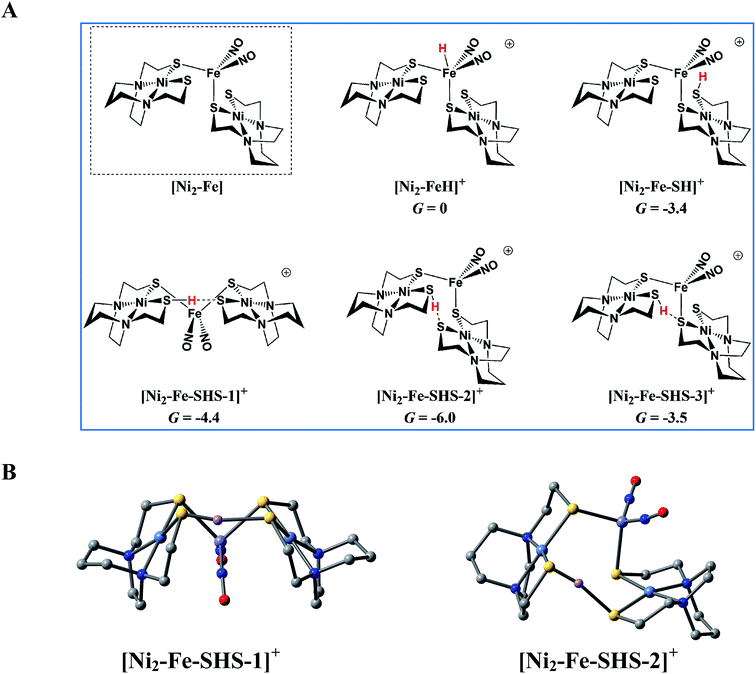
According to the computations, in the reduced [Ni2–Fe]0 the “free” thiolate competes with the reduced {Fe(NO)2}10 unit for the incoming proton (Fig. 8A); in addition, by rotation around an Fe–S bond, the two NiN2S2 ligands may orient their “free” thiolate sulfurs to pinch the proton, i.e., consequently forming a strong hydrogen bond (Fig. 8A and B). Spectroscopic evidence supports protonation on S even before reduction, i.e., in [Ni2–Fe]+, Fig. S27.† Two geometries of the pinched proton by two “free” thiolates, [Ni2–Fe–SHS–1]+ and [Ni2–Fe–SHS–2]+ can be achieved by either translating or rotating one NiN2S2 unit of [Ni2–Fe], respectively. Precedent in Dubois' Ni(P2N2)2 catalysts,51 a proton pinched between two amine N bases is relatively stable; in our case, the pinched proton is even more stable than a hydride on Fe(NO)2 (Fig. 8A). However, the mechanistic clue from the [Ni–Fe] complex50 indicates the requirement for a proton to be reduced into a hydride, by {Fe(NO)2}10, before the H2 can be produced by the proton–hydride coupling mechanism. Therefore, the formation of a stable pinched proton likely prevents the generation of the hydride and cuts off the catalytic cycle. The thiolate already bound to Fe(NO)2 also helps stabilize the proton on a “free” thiolate, to a smaller extent, with the example of [Ni2–Fe–SHS–3]+ (Fig. 8A).
Conclusions
Our collection of hydrogen evolution reaction catalysts is summarized in Fig. 9. While the small differences in donor units and acceptor units do not influence the overall structures of the S-bridged bimetallics; all have butterfly-like [M(μ-SR)2Fe] core and the potential for opening up sites for proton addition via the hemi-lability of the metallothiolate donors. Nevertheless, demonstrable and explicable differences are seen in their catalytic performances as indicated by catalytic potential, required acid strength, and TOF.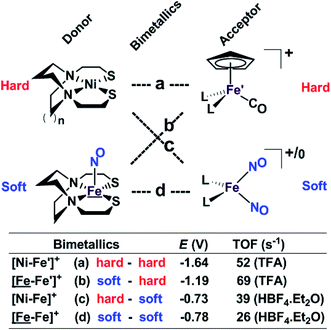 | ||
| Fig. 9 A comparative schematic for a matrix of bimetallic electrocatalysts containing hard/soft donor/acceptor units. | ||
Analogous to the HSAB (Hard and Soft (Lewis) Acids and Bases) concept, we offer an electronic parallel, “soft vs. hard donor/receiver units”, in this case directed towards the number of NO ligands in the bimetallics ranging from 0 to 3, with increasing flexibility (i.e., soft) of electronic structure within each unit. The non-innocence of the NO ligand confers electron uptake at milder potentials, which we have seen in both the donor units and acceptor units. Thus the incorporation of NO ligands on the acceptor units, the ‘hard–soft’ and ‘soft–soft’ electrocatalysts lead to energetically more accessible catalytic current, however, at the cost of a stronger acid and a lower TOF in comparison to the bimetallics with hard acceptor units.
While these electrocatalysts are only moderately efficient for H2 production, they are well-behaved and have demonstrated reproducibility. Two of the catalysts, c and d, with soft receivers, are isolated and crystallized in both oxidized and reduced forms at ambient conditions lending confidence to the presumed catalytic cycle.
Features in the electrochemical scans may be reasonably ascribed to protonation products whose identities are further described by computational chemistry. The resulting computational mechanisms identify key features that may guide future synthetic targets. For example, the hemi-lability of the S-donors may be optimized by steric constraints; the usefulness of the Fe(NO)2 unit as electron depot and protonation site with low redox potential, should encourage explorations with other redox-active, soft acceptors. The computations also suggest a mechanistic paradigm of heterolytic H−/H+, hydride–proton, coupling for bimetallics a, b and c from the chart, and reductive elimination from d arising in the soft–soft construct. Such a supposition derives from extreme electron delocalization in the trinitrosylated 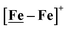 complex and argues that suitably constructed first row, bimetallic complexes may take on two-electron processes that emulate noble metals.
complex and argues that suitably constructed first row, bimetallic complexes may take on two-electron processes that emulate noble metals.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
We are thankful for financial support from the National Science Foundation (CHE-1266097, CHE-1665258 to M. Y. D., and CHE-1300787, CHE-1664866 to M. B. H.), the Robert A. Welch Foundation (A-0924 to M. Y. D., and A-0648 to M. B. H.). A portion of the salary of Pokhraj Ghosh was made possible by an NPRP award (NPRP 6-1184-1-224) from the Qatar National Research Fund (a member of Qatar Foundation). The authors acknowledge the Laboratory for Molecular Simulation at Texas A&M University for providing computing resources. Appreciation is expressed to Dr David Russell and Dr Doyong Kim for interpretation of the high resolution ESI-MS. The authors would also like to acknowledge Dr Allen M. Lunsford for helpful discussions.References
- W. Lubitz, H. Ogata, O. Rüdiger and E. Reijerse, Chem. Rev., 2014, 114, 4081 CrossRef CAS PubMed.
- M. Can, F. A. Armstrong and S. W. Ragsdale, Chem. Rev., 2014, 114, 4149 CrossRef CAS PubMed.
- Special issue on “Hydrogenases”, Eur. J. Inorg. Chem., 2011, 915 Search PubMed.
- Special issue on “Renewable Energy”, Chem. Soc. Rev., 2009, 38, 1–300 Search PubMed.
- Special issue on “Hydrogen”, Chem. Rev., 2007, 107, 3900–4435 Search PubMed.
- M. L. Helm, M. P. Stewart, R. M. Bullock, M. R. DuBois and D. L. DuBois, Science, 2011, 333, 863 CrossRef CAS PubMed.
- J. A. Denny and M. Y. Darensbourg, Chem. Rev., 2015, 115, 5248 CrossRef CAS PubMed.
- C. H. Hsieh, S. Ding, O. F. Erdem, D. J. Crouthers, T. Liu, C. C. L. McCrory, W. Lubitz, C. V. Popescu, J. H. Reibenspies, M. B. Hall and M. Y. Darensbourg, Nat. Commun., 2014, 5, 3684 CAS.
- S. Ding, P. Ghosh, A. M. Lunsford, N. Wang, N. Bhuvanesh, M. B. Hall and M. Y. Darensbourg, J. Am. Chem. Soc., 2016, 138, 12920 CrossRef CAS PubMed.
- P. Ghosh, M. Quiroz, N. Wang, N. Bhuvanesh and M. Y. Darensbourg, Dalton Trans., 2017, 46, 5617 RSC.
- W. Zhu, A. C. Marr, Q. Wang, F. Neese, D. J. E. Spencer, A. J. Blake, P. A. Cooke, C. Wilson and M. Schröder, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 18280 CrossRef CAS PubMed.
- S. Ogo, R. Kabe, K. Uehara, B. Kure, T. Nishimura, S. C. Menon, R. Harada, S. Fukuzumi, Y. Higuchi, T. Ohhara, T. Tamada and R. Kuroki, Science, 2007, 316, 585 CrossRef CAS PubMed.
- S. Ogo, K. Ichikawa, T. Kishima, T. Matsumoto, H. Nakai, K. Kusaka and T. Ohhara, Science, 2013, 339, 682 CrossRef CAS PubMed.
- T. Kishima, T. Matsumoto, H. Nakai, S. Hayami, T. Ohta and S. Ogo, Angew. Chem., Int. Ed., 2016, 55, 724 CrossRef CAS PubMed.
- D. Brazzolotto, M. Gennari, N. Queyriaux, T. R. Simmons, J. Pécaut, S. Demeshko, F. Meyer, M. Orio, V. Artero and C. Duboc, Nat. Chem., 2016, 8, 1054 CrossRef CAS PubMed.
- S. Canaguier, M. Field, Y. Oudart, J. Pecaut, M. Fontecave and V. Artero, Chem. Commun., 2010, 46, 5876 RSC.
- M. A. Reynolds, T. B. Rauchfuss and S. R. Wilson, Organometallics, 2003, 22, 1619 CrossRef CAS.
- D. Schilter, J. M. Camara, M. T. Huynh, S. Hammes-Schiffer and T. B. Rauchfuss, Chem. Rev., 2016, 116, 8693 CrossRef CAS PubMed.
- K. Weber, Ö. F. Erdem, E. Bill, T. Weyhermüller and W. Lubitz, Inorg. Chem., 2014, 53, 6329 CrossRef CAS PubMed.
- D. Yang, Y. Li, L. Su, B. Wang and J. Qu, Eur. J. Inorg. Chem., 2015, 2015, 2965 CrossRef CAS.
- N. T. Nguyen, Y. Mori, T. Matsumoto, T. Yatabe, R. Kabe, H. Nakai, K. S. Yoon and S. Ogo, Chem. Commun., 2014, 50, 13385 RSC.
- T. Matsumoto, R. Kabe, K. Nonaka, T. Ando, K.-S. Yoon, H. Nakai and S. Ogo, Inorg. Chem., 2011, 50, 8902 CrossRef CAS PubMed.
- K. Ichikawa, T. Matsumoto and S. Ogo, Dalton Trans., 2009, 4304 RSC.
- K. Kim, T. Kishima, T. Matsumoto, H. Nakai and S. Ogo, Organometallics, 2013, 32, 79 CrossRef CAS.
- K. Kim, T. Matsumoto, A. Robertson, H. Nakai and S. Ogo, Chem.–Asian J., 2012, 7, 1394 CrossRef CAS PubMed.
- J. A. Denny and M. Y. Darensbourg, Coord. Chem. Rev., 2016, 324, 82 CrossRef CAS.
- C. H. Hsieh and M. Y. Darensbourg, J. Am. Chem. Soc., 2010, 132, 14118 CrossRef CAS PubMed.
- J. H. Enemark and R. D. Feltham, Coord. Chem. Rev., 1974, 13, 339 CrossRef CAS.
- A. Silakov, B. Wenk, E. Reijerse and W. Lubitz, Phys. Chem. Chem. Phys., 2009, 11, 6592 RSC.
- G. Berggren, A. Adamska, C. Lambertz, T. R. Simmons, J. Esselborn, M. Atta, S. Gambarelli, J. M. Mouesca, E. Reijerse, W. Lubitz, T. Happe, V. Artero and M. Fontecave, Nature, 2013, 499, 66 CrossRef CAS PubMed.
- J. Esselborn, C. Lambertz, A. Adamska-Venkatesh, T. Simmons, G. Berggren, J. Noth, J. Siebel, A. Hemschemeier, V. Artero, E. Reijerse, M. Fontecave, W. Lubitz and T. Happe, Nat. Chem. Biol., 2013, 9, 607 CrossRef CAS PubMed.
- J. F. Siebel, A. Adamska-Venkatesh, K. Weber, S. Rumpel, E. Reijerse and W. Lubitz, Biochemistry, 2015, 54, 1474 CrossRef CAS PubMed.
- J. Esselborn, N. Muraki, K. Klein, V. Engelbrecht, N. Metzler-Nolte, U. P. Apfel, E. Hofmann, G. Kurisu and T. Happe, Chem. Sci., 2016, 7, 959 RSC.
- W. F. Liaw, C. Y. Chiang, G. H. Lee, S. M. Peng, C. H. Lai and M. Y. Darensbourg, Inorg. Chem., 2000, 39, 480 CrossRef CAS PubMed.
- C. H. Hsieh, R. B. Chupik, S. M. Brothers, M. B. Hall and M. Y. Darensbourg, Dalton Trans., 2011, 40, 6047 RSC.
- F. Osterloh, W. Saak, D. Haase and S. Pohl, Chem. Commun., 1997, 979 RSC.
- J. A. W. Verhagen, M. Lutz, A. L. Spek and E. Bouwman, Eur. J. Chem., 2003, 3968 CAS.
- C. H. Hsieh, S. M. Brothers, J. H. Reibenspies, M. B. Hall, C. V. Popescu and M. Y. Darensbourg, Inorg. Chem., 2013, 52, 2119 CrossRef CAS PubMed.
- M. V. Rampersad, S. P. Jeffery, M. L. Golden, J. Lee, J. H. Reibenspies, D. J. Darensbourg and M. Y. Darensbourg, J. Am. Chem. Soc., 2005, 127, 17323 CrossRef CAS PubMed.
- G. B. Richter-Addo and P. Legzdins, Metal Nitrosyls, 1992, ch. 5 Search PubMed.
- T. A. Pinder, S. K. Montalvo, A. M. Lunsford, C. H. Hsieh, J. H. Reibenspies and M. Y. Darensbourg, Dalton Trans., 2014, 43, 138 RSC.
- B. P. Hay and R. D. Hancock, Coord. Chem. Rev., 2001, 212, 61 CrossRef CAS.
- M. B. Hall, Inorg. Chem., 1978, 17, 2261 CrossRef CAS.
- D. J. Crouthers, S. Ding, J. A. Denny, R. D. Bethel, C. H. Hsieh, M. B. Hall and M. Y. Darensbourg, Angew. Chem., 2015, 127, 11254 CrossRef.
- A. M. Appel and M. L. Helm, ACS Catal., 2014, 4, 630 CrossRef CAS.
- S. Wiese, U. J. Kilgore, M. H. Ho, S. Raugei, D. L. DuBois, R. M. Bullock and M. L. Helm, ACS Catal., 2013, 3, 2527 CrossRef CAS.
- V. Fourmond, P. A. Jacques, M. Fontecave and V. Artero, Inorg. Chem., 2010, 49, 10338 CrossRef CAS PubMed.
- S. Daniele, P. Ugo, G. A. Mazzocchin and G. Bontempelli, Anal. Chim. Acta, 1985, 173, 141 CrossRef CAS.
- G. A. N. Felton, R. S. Glass, D. L. Lichtenberger and D. H. Evans, Inorg. Chem., 2006, 45, 9181 CrossRef CAS PubMed.
- S. Ding, P. Ghosh, M. Y. Darensbourg and M. B. Hall, Proc. Natl. Acad. Sci. U. S. A., 2017 DOI:10.1073/pnas.1710475114 , Accepted manuscript..
- D. L. DuBois, Inorg. Chem., 2014, 53, 3935 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental, spectroscopic, additional electrochemical and computational details, X-ray crystallographic data (CIF) from the structure of the complexes [Ni–Fe]0, [Ni2–Fe2]2+, [Ni2–Fe]+, and computational coordinates are available. CCDC crystallographic data for the complexes [Ni–Fe]0, [Ni2–Fe2]2+ and [Ni2–Fe]+ were deposited in the Cambridge Crystallographic Data Centre. CCDC [Ni–Fe]0 (CCDC 1045461), [Ni2–Fe2]2+ (CCDC 1045460) and [Ni2–Fe]+ (CCDC 1565539). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7sc03378h |
| This journal is © The Royal Society of Chemistry 2017 |