Cesium carbonate promoted cascade reaction involving DMF as a reactant for the synthesis of dihydropyrrolizino[3,2-b]indol-10-ones†
Qianqian
Zhang
a,
Chuanjun
Song
 *a,
He
Huang
a,
Kun
Zhang
a and
Junbiao
Chang
*ab
*a,
He
Huang
a,
Kun
Zhang
a and
Junbiao
Chang
*ab
aCollege of Chemistry and Molecular Engineering, Zhengzhou University, Zhengzhou 450001, China. E-mail: chjsong@zzu.edu.cn
bCollaborative Innovation Centre of New Drug Research and Safety Evaluation, Henan Province, Zhengzhou 450001, China. E-mail: changjunbiao@zzu.edu.cn
First published on 22nd September 2017
Abstract
We presented a cesium carbonate promoted cascade reaction of N-tosyl-2-(2-bromophenylacetyl)pyrroles with DMF to synthesize dihydropyrrolizino[3,2-b]indol-10-ones. The transformation involved three successive bond formations without the aid of an exogenous transition metal catalyst or ligand.
Introduction
Transition metal catalyzed cross-coupling reactions for carbon–carbon and carbon–heteroatom bond formation1 have become the most powerful tool in modern organic synthesis. Despite the remarkable advances, however, transition metal catalyzed cross-coupling reactions still confront challenges such as expensiveness of metal catalysts and ligands, high catalyst loading, and difficulties in removing trace amounts of toxic transition metal residues from the products. Therefore, the development of transition metal free protocols to fulfill the classic transition metal catalyzed cross-coupling reactions is highly appealing.2 Pioneered by Itami's initial report in 2008,3a great progress has been made in the field of transition metal free biaryl couplings.3 However, there are only a few reports on transition metal free carbon–heteroatom bond forming reactions.4N,N-Dimethylformamide (DMF) is an aprotic polar solvent commonly employed in organic reactions. Besides, DMF has been used as a promising reagent in organic transformation as a source of various units such as –CN, –CHO, –CONMe2, –OCHO, –NMe2, –O, etc. in transition metal catalyzed reactions.5,6 Herein, we report novel base promoted cascade reactions involving DMF as a reactant/solvent for the construction of dihydropyrrolizino[3,2-b]indol-10-ones.
Results and discussion
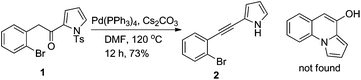
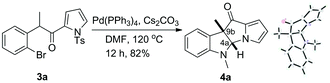
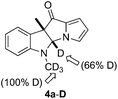
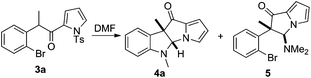
Previously, we developed a valuable approach toward the synthesis of 9H-pyrrolo[1,2-a]indol-9-ones via detosylation and in situ palladium catalyzed C–N bond formation of 2-bromophenyl N-tosylpyrrol-2-yl ketones.7 We further envisioned that under the reaction conditions, the one-carbon homologue 2-(2-bromophenyl)acetyl-N-tosylpyrroles, which was easily accessed by TFAA-mediated acylation of N-tosylpyrrole with 2-(2-bromophenyl)acetic acids,8 might give rise to the formation of pyrrolo[1,2-a]quinolines, a class of compounds possessing important biological activities9 (Scheme 1). However, alkyne 2 was the only product obtained after heating a mixture of 1, Pd(PPh3)4, Cs2CO3 and DMF at 120 °C for 12 h. Literature search indicated that a similar transformation was previously reported.10To bypass alkyne formation, we next subjected 3a to the aforementioned reaction conditions (Scheme 2). The reaction proceeded cleanly and resulted, quite surprisingly, in the formation of dihydropyrrolizino[3,2-b]indol-10-one 4a in 82% isolated yield. The structure of 4a was unambiguously confirmed by X-ray single crystal diffraction,11 and no trace of the 4a,9b-trans isomer was evident by NMR. Repetition of the reaction in deuterated DMF clearly indicated that the product 4a-D arose from the reaction of 3a with the solvent (Fig. 1).
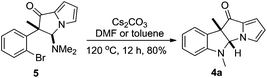
Inspired by the results, we then turned our attention to the synthesis of dihydropyrrolizino[3,2-b]indol-10-ones. Using 3a as a substrate, the results for reaction condition optimization are shown in Table 1. The reaction proceeded smoothly in the presence of a variety of palladium catalysts and Cs2CO3 as the base to give 4a in good to excellent isolated yields (entries 1–5). It was subsequently found that Cs2CO3 alone, without any exogenous palladium catalyst, could efficiently promote the reaction to provide 4a in 72% isolated yield, together with a small amount of dihydropyrrolizinone 5 (entry 6). The relative stereochemistry of 5 was assigned on the basis of NOE correlation. Extension of the reaction time to 24 h resulted in the exclusive formation of 4a in 87% isolated yield (entry 7). Furthermore, the isolated 5 could be transformed into 4a by exposure to the reaction conditions, indicating that 5 was an intermediate for the formation of 4a from 3a (Scheme 3). The bases were then screened and Cs2CO3 remained the best choice (entries 7–12). With Cs2CO3 as the optimized base, it was found that the reaction could hardly be driven to completion at temperatures below 120 °C (entries 13–15). Although elevated temperature favored the formation of 4a, no improvement was observed at a temperature higher than 120 °C (entry 16 vs. 7).
| Entry | Catalysta | Baseb | Temp. (°C) | 4a Yieldc (%) | 5 Yieldc (%) |
|---|---|---|---|---|---|
| a 0.05 equiv. b 2.0 equiv. c Isolated yield. d Reaction time: 12 h. e Reaction time: 24 h. f The starting material was completely reacted. g No reaction, starting material recovered. h 10% starting material recovered. i Another product was also isolated (structure not determined), which on heating could be transformed into 5 (80 °C) or 4a (120 °C). | |||||
| 1d | Pd(PPh3)4 | Cs2CO3 | 120 | 82 | — |
| 2d | Pd2(dba)3 | Cs2CO3 | 120 | 84 | — |
| 3d | Pd[P(tBu)3]2 | Cs2CO3 | 120 | 63 | — |
| 4d | Pd(PPh3)2Cl2 | Cs2CO3 | 120 | 80 | — |
| 5d | Pd(dppf)Cl2 | Cs2CO3 | 120 | 63 | — |
| 6d | — | Cs2CO3 | 120 | 72 | 7 |
| 7e | — | Cs2CO3 | 120 | 87 | — |
| 8e | — | K2CO3 | 120 | 67 | — |
| 9e,f | — | KOtBu | 120 | — | — |
| 10e,g | — | DBU | 120 | — | — |
| 11e,h | — | K3PO4 | 120 | 40 | — |
| 12e | — | LiHMDS | 120 | 20 | — |
| 13e,i | — | Cs2CO3 | 80 | — | 28 |
| 14e | — | Cs2CO3 | 100 | 41 | 20 |
| 15e | — | Cs2CO3 | 110 | 72 | 14 |
| 16e | — | Cs2CO3 | 130 | 86 | — |
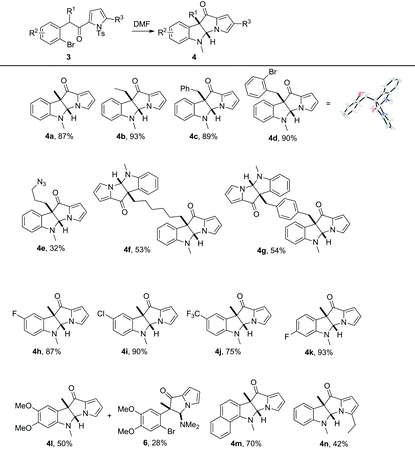
Next, the substrate scope was explored and the results are shown in Table 2. When the methyl substituent in 3a was replaced with a more bulky ethyl, benzyl or 2-bromobenzyl group, the products 4b–d could be obtained in excellent yields. The structure of 4d was unambiguously confirmed by X-ray single crystal diffraction12 and no trace of the alternative [6-6-5-5]-tetrahydropyrrolizino[3,2-b]quinolin-11-one was observed. The reaction of the substrate with a 3-azidopropyl group was less satisfactory and gave a mixture of products from which 4e was isolated in 32% yield. Under the reaction conditions, both dimeric products 4f and 4g could be obtained eventlessly in moderate yield. A variety of substituted bromobenzene derivatives, as well as bromonaphthalene could all participate in the desired reaction to give 4h–m in good to excellent yields. It should be indicated that the substrate bearing two electron-donating methoxy groups reacted rather slowly. Apart from 4l (50%), 28% of the intermediate 6 was also isolated. Substituted pyrrolyl derivative 4n could also be successfully synthesized, but in 42% isolated yield only, probably due to increased steric hindrance for the nucleophilic attack of pyrrolyl nitrogen to DMF.
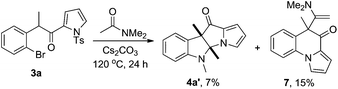
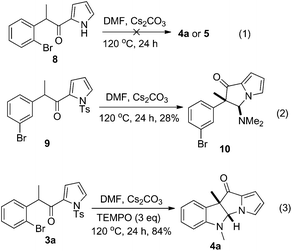
The limitations of the current method were revealed when we were testing the reaction of 3a with other amides. The reaction of 3a with the enolizable N,N-dimethylacetamide gave a mixture of products. The desired 4a′ could be isolated, but only in a disappointing 7% yield (Scheme 4). Another product separated from the reaction system was pyrrolo[1,2-a]quinolin-4-one 7 (15%). The reaction of 3a with N-methylformamide or N,N-dimethylbenzamide (dioxane as a solvent) also failed to deliver the desired dihydropyrrolizino[3,2-b]indol-10-ones. The detosylation product 8 (Scheme 5) was exclusively formed in the former case, while no isolable product could be obtained in the latter case.
Finally, to gain a deep insight into the reaction mechanism, we carried out some control experiments (Scheme 5). First, the unprotected acylpyrrole 8 did not react with DMF under the reaction conditions to provide any trace of 4a or 5, indicating that the tosyl protecting group is crucial to the formation of 5. Second, the reaction of 9 with DMF gave rise to the formation of dihydropyrrolizinone 10 (28%), together with 15% of the unreacted starting material recovered. No trace of 4a was observed. This result ruled out the mechanism for C–N bond formation via a benzyne intermediate. Third, when the reaction of 3a with DMF was performed in the presence of a radical scavenger (TEMPO), the yield of the product 4a was not reduced. This result ruled out the mechanism for C–N bond formation via a radical pathway.
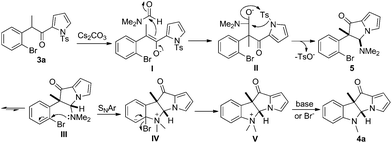
Based on the results obtained above, a plausible mechanism is depicted in Scheme 6. The enolization of 3a provided I which attacked DMF to give intermediate II. Intramolecular tosyl group transfer and nucleophilic substitution provided 5 in which the methyl and dimethylamino groups were in a cis relationship. 5 was in equilibrium with the sterically disfavored III. Once III was formed, the dimethylamino group was in an ideal position to displace the bromide following a typical SNAr mechanism to give V.4d,g,13 Finally, nucleophilic attack of the methyl group attached on nitrogen with the displaced bromide or base furnished 4a.
Conclusions
In summary, we have developed an efficient and environmentally friendly Cs2CO3-mediated cascade reaction for the synthesis of dihydropyrrolizino[3,2-b]indol-10-ones. The reaction mechanism involved consecutive nucleophilic attack of DMF with enolate and pyrrolyl nitrogen, followed by transition metal free C–N bond formation.Experimental section
General
Melting points were determined on an XT4A hot-stage apparatus and are uncorrected. IR spectra were obtained using a PerkinElmer FT/IR spectrometer. 1H and 13C NMR spectra were obtained on an Agilent AV400 instrument. High-resolution mass spectra were recorded on a Micromass Q-TOF mass spectrometer. The X-ray diffraction (XRD) patterns of the samples were recorded on a Rigaku B/Max-RB diffractometer.2-[(2-Bromophenyl)ethynyl]-1H-pyrrole (2)
A mixture of 2-(2-bromophenyl)-1-(1-tosyl-1H-pyrrol-2-yl)ethan-1-one (167 mg, 0.4 mmol), Cs2CO3 (261 mg, 0.8 mmol), Pd(PPh3)4 (5 mg, 0.004 mmol) and DMF (4.0 mL) under nitrogen was heated at 120 °C for 12 h. The cooled mixture was partitioned between EtOAc (10 mL) and H2O (20 mL). The separated aqueous phase was extracted with EtOAc (3 × 10 mL). The combined organic extracts were washed with brine (30 mL), then dried (Na2SO4), filtered, and evaporated in vacuo. The residue was purified by column chromatography on silica gel (5% EtOAc in petroleum ether) to afford 2 (72 mg, 73%) as a gray oil; 1H NMR (CDCl3, 400 MHz): δ = 8.45 (s, 1H), 7.58 (dd, J = 8.1, 0.9 Hz, 1H), 7.49 (dd, J = 7.7, 1.6 Hz, 1H), 7.26 (m, 1H), 7.14 (td, J = 7.8, 1.6 Hz, 1H), 6.82 (m, 1H), 6.60 (m, 1H), 6.24 (m, 1H) ppm; 13C NMR (CDCl3, 100 MHz): δ = 132.9, 132.5, 129.2, 127.2, 125.6, 125.0, 120.2, 115.6, 112.7, 109.8, 89.3, 86.7 ppm; IR (neat, cm−1) νmax 3416, 2204, 1431, 1043, 1088, 1025; HRMS (ESI): m/z calcd for C12H979BrN [M + H]+: 245.9913; found 245.9917.General procedure for the preparation of 4a–n, 5, 6 and 10
A sealed tube was charged with 2-(2-bromophenyl)-1-(N-tosyl-2-pyrrolyl)propan-1-one (1.0 mmol), Cs2CO3 (2.0 mmol) and DMF (10 mL). The reaction system was recharged with nitrogen three times. The reaction mixture was heated at 120 °C for 24 h and cooled. The mixture was partitioned between EtOAc (20 mL) and H2O (40 mL). The separated aqueous phase was extracted with EtOAc (3 × 20 mL). The combined organic extracts were washed with brine (100 mL), then dried (Na2SO4), filtered, and evaporated in vacuo. The residue was purified by column chromatography on silica gel (petroleum ether/EtOAc) to afford the products 4a–n, 5, 6 and 10.5,9b-Dimethyl-5,9b-dihydropyrrolizino[3,2-b]indol-10(4aH)-one (4a)
The title compound was prepared according to the general procedure by stirring a mixture of 2-(2-bromophenyl)-1-(1-tosyl-1H-pyrrol-2-yl)propan-1-one (173 mg, 0.4 mmol), Cs2CO3 (261 mg, 0.8 mmol) and DMF (4.0 mL) at 120 °C for 24 h. The crude product was purified by column chromatography on silica gel (7% EtOAc in petroleum ether) to afford 4a (83 mg, 87%) as a colorless solid; mp 145–146 °C; 1H NMR (DMSO-d6, 400 MHz): δ = 7.59 (d, J = 0.8 Hz, 1H), 7.17 (d, J = 7.3 Hz, 1H), 7.12 (t, J = 7.5 Hz, 1H), 6.71–6.68 (m, 2H), 6.58 (dd, J = 3.6, 2.4 Hz, 1H), 6.52 (d, J = 7.9 Hz, 1H), 5.92 (s, 1H), 3.16 (s, 3H), 1.52 (s, 3H) ppm; 13C NMR (DMSO-d6, 100 MHz): δ = 188.8, 148.5, 130.4, 129.2, 128.8, 123.9, 122.7, 118.1, 117.2, 107.9, 106.5, 83.2, 62.0, 31.8, 20.1 ppm; IR (neat, cm−1) νmax 1688, 1493, 1360, 1279, 1217, 1048; HRMS (ESI): m/z calcd for C15H14N2ONa [M + Na]+: 261.0998; found 261.0996.9b-Methyl-5-(methyl-d3)-5,9b-dihydropyrrolizino[3,2-b]indol-10(4aH)-one-4a-d (4a-D)
The title compound was prepared according to the general procedure by stirring a mixture of 2-(2-bromophenyl)-1-(1-tosyl-1H-pyrrol-2-yl)propan-1-one (173 mg, 0.4 mmol), Cs2CO3 (261 mg, 0.8 mmol) and DMF-d7 (2.0 mL) at 120 °C for 24 h. The crude product was purified by column chromatography on silica gel (7% EtOAc in petroleum ether) to afford 4a-D (74 mg, 76%) as a colorless solid; mp 132–133 °C; 1H NMR (CDCl3, 400 MHz): δ = 7.36 (dd, J = 7.4, 0.8 Hz, 1H), 7.17–7.12 (m, 2H), 6.76 (td, J = 7.5, 0.9 Hz, 1H), 6.73 (dd, J = 3.9, 1.0 Hz, 1H), 6.54 (dd, J = 3.9, 2.3 Hz, 1H), 6.42 (d, J = 7.9 Hz, 1H), 5.61 (s, 0.34H), 1.64 (s, 3H) ppm; 13C NMR (CDCl3, 100 MHz): δ = 189.5, 148.4, 131.5, 129.4, 129.0, 123.6, 122.2, 119.0, 117.6, 108.7, 106.4, 83.8 (t, JD–C = 25.4 Hz), 62.8, 31.4 (m), 20.6 ppm; IR (neat, cm−1) νmax 1688, 1601, 1487, 1359, 1288, 1047, 1011; HRMS (ESI): m/z calcd for C15H10D4N2ONa [M + Na]+: 265.1249; found 265.1246.9b-Ethyl-5-methyl-5,9b-dihydropyrrolizino[3,2-b]indol-10(4aH)-one (4b)
The title compound was prepared according to the general procedure by stirring a mixture of 2-(2-bromophenyl)-1-(1-tosyl-1H-pyrrol-2-yl)butan-1-one (179 mg, 0.4 mmol), Cs2CO3 (261 mg, 0.8 mmol) and DMF (4.0 mL) at 120 °C for 24 h. The crude product was purified by column chromatography on silica gel (7% EtOAc in petroleum ether) to afford 4b (94 mg, 93%) as a colorless solid; mp 137–138 °C; 1H NMR (DMSO-d6, 400 MHz): δ = 7.60 (d, J = 1.8 Hz, 1H), 7.16–7.10 (m, 2H), 6.70–6.66 (m, 2H), 6.57 (dd, J = 3.8, 2.4 Hz, 1H), 6.51 (d, J = 7.8 Hz, 1H), 6.05 (s, 1H), 3.15 (s, 3H), 2.05 (dq, J = 14.4, 7.2 Hz, 1H), 1.90 (dq, J = 14.4, 7.2 Hz, 1H), 0.77 (t, J = 7.4 Hz, 3H) ppm; 13C NMR (DMSO-d6, 100 MHz): δ = 188.6, 149.0, 131.0, 129.3, 127.2, 124.0, 122.8, 118.0, 117.1, 107.7, 106.5, 80.7, 67.1, 31.8, 26.7, 8.8 ppm; IR (neat, cm−1) νmax 1682, 1604, 1525, 1492, 1455, 1275, 1219; HRMS (ESI): m/z calcd for C16H16N2ONa [M + Na]+: 275.1155; found 275.1156.9b-Benzyl-5-methyl-5,9b-dihydropyrrolizino[3,2-b]indol-10(4aH)-one (4c)
The title compound was prepared according to the general procedure by stirring a mixture of 2-(2-bromophenyl)-3-phenyl-1-(1-tosyl-1H-pyrrol-2-yl)propan-1-one (203 mg, 0.4 mmol), Cs2CO3 (261 mg, 0.8 mmol) and DMF (4.0 mL) at 120 °C for 24 h. The crude product was purified by column chromatography on silica gel (7% EtOAc in petroleum ether) to afford 4c (112 mg, 89%) as a colorless solid; mp 154–155 °C; 1H NMR (CDCl3, 400 MHz): δ = 7.49 (dd, J = 7.5, 1.2 Hz, 1H), 7.19–7.12 (m, 4H), 7.04–7.07 (m, 2H), 7.01 (dd, J = 2.2, 0.8 Hz, 1H), 6.81 (td, J = 7.5, 0.7 Hz, 1H), 6.68 (dd, J = 3.9, 0.8 Hz, 1H), 6.45 (dd, J = 3.9, 2.2 Hz, 1H), 6.38 (d, J = 7.9 Hz, 1H), 5.62 (s, 1H), 3.62 (d, J = 14.0 Hz, 1H), 3.12 (d, J = 14.0 Hz, 1H), 3.00 (s, 3H) ppm; 13C NMR (CDCl3, 100 MHz): δ = 188.5, 148.7, 136.6, 132.0, 129.7, 129.6, 128.5, 128.1, 127.0, 123.9, 122.4, 119.1, 117.4, 108.5, 107.0, 80.9, 68.2, 40.0, 32.5 ppm; IR (neat, cm−1) νmax 1697, 1487, 1367, 1281, 1222, 1061, 1024; HRMS (ESI): m/z calcd for C21H18N2ONa [M + Na]+: 337.1311; found 337.1313.9b-(2-Bromobenzyl)-5-methyl-5,9b-dihydropyrrolizino[3,2-b]indol-10(4aH)-one (4d)
The title compound was prepared according to the general procedure by stirring a mixture of 2,3-bis(2-bromophenyl)-1-(1-tosyl-1H-pyrrol-2-yl)propan-1-one (235 mg, 0.4 mmol), Cs2CO3 (261 mg, 0.8 mmol) and DMF (4.0 mL) at 120 °C for 24 h. The crude product was purified by column chromatography on silica gel (7% EtOAc in petroleum ether) to afford 4d (142 mg, 90%) as a colorless solid; mp 135–136 °C; 1H NMR (CDCl3, 400 MHz): δ = 7.51 (dd, J = 7.9, 1.3 Hz, 1H), 7.46 (dd, J = 7.5, 0.9 Hz, 1H), 7.20–7.16 (m, 2H), 7.08 (td, J = 7.5, 1.4 Hz, 1H), 7.04–6.99 (m, 2H), 6.80 (td, J = 7.5, 0.8 Hz, 1H), 6.68 (dd, J = 3.9, 1.0 Hz, 1H), 6.45 (dd, J = 3.9, 2.3 Hz, 1H), 6.42 (d, J = 7.9 Hz, 1H), 5.76 (s, 1H), 3.82 (d, J = 14.2 Hz, 1H), 3.37 (d, J = 14.2 Hz, 1H), 3.11 (s, 3H) ppm; 13C NMR (CDCl3, 100 MHz): δ = 188.1, 148.5, 136.3, 133.0, 132.0, 132.0, 129.8, 128.8, 128.0, 127.9, 125.4, 123.8, 122.5, 119.1, 117.5, 108.6, 107.0, 80.8, 68.2, 38.6, 32.6 ppm; IR (neat, cm−1) νmax 1698, 1488, 1365, 1277, 1215, 1021; HRMS (ESI): m/z calcd for C21H1779BrN2ONa [M + Na]+: 415.0416; found 415.0429.9b-(3-Azidopropyl)-5-methyl-5,9b-dihydropyrrolizino[3,2-b]indol-10(4aH)-one (4e)
The title compound was prepared according to the general procedure by stirring a mixture of 5-azido-2-(2-bromophenyl)-1-(1-tosyl-1H-pyrrol-2-yl)pentan-1-one (200 mg, 0.4 mmol), Cs2CO3 (261 mg, 0.8 mmol) and DMF (4.0 mL) at 120 °C for 24 h. The crude product was purified by column chromatography on silica gel (3% EtOAc in petroleum ether) to afford 4e (39 mg, 32%) as a colorless solid; mp 102–103 °C; 1H NMR (CDCl3, 400 MHz): δ = 7.33 (dd, J = 7.4, 0.7 Hz, 1H), 7.18–7.14 (m, 2H), 6.77 (td, J = 7.4, 0.6 Hz, 1H), 6.73 (dd, J = 3.9, 0.8 Hz, 1H), 6.54 (dd, J = 3.9, 2.4 Hz, 1H), 6.42 (d, J = 7.9 Hz, 1H), 5.72 (s, 1H), 3.31–3.20 (m, 2H), 3.17 (s, 3H), 2.19–2.08 (m, 2H), 1.61–1.40 (m, 2H) ppm; 13C NMR (CDCl3, 100 MHz): δ = 188.5, 148.7, 131.8, 129.7, 127.1, 123.7, 122.5, 119.2, 117.7, 108.8, 106.6, 81.8, 66.8, 51.4, 32.2, 31.4, 24.5 ppm; IR (neat, cm−1) νmax 2093, 1694, 1490, 1364, 1283, 1245, 1220, 1055; HRMS (ESI): m/z calcd for C17H17N5ONa [M + Na]+: 330.1325; found 330.1340.9b,9b′-(Hexane-1,6-diyl)bis[5-methyl-5,9b-dihydropyrrolizino[3,2-b]indol-10(4aH)-one] (4f)
The title compound was prepared according to the general procedure by stirring a mixture of 2,9-bis(2-bromophenyl)-1,10-bis(1-tosyl-1H-pyrrol-2-yl)decane-1,10-dione (367 mg, 0.4 mmol), Cs2CO3 (261 mg, 0.8 mmol) and DMF (4.0 mL) at 120 °C for 24 h. The crude product was purified by column chromatography on silica gel (3% EtOAc in petroleum ether) to afford 4f (113 mg, 53%) as a colorless solid; mp 176–177 °C; 1H NMR (CDCl3, 400 MHz): δ = 7.30 (d, J = 7.4 Hz, 2H), 7.15–7.11 (m, 4H), 6.74 (t, J = 7.5 Hz, 2H), 6.70 (dd, J = 3.9, 0.9 Hz, 2H), 6.52 (dd, J = 7.6, 2.3 Hz, 2H), 6.39 (dd, J = 7.8, 3.3 Hz, 2H), 5.70 (s, 2H), 3.16 (s, 6H), 2.10–1.90 (m, 4H), 1.28–1.17 (m, 8H) ppm; 13C NMR (CDCl3, 100 MHz): δ = 189.3, 148.6, 131.9, 129.4, 127.8, 123.6, 122.3, 119.0, 117.5, 108.5, 106.4, 81.8, 67.4, 34.3, 32.3, 29.4, 24.5 ppm; IR (neat, cm−1) νmax 1691, 1278, 1121, 1054; HRMS (ESI): m/z calcd for C34H34N4O2Na [M + Na]+: 553.2574; found 553.2574.9b,9b′-[1,4-Phenylenebis(methylene)]bis{5-methyl-5,9b-dihydropyrrolizino[3,2-b]indol-10(4aH)-one} (4g)
The title compound was prepared according to the general procedure by stirring a mixture of 2-(2-bromophenyl)-3-(4-(2-(2-bromophenyl)-3-oxo-3-(1-tosyl-1H-pyrrol-2-yl)propyl)phenyl)-1-(1-tosyl-1H-pyrrol-2-yl)propan-1-one (375 mg, 0.4 mmol), Cs2CO3 (261 mg, 0.8 mmol) and DMF (4.0 mL) at 120 °C for 24 h. The crude product was purified by column chromatography on silica gel (3% EtOAc in petroleum ether) to afford 4g (119 mg, 54%) as a colorless solid; mp 226–228 °C; 1H NMR (CDCl3, 400 MHz): δ = 7.43 (d, J = 7.3 Hz, 2H), 7.13 (t, J = 7.6 Hz, 2H), 6.96 (m, 2H), 6.85 (s, 4H), 6.77 (t, J = 7.4 Hz, 2H), 6.66 (d, J = 3.7 Hz, 2H), 6.44 (m, 2H), 6.32 (d, J = 7.9 Hz, 2H), 5.49 (s, 2H), 3.47 (d, J = 14.0 Hz, 2H), 3.04 (d, J = 14.0 Hz, 2H), 2.90 (s, 6H) ppm; 13C NMR (CDCl3, 100 MHz): δ = 188.4, 148.8, 135.1, 131.9, 129.7, 129.6, 127.9, 123.8, 122.3, 119.1, 117.4, 108.5, 107.0, 81.0, 68.1, 39.6, 32.6 ppm; IR (neat, cm−1) νmax 1687, 1607, 1497, 1359, 1280, 1225; HRMS (ESI): m/z calcd for C36H30N4O2Na [M + Na]+: 573.2261; found 573.2248.8-Fluoro-5,9b-dimethyl-5,9b-dihydropyrrolizino[3,2-b]indol-10(4aH)-one (4h)
The title compound was prepared according to the general procedure by stirring a mixture of 2-(2-bromo-5-fluorophenyl)-1-(1-tosyl-1H-pyrrol-2-yl)propan-1-one (180 mg, 0.4 mmol), Cs2CO3 (261 mg, 0.8 mmol) and DMF (4.0 mL) at 120 °C for 24 h. The crude product was purified by column chromatography on silica gel (7% EtOAc in petroleum ether) to afford 4h (89 mg, 87%) as a colorless solid; mp 108–109 °C; 1H NMR (CDCl3, 400 MHz): δ = 7.16 (dd, J = 2.4, 0.9 Hz, 1H), 7.09 (dd, J = 8.1, 2.6 Hz, 1H), 6.83 (td, J = 8.8, 2.4 Hz, 1H), 6.75 (dd, J = 3.9, 0.9 Hz, 1H), 6.56 (dd, J = 3.9, 2.4 Hz, 1H), 6.30 (dd, J = 8.6, 4.0 Hz, 1H), 5.62 (s, 1H), 3.16 (s, 3H), 1.63 (s, 3H) ppm; 13C NMR (CDCl3, 100 MHz): δ = 188.8, 157.2 (d, JF–C = 235.1 Hz), 144.6 (d, JF–C = 1.4 Hz), 131.2, 130.4 (d, JF–C = 8.3 Hz), 122.4, 117.8, 115.4 (d, JF–C = 23.3 Hz), 111.3 (d, JF–C = 24.9 Hz), 109.0, 106.7 (d, JF–C = 8.1 Hz), 84.8, 62.8 (d, JF–C = 2.0 Hz), 32.8, 20.5 ppm; IR (neat, cm−1) νmax 1693, 1494, 1364, 1288, 1265, 1225, 1056, 1008; HRMS (ESI): m/z calcd for C15H13FN2ONa [M + Na]+: 279.0904; found 279.0896.8-Chloro-5,9b-dimethyl-5,9b-dihydropyrrolizino[3,2-b]indol-10(4aH)-one (4i)
The title compound was prepared according to the general procedure by stirring a mixture of 2-(2-bromo-5-chlorophenyl)-1-(1-tosyl-1H-pyrrol-2-yl)propan-1-one (187 mg, 0.4 mmol), Cs2CO3 (261 mg, 0.8 mmol) and DMF (4.0 mL) at 120 °C for 24 h. The crude product was purified by column chromatography on silica gel (7% EtOAc in petroleum ether) to afford 4i (98 mg, 90%) as a colorless solid; mp 143–145 °C; 1H NMR (CDCl3, 400 MHz): δ = 7.31 (d, J = 2.2 Hz, 1H), 7.16 (dd, J = 2.4, 0.9 Hz, 1H), 7.07 (dd, J = 8.4, 2.2 Hz, 1H), 6.73 (dd, J = 3.9, 0.9 Hz, 1H), 6.55 (dd, J = 3.9, 2.4 Hz, 1H), 6.30 (d, J = 8.4 Hz, 1H), 5.62 (s, 1H), 3.15 (s, 3H), 1.61 (s, 3H) ppm; 13C NMR (CDCl3, 100 MHz): δ = 188.6, 147.0, 131.1, 130.6, 129.1, 123.8, 123.7, 122.4, 117.9, 109.0, 107.2, 84.3, 62.7, 32.3, 20.6 ppm; IR (neat, cm−1) νmax 1698, 1493, 1369, 1271, 1055; HRMS (ESI): m/z calcd for C15H1335ClN2ONa [M + Na]+: 295.0609; found 295.0606.5,9b-Dimethyl-8-(trifluoromethyl)-5,9b-dihydropyrrolizino[3,2-b]indol-10(4aH)-one (4j)
The title compound was prepared according to the general procedure by stirring a mixture of 2-(2-bromo-5-(trifluoromethyl)phenyl)-1-(1-tosyl-1H-pyrrol-2-yl)propan-1-one (200 mg, 0.4 mmol), Cs2CO3 (261 mg, 0.8 mmol) and DMF (4.0 mL) at 120 °C for 24 h. The crude product was purified by column chromatography on silica gel (7% EtOAc in petroleum ether) to afford 4j (91 mg, 75%) as a colorless solid; mp 165–166 °C; 1H NMR (CDCl3, 400 MHz): δ = 7.57 (d, J = 1.2 Hz, 1H), 7.40 (dd, J = 8.3, 1.2 Hz, 1H), 7.18 (dd, J = 2.4, 0.9 Hz, 1H), 6.75 (dd, J = 3.9, 0.9 Hz, 1H), 6.57 (dd, J = 3.9, 2.4 Hz, 1H), 6.42 (d, J = 8.3 Hz, 1H), 5.69 (s, 1H), 3.22 (s, 3H), 1.66 (s, 3H) ppm; 13C NMR (CDCl3, 100 MHz): δ = 188.4, 150.8, 131.0, 129.3, 127.3 (q, JF–C = 3.8 Hz), 124.9 (q, JF–C = 269.1 Hz), 122.3, 120.9 (q, JF–C = 32.4 Hz), 120.8 (q, JF–C = 3.6 Hz), 118.1, 109.3, 105.6, 84.2, 62.5, 31.9, 20.8 ppm; IR (neat, cm−1) νmax 1696, 1618, 1321, 1279, 1095, 1068, 1056; HRMS (ESI): m/z calcd for C16H13F3N2ONa [M + Na]+: 329.0872; found 329.0873.7-Fluoro-5,9b-dimethyl-5,9b-dihydropyrrolizino[3,2-b]indol-10(4aH)-one (4k)
The title compound was prepared according to the general procedure by stirring a mixture of 2-(2-bromo-4-fluorophenyl)-1-(1-tosyl-1H-pyrrol-2-yl)propan-1-one (180 mg, 0.4 mmol), Cs2CO3 (261 mg, 0.8 mmol) and DMF (4.0 mL) at 120 °C for 24 h. The crude product was purified by column chromatography on silica gel (7% EtOAc in petroleum ether) to afford 4k (95 mg, 93%) as a colorless solid; mp 164–165 °C; 1H NMR (CDCl3, 400 MHz): δ = 7.25 (dd, J = 8.2, 5.5 Hz, 1H), 7.15 (dd, J = 2.3, 0.8 Hz, 1H), 6.75 (dd, J = 3.9, 0.8 Hz, 1H), 6.56 (dd, J = 3.9, 2.3 Hz, 1H), 6.41 (ddd, J = 9.5, 8.2, 2.3 Hz, 1H), 6.11 (dd, J = 9.8, 2.3 Hz, 1H), 5.64 (s, 1H), 3.16 (s, 3H), 1.62 (s, 3H) ppm; 13C NMR (CDCl3, 100 MHz): δ = 189.2, 164.8 (d, JF–C = 242.1 Hz), 149.9 (d, JF–C = 11.9 Hz), 131.3, 124.5 (d, JF–C = 2.2 Hz), 124.3 (d, JF–C = 10.7 Hz), 122.1, 117.8, 109.0, 105.0 (d, JF–C = 22.9 Hz), 94.6 (d, JF–C = 27.4 Hz), 84.7, 62.3, 32.1, 20.8 ppm; IR (neat, cm−1) νmax 1691, 1495, 1244, 1219, 1092, 1051, 1017; HRMS (ESI): m/z calcd for C15H13FN2ONa [M + Na]+: 279.0904; found 279.0906.7,8-Dimethoxy-5,9b-dimethyl-5,9b-dihydropyrrolizino[3,2-b]indol-10(4aH)-one (4l) and 2-(2-bromo-4,5-dimethoxyphenyl)-2-methyl-3-(dimethylamino)-2,3-dihydro-1H-pyrrolizin-1-one (6)
A mixture of 2-(2-bromo-4,5-dimethoxyphenyl)-1-(1-tosyl-1H-pyrrol-2-yl)propan-1-one (197 mg, 0.4 mmol), Cs2CO3 (261 mg, 0.8 mmol) and DMF (4.0 mL) was stirred at 120 °C for 24 h. The crude product was purified by column chromatography on silica gel (3% EtOAc in petroleum ether) to afford 4l (60 mg, 50%) and 6 (44 mg, 28%) both as colorless solids.Compound 4l showed: mp 137–138 °C; 1H NMR (CDCl3, 400 MHz): δ = 7.14 (d, J = 2.4 Hz, 1H), 6.96 (s, 1H), 6.73 (d, J = 3.8 Hz, 1H), 6.55 (dd, J = 3.8, 2.4 Hz, 1H), 6.09 (s, 1H), 5.57 (s, 1H), 3.83 (s, 3H), 3.82 (s, 3H), 3.17 (s, 3H), 1.62 (s, 3H) ppm; 13C NMR (CDCl3, 100 MHz): δ = 189.7, 150.9, 143.0, 142.8, 131.4, 122.2, 119.7, 117.5, 108.6, 108.4, 93.1, 85.1, 62.8, 57.1, 56.3, 33.3, 20.5 ppm; IR (neat, cm−1) νmax 1690, 1506, 1220, 1205, 1050, 1022; HRMS (ESI): m/z calcd for C17H19N2O3 [M + H]+: 299.1390; found 299.1386.
Compound 6 showed: mp 91–92 °C; 1H NMR (CDCl3, 400 MHz): δ = 7.07 (s, 1H), 6.99 (d, J = 2.2 Hz, 1H), 6.83 (d, J = 3.9 Hz, 1H), 6.72 (s, 1H), 6.50 (dd, J = 3.9, 2.2 Hz, 1H), 5.75 (s, 1H), 3.83 (s, 3H), 3.74 (s, 3H), 2.26 (s, 6H), 1.75 (s, 3H) ppm; 13C NMR (CDCl3, 100 MHz): δ = 193.0, 148.3, 146.9, 134.6, 132.7, 123.6, 118.2, 116.4, 112.7, 112.6, 108.0, 84.1, 62.5, 56.2, 56.0, 40.4, 18.1 ppm; IR (neat, cm−1) νmax 1698, 1500, 1361, 1249, 1204, 1162; HRMS (ESI): m/z calcd for C18H2279BrN2O3 [M + H]+: 393.0808; found 393.0818.
6b,12-Dimethyl-11a,12-dihydrobenzo[g]pyrrolizino[3,2-b]indol-7(6bH)-one (4m)
The title compound was prepared according to the general procedure by stirring a mixture of 2-(1-bromonaphthalen-2-yl)-1-(1-tosyl-1H-pyrrol-2-yl)propan-1-one (193 mg, 0.4 mmol), Cs2CO3 (261 mg, 0.8 mmol) and DMF (4.0 mL) at 120 °C for 24 h. The crude product was purified by column chromatography on silica gel (3% EtOAc in petroleum ether) to afford 4m (81 mg, 70%) as a colorless solid; mp 216–218 °C; 1H NMR (CDCl3, 400 MHz): δ = 8.02 (m, 1H), 7.78 (m, 1H), 7.53 (d, J = 8.3 Hz, 1H), 7.44 (d, J = 8.3 Hz, 1H), 7.40–7.37 (m, 2H), 7.25 (m, 1H), 6.67 (dd, J = 3.9, 0.9 Hz, 1H), 6.53 (dd, J = 3.9, 2.4 Hz, 1H), 5.53 (s, 1H), 3.56 (s, 3H), 1.74 (s, 3H) ppm; 13C NMR (CDCl3, 100 MHz): δ = 189.6, 145.6, 135.4, 130.5, 129.1, 127.2, 125.7, 125.2, 123.0, 122.8, 122.1, 121.2, 117.7, 108.3, 88.8, 63.8, 42.4, 21.7 ppm; IR (neat, cm−1) νmax 1687, 1362, 1303, 1277, 1058; HRMS (ESI): m/z calcd for C19H16N2ONa [M + Na]+: 311.1155; found 311.1151.3-Ethyl-5,9b-dimethyl-5,9b-dihydropyrrolizino[3,2-b]indol-10(4aH)-one (4n)
The title compound was prepared according to the general procedure by stirring a mixture of 2-(2-bromophenyl)-1-(5-ethyl-1-tosyl-1H-pyrrol-2-yl)propan-1-one (184 mg, 0.4 mmol), Cs2CO3 (261 mg, 0.8 mmol) and DMF (4.0 mL) at 120 °C for 24 h. The crude product was purified by column chromatography on silica gel (3% EtOAc in petroleum ether) to afford 4n (45 mg, 42%) as a colorless solid; mp 71–72 °C; 1H NMR (CDCl3, 400 MHz): δ = 7.39 (d, J = 7.4 Hz, 1H), 7.17 (t, J = 7.7 Hz, 1H), 6.82 (t, J = 7.4 Hz, 1H), 6.72 (d, J = 3.9 Hz, 1H), 6.52 (d, J = 7.9 Hz, 1H), 6.34 (d, J = 3.9 Hz, 1H), 5.47 (s, 1H), 3.24 (s, 3H), 2.84 (q, J = 7.5 Hz, 2H), 1.64 (s, 3H), 1.37 (t, J = 7.5 Hz, 3H) ppm; 13C NMR (CDCl3, 100 MHz): δ = 188.8, 150.1, 139.5, 130.0, 129.9, 129.3, 123.6, 120.0, 115.3, 109.6, 108.8, 86.3, 63.6, 37.4, 22.4, 20.7, 12.8 ppm; IR (neat, cm−1) νmax 1687, 1487, 1355, 1274, 1260, 1222, 1017; HRMS (ESI): m/z calcd for C17H19N2O [M + H]+: 267.1492; found 267.1494.2-(2-Bromophenyl)-2-methyl-3-(dimethylamino)-2,3-dihydro-1H-pyrrolizin-1-one (5)
The title compound was prepared according to the general procedure by stirring a mixture of 2-(2-bromophenyl)-1-(1-tosyl-1H-pyrrol-2-yl)propan-1-one (173 mg, 0.4 mmol), Cs2CO3 (261 mg, 0.8 mmol) and DMF (4.0 mL) at 80 °C for 24 h. The crude product was purified by column chromatography on silica gel (3% EtOAc in petroleum ether) to afford 5 (37 mg, 28%) as a colorless solid; mp 130–132 °C; 1H NMR (CDCl3, 400 MHz): δ = 7.62 (dd, J = 7.8, 1.4 Hz, 1H), 7.22–7.14 (m, 2H), 7.06 (td, J = 7.8, 2.0 Hz, 1H), 6.98 (dd, J = 2.2, 1.0 Hz, 1H), 6.83 (dd, J = 4.0, 1.0 Hz, 1H), 6.49 (dd, J = 4.0, 2.2 Hz, 1H), 5.74 (s, 1H), 2.26 (s, 6H), 1.78 (s, 3H) ppm; 13C NMR (CDCl3, 100 MHz): δ = 191.8, 141.5, 134.7, 131.8, 128.7, 127.8, 126.8, 122.5, 121.9, 115.4, 107.1, 82.9, 62.0, 39.4, 17.0 ppm; IR (neat, cm−1) νmax 1684, 1362, 1053, 1038, 1016; HRMS (ESI): m/z calcd for C16H1779BrN2ONa [M + Na]+: 355.0416; found 355.0415.2-(3-Bromophenyl)-2-methyl-3-(dimethylamino)-2,3-dihydro-1H-pyrrolizin-1-one (10)
The title compound was prepared according to the general procedure by stirring a mixture of 2-(3-bromophenyl)-1-(1-tosyl-1H-pyrrol-2-yl)propan-1-one (173 mg, 0.4 mmol), Cs2CO3 (261 mg, 0.8 mmol) and DMF (4.0 mL) at 120 °C for 24 h. The crude product was purified by column chromatography on silica gel (3% EtOAc in petroleum ether) to afford 10 (37 mg, 28%) as a colorless solid; mp 78–79 °C; 1H NMR (CDCl3, 400 MHz): δ = 7.36 (m, 1H), 7.33 (dt, J = 7.3, 1.8 Hz, 1H), 7.14–7.08 (m, 2H), 7.06 (dd, J = 2.3, 1.0 Hz, 1H), 6.82 (dd, J = 3.9, 1.0 Hz, 1H), 6.54 (dd, J = 3.9, 2.3 Hz, 1H), 5.30 (s, 1H), 2.24 (s, 6H), 1.62 (s, 3H) ppm; 13C NMR (CDCl3, 100 MHz): δ = 191.7, 147.0, 132.4, 130.4, 130.2, 129.2, 124.6, 123.9, 123.0, 116.8, 108.7, 85.9, 61.3, 40.8, 18.6 ppm; IR (neat, cm−1) νmax 1693, 1527, 1363, 1269, 1047, 1035; HRMS (ESI): m/z calcd for C16H1779BrN2ONa [M + Na]+: 355.0416; found 355.0414.4a,5,9b-Trimethyl-5,9b-dihydropyrrolizino[3,2-b]indol-10(4aH)-one (4a′) and 5-methyl-5-[1-(dimethylamino)vinyl]pyrrolo[1,2-a]quinolin-4(5H)-one (7)
A mixture of 2-(2-bromophenyl)-1-(1-tosyl-1H-pyrrol-2-yl)propan-1-one (173 mg, 0.4 mmol), Cs2CO3 (261 mg, 0.8 mmol) and N,N-dimethylacetamide (4.0 mL) was stirred at 120 °C for 24 h. The crude product was purified by column chromatography on silica gel (7% EtOAc in petroleum ether) to afford 4a′ (7 mg, 7%) and 7 (16 mg, 15%) both as colorless solids.Compound 4a′ showed: mp 117–118 °C; 1H NMR (CDCl3, 400 MHz): δ = 7.34 (d, J = 7.4 Hz, 1H), 7.12 (td, J = 7.7, 1.2 Hz, 1H), 7.10 (dd, J = 2.3, 0.9 Hz, 1H), 6.75 (t, J = 7.6 Hz, 1H), 6.70 (d, J = 3.9 Hz, 1H), 6.49 (dd, J = 3.9, 2.3 Hz, 1H), 6.39 (d, J = 7.9 Hz, 1H), 3.06 (s, 3H), 1.77 (s, 3H), 1.53 (s, 3H) ppm; 13C NMR (CDCl3, 100 MHz): δ = 189.1, 147.8, 131.3, 129.2, 128.8, 123.5, 120.8, 119.1, 116.9, 108.2, 106.3, 86.2, 65.4, 28.7, 21.0, 17.6 ppm; IR (neat, cm−1) νmax 1682, 1603, 1530, 1488, 1362, 1291, 1019; HRMS (ESI): m/z calcd for C16H17N2O [M + H]+: 253.1335; found 253.1325.
Compound 7 showed: mp 86–88 °C; 1H NMR (CDCl3, 400 MHz): δ = 7.52 (dd, J = 7.6, 1.6 Hz, 1H), 7.34–7.25 (m, 2H), 7.22 (dt, J = 7.6, 1.6 Hz, 1H), 7.16 (dd, J = 2.6, 1.0 Hz, 1H), 6.74 (d, J = 3.6 Hz, 1H), 6.55 (dd, J = 3.6, 2.6 Hz, 1H), 4.96 (d, J = 2.4 Hz, 1H), 4.25 (d, J = 2.4 Hz, 1H), 2.22 (s, 3H), 2.06 (s, 3H), 1.74 (s, 3H) ppm; 13C NMR (CDCl3, 100 MHz): δ = 189.8, 152.1, 150.3, 140.7, 133.2, 129.0, 127.5, 126.6, 124.4, 116.9, 115.6, 106.5, 90.0, 57.8, 45.6, 43.5, 23.7 ppm; IR (neat, cm−1) νmax 1693, 1658, 1450, 1417, 1370, 1305; HRMS (ESI): m/z calcd for C17H19N2O [M + H]+: 267.1492; found 267.1485.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful to the NSFC (#81330075; #21172202) for financial support.Notes and references
- For reviews, see: (a) K. C. Nicolaou, P. G. Bulger and D. Sarlah, Angew. Chem., Int. Ed., 2005, 44, 4442 CrossRef CAS PubMed; (b) L.-M. Xu, B.-J. Li, Z. Yang and Z.-J. Shi, Chem. Soc. Rev., 2010, 39, 712 RSC; (c) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef CAS PubMed; (d) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef CAS PubMed.
- For reviews, see: (a) C.-L. Sun and Z.-J. Shi, Chem. Rev., 2014, 114, 9219 CrossRef CAS PubMed; (b) V. P. Mehta and B. Punji, RSC Adv., 2013, 3, 11957 RSC.
- For selected examples, see: (a) S. Yanagisawa, K. Ueda, T. Taniguchi and K. Itami, Org. Lett., 2008, 10, 4673 CrossRef CAS PubMed; (b) C.-L. Sun, Y.-F. Gu, W.-P. Huang and Z.-J. Shi, Chem. Commun., 2011, 47, 9813 RSC; (c) A. Studer and D. P. Curran, Angew. Chem., Int. Ed., 2011, 50, 5018 CrossRef CAS PubMed; (d) M. Rueping, M. Leiendecker, A. Das, T. Poisson and L. Bui, Chem. Commun., 2011, 47, 10629 RSC; (e) E. Shirakawa, K.-I. Itoh, T. Higashino and T. Hayashi, J. Am. Chem. Soc., 2010, 132, 15537 CrossRef CAS PubMed; (f) D. S. Roman, Y. Takahashi and A. B. Charette, Org. Lett., 2011, 13, 3242 CrossRef CAS PubMed.
- (a) M. Beller, C. Breindl, T. H. Riermeier and A. Tillack, J. Org. Chem., 2001, 66, 1403 CrossRef CAS PubMed; (b) L. Shi, M. Wang, C.-A. Fan, F.-M. Zhang and Y.-Q. Tu, Org. Lett., 2003, 5, 3515 CrossRef CAS PubMed; (c) J. L. Bolliger and C. M. Frech, Tetrahedron, 2009, 65, 1180 CrossRef CAS; (d) Y. Yuan, I. Thomé, S. H. Kim, D. Chen, A. Beyer, J. Bonnamour, E. Zuidema, S. Chang and C. Bolm, Adv. Synth. Catal., 2010, 352, 2892 CrossRef CAS; (e) I. Thomé and C. Bolm, Org. Lett., 2012, 14, 1892 CrossRef PubMed; (f) H. Baars, A. Beyer, S. V. Kohlhepp and C. Bolm, Org. Lett., 2014, 16, 536 CrossRef CAS PubMed; (g) R. Singha, A. Ahmed, Y. Nuree, M. Ghosh and J. K. Ray, RSC Adv., 2015, 5, 50174 RSC.
- For reviews, see: (a) J. Muzart, Tetrahedron, 2009, 65, 8313 CrossRef CAS; (b) S. Ding and N. Jiao, Angew. Chem., Int. Ed., 2012, 51, 9226 CrossRef CAS PubMed.
- For selected recent examples, see: (a) Y. Li, D. Xue, W. Lu, C. Wang, Z.-T. Liu and J. Xiao, Org. Lett., 2014, 16, 66 CrossRef CAS PubMed; (b) L. Liu, D. Zhang-Negrerie, Y. Du and K. Zhao, Org. Lett., 2014, 16, 436 CrossRef CAS PubMed; (c) D. J. Jung, H. J. Jeon, J. H. Kim, Y. Kim and S.-G. Lee, Org. Lett., 2014, 16, 2208 CrossRef CAS PubMed; (d) M.-N. Zhao, R.-R. Hui, Z.-H. Ren, Y.-Y. Wang and Z.-H. Guan, Org. Lett., 2014, 16, 3082 CrossRef CAS PubMed; (e) X. Xu, M. Zhang, H. Jiang, J. Zheng and Y. Li, Org. Lett., 2014, 16, 3540 CrossRef CAS PubMed; (f) D. N. Rao, S. Rasheed and P. Das, Org. Lett., 2016, 18, 3142 CrossRef PubMed; (g) W. Liu, C. Chen and P. Zhou, J. Org. Chem., 2017, 82, 2219 CrossRef CAS PubMed; (h) J. B. Wang, Y. L. Li and J. Deng, Adv. Synth. Catal., 2017, 359 DOI:10.1002/adsc.201700584; (i) W. Liu, H. Tan, C. Chen and Y. Pan, Adv. Synth. Catal., 2017, 359, 1594 CrossRef CAS.
- (a) S. Wang, Q. Yang, J. Dong, C. Li, L. Sun, C. Song and J. Chang, Eur. J. Org. Chem., 2013, 7631 CrossRef CAS; (b) J. Chang, L. Sun, J. Dong, Z. Shen, Y. Zhang, J. Wu, R. Wang, J. Wang and C. Song, Synlett, 2012, 2704 CrossRef CAS.
- C. Song, D. W. Knight and M. A. Whatton, Tetrahedron Lett., 2004, 45, 9573 CrossRef CAS.
- (a) K. Tsuji, H. Tsubouchi and H. Ishikawa, Chem. Pharm. Bull., 1995, 43, 1678 CrossRef CAS PubMed; (b) S. M. Gomha and K. M. Dawood, J. Chem. Res., 2014, 38, 515 CrossRef CAS.
- R. Soley, F. Albericio and M. Álvarez, Synthesis, 2007, 1559 CAS.
- Crystallographic data for structural analysis have been deposited with the Cambridge Crystallographic Data Center, CCDC reference number 1542588.†.
- Crystallographic data for structural analysis have been deposited with the Cambridge Crystallographic Data Center, CCDC reference number 1543830.†.
- (a) F. Diness and D. P. Fairlie, Angew. Chem., Int. Ed., 2012, 51, 8012 CrossRef CAS PubMed; (b) D. Dehe, I. Munstein, A. Reis and W. R. Thiel, J. Org. Chem., 2011, 76, 1151 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1542588 and 1543830. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c7qo00771j |
| This journal is © the Partner Organisations 2018 |