Open Access Article
Open Access ArticleHesperetin inhibits Eca-109 cell proliferation and invasion by suppressing the PI3K/AKT signaling pathway and synergistically enhances the anti-tumor effect of 5-fluorouracil on esophageal cancer in vitro and in vivo
Dandan Wu ,
Jiao Li
,
Jiao Li ,
Xue Hu
,
Xue Hu ,
Jingjing Ma
,
Jingjing Ma and
Weiguo Dong
and
Weiguo Dong *
*
Department of Gastroenterology, Renmin Hospital of Wuhan University, 238 Jiefang Road, Wuhan 430060, Hubei Province, China. E-mail: dwg@whu.edu.cn
First published on 6th July 2018
Abstract
Previously, we reported that hesperetin exhibited pro-apoptotic activity against esophageal cancer in vitro and in vivo. Here, we examined whether hesperetin inhibits cell proliferation and invasion and synergistically enhances the anti-tumor effect of 5-fluorouracil (5-FU) in esophageal cancer. Flow cytometry, EdU staining, and transwell invasion assays using Eca-109 cells showed that hesperetin induced cell cycle arrest at the G0/G1 phase and inhibited cell proliferation and invasion significantly. Moreover, hesperetin suppressed the expression of phosphorylated PI3K/AKT, cyclin D1, MMP-2, and MMP-9 and increased phosphorylated PTEN and p21 in Eca-109 cells. Hesperetin combined with 5-FU inhibited cell growth more effectively than did either drug alone in Eca-109 cells and in a xenograft mouse model. Hoechst 33258, Annexin V-PE/7-ADD double staining and TUNEL assay showed more apoptotic cells in the combination treatment group than in either individual treatment group. The combination down-regulated protein levels of Bcl-2 and up-regulated those of Bax, cleaved caspase-3, and cleaved caspase-9 more effectively than did either drug alone. These data suggest that hesperetin inhibited esophageal cancer cell proliferation and invasion by suppressing the PI3K/AKT signaling pathway. In conclusion, 5-FU and hesperetin exerted synergistic antitumor effects in vivo and in vitro and could constitute a novel therapeutic approach for esophageal cancer.
Introduction
Esophageal cancer is one of the most common gastrointestinal tract malignancies, with an estimated 455![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 new cancer cases and 400
800 new cancer cases and 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 deaths worldwide in 2012.1 Squamous cell carcinoma and adenocarcinoma are the main types of esophageal cancer; of these cases, 90% are squamous cell carcinoma. Owing to the lack of obvious early symptoms and the lack of effective early detection approaches, individuals with esophageal cancer are often diagnosed at advanced stages, and more than half of them present with metastases.2 In spite of the advances in surgical methods combined with chemotherapy and/or radiotherapy, the overall mortality rate remains high.3 The overall 5 year survival rate is around 20% but can drop to below 4% with metastases.4,5 There remains a need for efficient treatment modalities to inhibit tumor cell growth and restrain the metastatic capability of esophageal cancer cells.
200 deaths worldwide in 2012.1 Squamous cell carcinoma and adenocarcinoma are the main types of esophageal cancer; of these cases, 90% are squamous cell carcinoma. Owing to the lack of obvious early symptoms and the lack of effective early detection approaches, individuals with esophageal cancer are often diagnosed at advanced stages, and more than half of them present with metastases.2 In spite of the advances in surgical methods combined with chemotherapy and/or radiotherapy, the overall mortality rate remains high.3 The overall 5 year survival rate is around 20% but can drop to below 4% with metastases.4,5 There remains a need for efficient treatment modalities to inhibit tumor cell growth and restrain the metastatic capability of esophageal cancer cells.
Hesperetin, belonging to the flavanone class of flavonoids, is found abundantly in citrus fruits. It has been widely studied for its anticancer, antioxidant, and anti-inflammatory properties. Many studies have demonstrated that hesperetin can be an effective antitumor agent by inhibition of cell growth and invasion and induction of cell apoptosis in various cancers.6 Choi reported that hesperetin induced G1-phase cell cycle arrest in MCF-7 human breast cancer cells by regulating CDK4 and p21 expression.7 Alshatwi et al. showed that the anticancer effect of hesperetin on human cervical cancer cells is mediated through the cell cycle arrest, death receptor, and mitochondrial pathways.8 We also have demonstrated that hesperetin can induce apoptosis of esophageal cancer cells via a mitochondrial pathway mediated by increasing intracellular reactive oxygen species (ROS).9 ROS can also act as a signal transducer to modulate many other signaling pathways, such as the ERK, JNK, MAPK, and Akt pathways.10–14
In particular, the PI3K/Akt pathway is frequently activated in diverse cancers, including esophageal cancer, and is involved in regulating many physiologic processes, such as cell survival, differentiation, and apoptosis.15,16 PI3K activation could catalyze the synthesis of lipid second messenger phosphatidylinositol-3,4,5-trisphosphate (PIP3), which subsequently recruits and activates Akt. An overactive PI3K/Akt pathway could reduce the process of apoptosis by phosphorylating apoptotic signaling proteins directly and promote tumor cell cycle progression by modulating the activity of transcription factors. Phosphatase and tensin homolog (PTEN), a tumor suppressor, can negatively regulate the synthesis of PIP3 and thereby inhibits the activation of the Akt survival signaling pathway.17 Suppressing the PI3K/Akt signaling pathway may be an effective treatment for esophageal cancer. Some studies have shown that hesperetin can modulate the PI3K/Akt signaling pathway.18–20 We were therefore interested in studying the anti-proliferation and anti-invasion efficacy of hesperetin in esophageal cancer cells via inhibition of the PI3K/Akt pathway.
5-Fluorouracil (5-FU), belonging to the class of anti-metabolism, cycle specific drugs, mainly inhibits the synthesis of DNA and RNA. 5-FU plays a role in several pathways, but principally as a thymidylate synthase (TS) inhibitor which is responsible for the synthesis of the pyrimidine thymidine. Pyrimidine thymidine is a nucleoside required for DNA replication.21 For the past several decades, 5-FU has been a first-line treatment for esophageal cancer.3 However, single-agent chemotherapy is not ideal for the treatment of esophageal cancer because of the frequent occurrence of cell toxicity, drug resistance, and other adverse effects. 5-FU-based drug combination therapy has been employed in efforts to reduce toxicity and resistance.22 Although combination therapy has yielded better adverse effect profiles, drug resistance and cell toxicity remain major issues. New combination regimens with greater therapeutic effects and lower toxicity are urgently needed. In the previous study,9 we found that hesperetin induced esophageal cancer cell apoptosis by promoting ROS production, regulating the expression of Bcl-2 protein family, and then activating mitochondria-mediated apoptosis pathway. Hesperetin might make tumor cells more sensitive to other chemotherapeutic drugs or stimuli. As 5-FU and hesperetin act on different targets of esophageal cancer cells, we predicted that the combined use of these two drugs can improve the curative effect. In this study, we also evaluated the synergistic effect of hesperetin and 5-FU against esophageal cancer in vitro and in vivo.
Materials and methods
Cell culture
The human esophageal squamous cell carcinoma cell line Eca-109 was obtained from the China Center for Type Culture Collection (CCTCC). The cells were cultured in Dulbecco modified Eagle medium/F-12 medium (HyClone) supplemented with 10% fetal bovine serum (Gibco) plus 1% antibiotics (100 U ml−1 penicillin and 100 μg ml−1 streptomycin) in a humidified incubator containing 5% CO2 at 37 °C.Reagents
Hesperetin was purchased from Sigma-Aldrich and dissolved in dimethyl sulfoxide (DMSO) as previously described.9 PTEN, phosphorylated PTEN, PI3K, Akt, phosphorylated Akt, procaspase-3, cleaved caspase-3, procaspase-9, cleaved caspase-9, Bax, Bcl-2, cyclin D1, MMP-2, MMP-9, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) rabbit monoclonal antibodies were purchased from Cell Signaling Technology. Horseradish peroxidase-conjugated anti-rabbit immunoglobulin G was purchased from LI-COR Biosciences.EdU assay
Cell proliferation was measured by using the Cell-Light EdU Apollo 488 In Vitro Imaging kit (RiboBio). EdU is a thymidine analogue which can substitute for thymidine to penetrate into the DNA molecule during DNA replication period. Briefly, Eca-109 cells were treated with various concentrations of hesperetin (0, 100, 200, 300 μM) for 24 h. Then, the cells were digested and seeded into a 96-well plate (2 × 104 cells per well) supported with 10 μM EdU-containing medium. After incubation for 24 h, the cells were fixed, incubated with glycine, and EdU-stained following the manufacturer's protocol. Subsequently, the cells were co-stained with Hoechst 33342 for 30 min. The proportion of the cells that incorporated EdU was determined with a fluorescent microscope.Cell cycle assay
Cell cycle analysis was detected by flow cytometry. Briefly, Eca-109 cells were allowed to adhere in six-well plates. Before treatment with hesperetin, the cells were cultured in serum-free culture medium for 12 h. After 24 h of incubation, the cells were harvested and fixed with 70% ice-cold ethanol overnight at 4 °C, followed by propidium iodide (PI) staining. The different cell cycle phases were analyzed using a FACScan flow cytometer (BD FACSCalibur). The data presented are representative of those obtained in at least three independent experiments.Transwell invasion assay
After treated with different concentrations of hesperetin (0, 100, 200, 300 μM) for 24 h, the Eca-109 cells were digested and suspended. A total of 100 μl of the cell suspension (1 × 104 cells) was seeded into the upper chamber of a Transwell insert (polycarbonate membranes with 8 μm pores; Corning) precoated with Matrigel (BD Biosciences). 24 h later, the cells in the upper chamber were scrubbed out with cotton swabs. Then, the inserts were fixed with 4% paraformaldehyde for 15 min and stained with 0.1% crystal violet for 5 min. The cells passing through the chamber were observed under an inverted microscope, and eight fields of view were randomly selected to count cells.Cell viability assay
Cell proliferation and viability were assessed using CCK-8 assay (Dojindo) as previously described. Briefly, Eca-109 cells (2 × 104 cells per well) were seeded into a 96-well plate. The cells were allowed to adhere for 24 h and then incubated with 0, 25, 50, 100, 200, 400, or 800 μM hesperetin; 0, 2.5, 5, 10, 20, 40, or 80 μM 5-FU; or the combination of hesperetin and 5-FU for 24 and 48 h, separately. Afterwards, 10 μl of CCK-8 dye was added to each well. A microplate reader (PerkinElmer) was used to measure the absorbance of each sample at 450 nm.Apoptosis detection
Firstly, cell apoptosis was detected by employing Hoechst 33258 dye (Beyotime) as previously described. The cells were treated with hesperetin, 5-FU, or both for 48 h. Subsequently, cells were fixed and stained. The images were observed under an inverted fluorescence microscope (Olympus). Eight random fields of view were selected to count cells. Then, Annexin V-PE/7-ADD double staining with flow cytometry was used to further detect cell apoptosis. After Eca-109 cells were exposed to different drugs for 24 h, they were co-stained with 5 μl Annexin V-PE and 10 μl 7-ADD prior to flow cytometric analysis. The density plots illustrate four cell populations (live, early apoptotic, late apoptotic and dead) according to their fluorescence characteristics. Live cells are both PE and 7-ADD negative, early apoptotic cells are PE positive and 7-ADD negative, late apoptotic are both positive, and dead cells are PE negative and 7-ADD positive.Western blot analysis
Eca-109 cells were collected after the indicated treatments, and total cell proteins were extracted in radioimmunoprecipitation assay (RIPA) buffer supplemented with protease inhibitor cocktails (PIC) for 30 min on ice. Protein concentrations were measured using a BCA protein assay kit (Beyotime) according to the manufacturer's instructions. The proteins were resolved by sodium dodecyl sulfate loading buffer and denatured at 95 °C for 5 min. A total of 30 μg proteins were separated on a 10% sodium dodecyl sulfate polyacrylamide gel using electrophoresis and then transferred onto Immobilon-PVDF membranes (Millipore) using a wet transfer system. Membranes were blocked for 1 h with 5% non-fat dry milk and then incubated with various primary antibodies (working concentration, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) overnight at 4 °C. After washed with TBST, the PVDF membranes were incubated with secondary antibody (horseradish peroxidase-conjugated anti-rabbit immunoglobulin G, 1
1000) overnight at 4 °C. After washed with TBST, the PVDF membranes were incubated with secondary antibody (horseradish peroxidase-conjugated anti-rabbit immunoglobulin G, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) for 1 h at room temperature. Immunoreactive protein bands were visualized with an Odyssey Infrared Imaging System (LI-COR Biosciences).
000) for 1 h at room temperature. Immunoreactive protein bands were visualized with an Odyssey Infrared Imaging System (LI-COR Biosciences).
Xenograft tumor model
Female BALB/c nude mice (5–6 weeks old) purchased from Hunan SJA Laboratory Animal Company were maintained under specific-pathogen-free conditions. Before tumor implantation, the mice were allowed to get acclimatized for 1 week. This study was performed in strict accordance with the NIH guidelines for the care and use of laboratory animals (NIH Publication no. 85-23 Rev. 1985) and was approved by the Animal Care and Use Committees of Renmin Hospital of Wuhan University (Wuhan, China). Briefly, 5 × 106 cells suspended in 200 μl of phosphate-buffered saline were inoculated subcutaneously into the upper right flank of the nude mice. When the tumor diameter reached approximately 5–6 mm, the mice were pooled and divided into four groups (six mice per group), then they received intraperitoneal injection of hesperetin (60 mg kg−1), 5-FU (10 mg kg−1), hesperetin (60 mg kg−1) plus 5-FU (10 mg kg−1), or saline every 3 days. Mouse weight and tumor volume were measured every 3 days. Tumor volume in mm3 was determined by measuring the longest diameter (D) and shortest diameter (d) and calculated as 0.5 × d2 × D. All the mice were euthanized after 3 weeks of treatment. Blood was collected to detect the levels of serum alanine aminotransferase, aspartate aminotransferase, blood urea nitrogen, and serum creatinine. The tumor xenografts were extracted for hematoxylin and eosin (HE) staining and terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay as described before.9 Other important organs, including livers, lungs, kidneys, and brains, were also harvested for HE staining to identify any metastases.Statistical analysis
Data were analyzed using an unpaired two-tailed Student t-test. A p value less than 0.05 was considered to indicate statistical significance.Results
Hesperetin inhibits Eca-109 cell proliferation
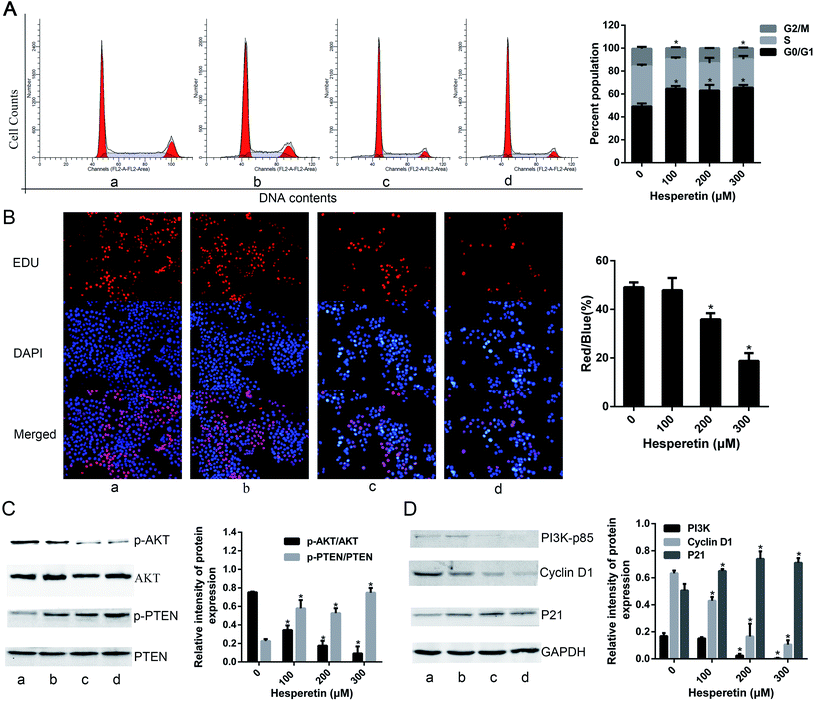
A previous study suggested that hesperetin inhibits Eca-109 cell growth in a concentration- and time-dependent manner. Here, we found that high-dose hesperetin (200 or 300 μM) induced cell cycle arrest at the G0/G1 phase in Eca-109 cells (Fig. 1A). We also examined the effect of hesperetin on cell proliferation using an EdU assay, which detects nucleotide analogue incorporation into replicating DNA in place of thymidine. As shown in Fig. 1B, 100 μM hesperetin did not inhibit EdU incorporation significantly, but the percentage of EdU-positive cells was much lower in cells treated by 200 or 300 μM hesperetin than in controls. To investigate the mechanisms underlying altered cell growth, western blot analysis was performed to determine protein expression associated with the PI3K/Akt signaling pathway. Fig. 1C showed that hesperetin notably down-regulated Akt phosphorylation in a dose-dependent manner while enhancing phosphorylation of PTEN. As shown in Fig. 1D, the expression of PI3K-p85 decreased after hesperetin treatment (p < 0.05). Cell cycle progression is controlled by cell cycle regulatory proteins that include p21 and cyclin D1, and we found that the expression levels of p21 significantly increased, while cyclin D1 decreased (Fig. 1D).Hesperetin inhibits Eca-109 cell invasion
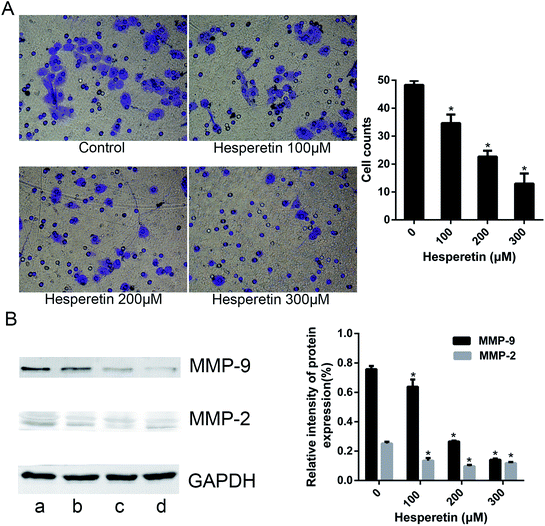
A transwell assay showed that the invasion abilities of Eca-109 cells were decreased significantly in those treated with hesperetin compared with controls (p < 0.05; Fig. 2A). As the concentration of hesperetin increased, the invaded cells became less. In addition, we evaluated the expression levels of the invasion-associated proteins MMP-2 and MMP-9. As Fig. 2B showed, MMP-2 and MMP-9 decreased dramatically after hesperetin treatment (p < 0.05).Hesperetin and 5-FU synergistically induce Eca-109 cell apoptosis
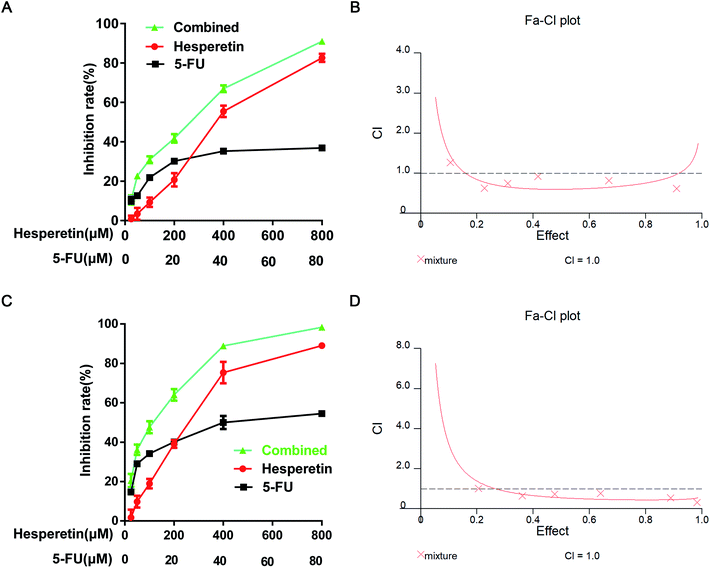
To investigate the synergistic effect of hesperetin and 5-FU on Eca-109 cells, the inhibitory effect of treatment on cell proliferation was determined by the CCK-8 assay. The cells were treated with hesperetin and 5-FU individually or in combination. As shown in Fig. 3A and C, hesperetin combined with 5-FU (molar ratio 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) inhibited Eca-109 cell proliferation more effectively than either compound alone did (p < 0.05). The Calcusyn 2.0 software analyzed the combination index (CI). Fig. 3B and D showed that CI was less than 1 in most concentration ranges, which meant hesperetin and 5-FU exerted synergistic anti-proliferative effect on Eca-109 cells.
1) inhibited Eca-109 cell proliferation more effectively than either compound alone did (p < 0.05). The Calcusyn 2.0 software analyzed the combination index (CI). Fig. 3B and D showed that CI was less than 1 in most concentration ranges, which meant hesperetin and 5-FU exerted synergistic anti-proliferative effect on Eca-109 cells.
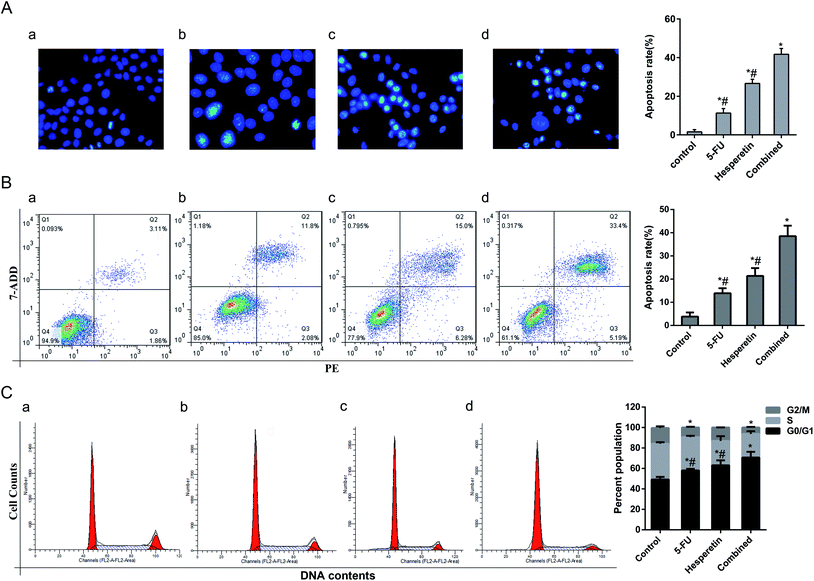
Cell apoptosis in each group was evaluated by Hoechst 33258 staining. The apoptotic cells were identified by nuclear morphology changes such as bright-blue fluorescent, condensed nuclei or fragmented nuclei. As shown in Fig. 4A, compared with the control group, more swollen and bright-blue fluorescent nuclei were seen in the group treated with 5-FU only, and there was more condensed chromatin in the group treated with hesperetin only. The percentage of apoptotic cells induced by the combined treatment was greater than that induced by either individual treatment (p < 0.05). We further verify the synergistic pro-apoptotic effect by applying Annexin V-PE/7-ADD double staining. The results showed that the apoptotic rate of the combined treatment group (38.25 ± 4.52%) was significantly higher than that of the single drug treatment group (200 μM hesperetin: 21.38 ± 3.35%; 20 μM 5-FU: 13.91 ± 2.16%) (p < 0.05) (Fig. 4B). Cell cycle distribution in each group was also analyzed (Fig. 4C). Flow cytometry showed that the combined treatment was associated with drastically increased G0/G1 phase accumulation, indicating that the combined treatment had a stronger effect than either individual treatment did.
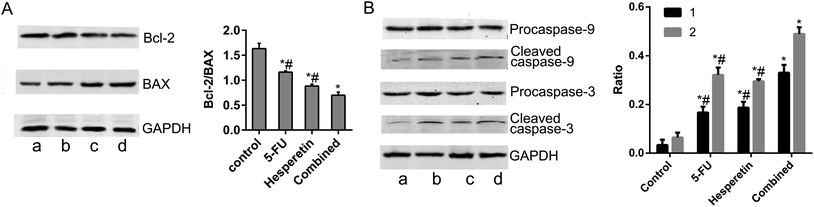
To identify the mechanism underlying apoptosis induced by hesperetin combined with 5-FU, we performed western blot analysis to examine the effects of each treatment on the mitochondrial apoptotic pathway. Fig. 5A showed that hesperetin and 5-FU individually or combined increased Bax expression and decreased Bcl-2 expression in the cells, decreasing the Bcl-2/Bax ratio. The decrease in the Bcl-2/Bax ratio was greater in the combined treatment group than that in either individual treatment group (p < 0.05). The expression levels of cleaved caspase-3 and -9 increased significantly in the individual and combined groups compared with the control group (Fig. 5B). The increase in these levels was also greater in the combined group than that in either individual treatment group (p < 0.05).
Hesperetin and 5-FU synergistically suppress tumor development in nude mice
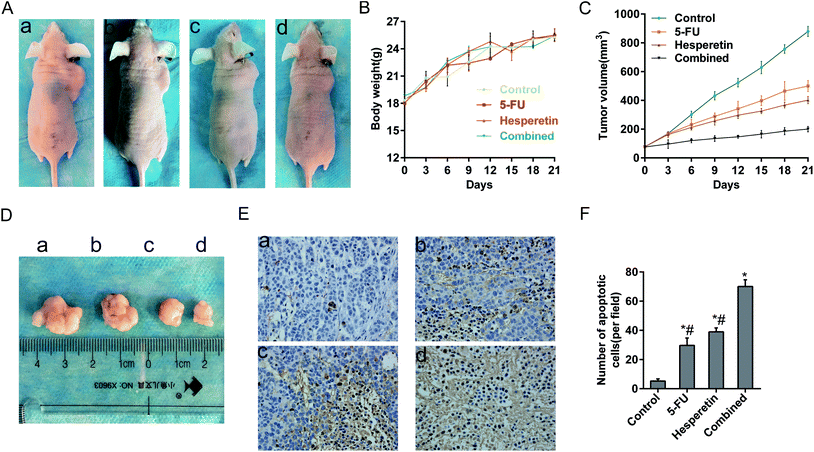
We next evaluated whether hesperetin combined with 5-FU exerted enhanced anti-tumor effects in vivo. All the mice grew Eca-109 tumor xenografts successfully. No adverse effects were observed in the mice's general appearance, weight, or blood serum alanine aminotransferase, serum aspartate aminotransferase, blood urea nitrogen levels, or serum creatinine levels (Fig. 6A and B, Table 1). As shown in Fig. 6C and D, tumors grew progressively in the control group during treatment period, however, tumor growth was markedly suppressed both in the single and combined treatment groups. Table 2 showed the inhibition rate of tumor volume in the combined treatment group (80.69%), 5-FU-only group (41.33%), and hesperetin-only group (55.72%) after 21 days of treatment, which clearly indicated that the combined treatment induced greater tumor growth suppression than did hesperetin or 5-FU alone. As shown in Table 3, the mean tumor weight in the combination group was significantly less than that in the hesperetin-only, 5-FU-only, or control group. HE staining (not shown) and TUNEL assay of the subcutaneous xenograft sections indicated that hesperetin and 5-FU individually and in combination induced significantly greater apoptosis than the control did (Fig. 6E), and the apoptotic rate in the combination treatment group was greater than that in either individual treatment group (Fig. 6F).| Group | ALT (U l−1) | AST (U l−1) | Urea (μmol l−1) | Cr (μmol l−1) |
|---|---|---|---|---|
| a Data are presented as mean (standard deviation). ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Cr, serum creatinine. No differences were observed in any laboratory value between the groups (p > 0.05). | ||||
| Control | 41.33(11.93) | 142.67(9.87) | 9.21(0.74) | 10.67(0.58) |
| 5-FU | 42.01(4.12) | 142.47(10.34) | 9.64(0.67) | 10.25(0.83) |
| Hesperetin | 40.53(2.57) | 143.35(7.79) | 8.33(1.18) | 11.5(0.5) |
| Combined | 40.78(2.83) | 142.98(2.83) | 9.36(0.38) | 10.83(1.04) |
| Group | Tumor volume (mm3) | Inhibition rate (%) |
|---|---|---|
| a Data are presented as mean (standard deviation) and inhibition rate. *p < 0.05 versus control; #p < 0.05 versus combination. | ||
| Control | 813 (46.79) | |
| 5-FU | 477 (80.25)*# | 41.33 |
| Hesperetin | 360 (66.36)*# | 55.72 |
| Combined | 157 (33.51)* | 80.69 |
| Group | Tumor weight (g) | Inhibition rate (%) |
|---|---|---|
| a Data are presented as mean (standard deviation) and inhibition rate. *p < 0.05 versus control; #p < 0.05 versus combined. | ||
| Control | 0.915 (0.144) | |
| 5-FU | 0.553 (0.021)*# | 39.56 |
| Hesperetin | 0.443 (0.033)*# | 51.58 |
| Combined | 0.273 (0.045)* | 70.16 |
Discussion
We investigated the effect of hesperetin on esophageal cancer cell proliferation and invasion abilities and assessed relevant molecular mechanisms, and we found that hesperetin not only inhibits Eca-109 cell proliferation and invasion through the PI3K/AKT pathway but also shows synergistic antitumor activity with chemotherapeutic agent 5-FU in vitro and in vivo in esophageal cancer.Through flow cytometry with PI staining, we found that hesperetin induced cell cycle arrest at the G0/G1 phase. And EdU assay showed that the number of EdU-positive cells decreased in a concentration-dependent manner. These two assays suggested that hesperetin could inhibit Eca-109 cell growth significantly. Furthermore, western blot analysis revealed that hesperetin functioned as a growth inhibitor of the PI3K/Akt signaling pathway, a well-characterized cell survival signaling pathway. In numerous cancer tissues and cells, this pathway is overactive, representing an opportunity for drug discovery.23 In the present study, we discovered that hesperetin treatment significantly inhibited PI3K and Akt activation and promoted PTEN phosphorylation. The results indicated that hesperetin was able to inhibit proliferation of Eca-109 cells through the PI3K/Akt pathway. Similarly, previous studies have shown that inhibiting the PI3K/Akt signaling pathway by other chemicals can prevent the growth of various cancer cells.24,25
The expression of cell cycle regulatory proteins was also detected. We found that hesperetin induced cell cycle arrest by increasing the expression levels of p21 and decreasing cyclin D1. Meanwhile, in addition to its proliferation-inhibiting function, hesperetin played a negative role in the regulation of cell invasion and metastasis. A vital step in cancer metastasis processes is the proteolytic degradation of the extracellular matrix by proteolytic enzymes such as MMPs, and we found that hesperetin significantly decreased the expression levels of MMP-2 and MMP-9. Many studies have reported that MMP-2 and MMP-9 expression were mediated by the PI3K/Akt pathway.26 Indeed, Akt activation can lead to cancer invasion and metastasis by stimulating the secretion of MMPs.
The prognosis of esophageal cancer remains poor because most patients present with advanced disease. Chemotherapy is one of the main therapeutic approaches for these patients, and cisplatin-based and/or 5-FU-based combination therapy is routinely used in the clinic.27 However, cisplatin- and 5-FU-related toxicity and resistance remain serious and common issues for many cancer patients. New combination therapies are being explored to maximize therapeutic advantages while reducing side effects and complications.28 Among various combination regimens, natural medicines with low toxicity and high efficiency are of particular interest.29 Here, we demonstrated that the combination of hesperetin and 5-FU achieved decreasing the dose of 5-FU, which is also effective and safe in the animal models tested.
We studied the synergistic effect of hesperetin and 5-FU on the cell growth and apoptosis of Eca-109 cells in vitro. We found that hesperetin and 5-FU individually exhibited anti-proliferative effects on Eca-109 cells and that these effects were significantly enhanced when the cells were treated with these drugs in combination. We also found that hesperetin and 5-FU individually induced significantly greater apoptosis compared with the control and that the combination had a synergistic pro-apoptotic effect. To date, two apoptotic pathways are best understood: the cell death receptor-mediated extrinsic pathway and the mitochondria-mediated intrinsic pathway. Hesperetin has been proven to induce the apoptosis of multiple cancer cells via the intrinsic pathway, including Eca-109 cells.9,30–32 Except as a TS inhibitor, 5-FU also acts as a pyrimidine analogue that incorporates into RNA and DNA, leading to activation of apoptosis involving expression changes of p53 and caspase-6.33 In this study, we found that the combination of hesperetin and 5-FU could induce Eca-109 cell apoptosis through the intrinsic pathway. The ratio of Bcl-2/Bax dramatically decreased in the combined treatment group compared with the individual treatment groups, while an increase was seen for the expression of cleaved caspase-9 and cleaved caspase-3.
Finally, the anti-proliferation effect on Eca-109 xenografts was also enhanced by the combined treatment. The TUNEL assay showed more apoptotic cells in the combined group than in the individual treatment groups or the control group. No obvious adverse effects, such as liver dysfunction and kidney dysfunction, were observed. Thus, hesperetin combined with 5-FU exerted a synergistic anti-cancer effect on esophageal cancer in vitro and in vivo.
In conclusion, hesperetin inhibits proliferation and invasion of esophageal cancer cells via the PI3K/Akt pathway, and the combination of hesperetin and 5-FU has synergistic anti-apoptotic effects on esophageal cancer cells via the mitochondria-mediated intrinsic pathway without notable toxic effects. Therefore, hesperetin combined with 5-FU may be an effective chemotherapeutic strategy for esophageal cancer.
Conflicts of interest
The authors have no conflicts of interest.Acknowledgements
This work was supported in part by National Natural Science Foundation of China (No. 81572426). This manuscript was edited by the Department of Scientific Publications of the University of Texas MD Anderson Cancer Center, which is supported in part by the National Institutes of Health through Cancer Center Support Grant P30CA016672.References
- L. A. Torre, F. Bray, R. L. Siegel, J. Ferlay, J. Lortet-Tieulent and A. Jemal, Global cancer statistics, Ca-Cancer J. Clin., 2012, 2015(65), 87–108, DOI:10.3322/caac.21262.
- R. Siegel, C. DeSantis, K. Virgo, K. Stein, A. Mariotto, T. Smith, D. Cooper, T. Gansler, C. Lerro, S. Fedewa, C. Lin, C. Leach and R. S. Cannady, et al., Cancer treatment and survivorship statistics, Ca-Cancer J. Clin., 2012, 2012(62), 220–241, DOI:10.3322/caac.21149.
- B. Keith, Mosby's Oncology Nursing Advisor: A Comprehensive Guide to Clinical Practice, 2016, vol. 58 Search PubMed.
- N. Howlader, A. M. Noone, M. Krapcho, J. Garshell, D. Miller, S. F. Altekruse, C. L. Kosary, M. Yu, J. Ruhl, Z. Tatalovich, A. Mariotto, D. R. Lewis and H. S. Chen, et al., SEER Cancer Statistics Review, 1975–2011, National Cancer Institute, Bethesda, MD, 2013, http://seer.cancer.gov/csr/1975_2011/, based on November, SEER data submission, posted to the SEER web site, April 2014 Search PubMed.
- H. Zeng, R. Zheng, Y. Guo, S. Zhang, X. Zou, N. Wang, L. Zhang, J. Tang, J. Chen, K. Wei, S. Huang, J. Wang and L. Yu, et al., Cancer survival in China, 2003–2005: a population-based study, Int. J. Cancer, 2015, 136, 1921–1930, DOI:10.1002/ijc.29227.
- A. Roohbakhsh, H. Parhiz, F. Soltani, R. Rezaee and M. Iranshahi, Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases, Life Sci., 2015, 124, 64–74, DOI:10.1016/j.lfs.2014.12.030.
- E. J. Choi, Hesperetin induced G1-phase cell cycle arrest in human breast cancer MCF-7 cells: involvement of CDK4 and p21, Nutr. Cancer, 2007, 59, 115–119, DOI:10.1080/01635580701419030.
- A. A. Alshatwi, E. Ramesh, V. S. Periasamy and P. Subash-Babu, The apoptotic effect of hesperetin on human cervical cancer cells is mediated through cell cycle arrest, death receptor, and mitochondrial pathways, Fundam. Clin. Pharmacol., 2013, 27, 581–592, DOI:10.1111/j.1472-8206.2012.01061.x.
- D. Wu, J. Zhang, J. Wang, J. Li, F. Liao and W. Dong, Hesperetin induces apoptosis of esophageal cancer cells via mitochondrial pathway mediated by the increased intracellular reactive oxygen species, Tumour Biol., 2016, 37, 3451–3459, DOI:10.1007/s13277-015-4176-6.
- K. Y. Kim, K. I. Park, S. H. Kim, S. N. Yu, S. G. Park, Y. W. Kim, Y. K. Seo, J. Y. Ma and S. C. Ahn, Inhibition of Autophagy Promotes Salinomycin-Induced Apoptosis via Reactive Oxygen Species-Mediated PI3K/AKT/mTOR and ERK/p38 MAPK-Dependent Signaling in Human Prostate Cancer Cells, Int. J. Mol. Sci., 2017, 18, 1088 CrossRef PubMed.
- I. Khan and S. C. Kang, Apoptotic Activity of Lactobacillus plantarum DGK-17-Fermented Soybean Seed Extract in Human Colon Cancer Cells via ROS-JNK Signaling Pathway, J. Food Sci., 2017, 82, 1475–1483 CrossRef PubMed.
- S. Palit, S. Kar, G. Sharma and P. K. Das, Hesperetin Induces Apoptosis in Breast Carcinoma by Triggering Accumulation of ROS and Activation of ASK1/JNK Pathway, J. Cell. Physiol., 2015, 230, 1729–1739, DOI:10.1002/jcp.24818.
- L. Cao, X. Chen, X. Xiao, Q. Ma and W. Li, Resveratrol inhibits hyperglycemia-driven ROS-induced invasion and migration of pancreatic cancer cells via suppression of the ERK and p38 MAPK signaling pathways, Int. J. Oncol., 2016, 49, 735–743, DOI:10.3892/ijo.2016.3559.
- J. Zhang, X. Wang, V. Vikash, Q. Ye, D. Wu, Y. Liu and W. Dong, ROS and ROS-Mediated Cellular Signaling, Oxid. Med. Cell. Longevity, 2016, 2016, 4350965, DOI:10.1155/2016/4350965.
- N. Saeed, R. Shridhar, S. Hoffe, K. Almhanna and K. L. Meredith, AKT expression is associated with degree of pathologic response in adenocarcinoma of the esophagus treated with neoadjuvant therapy, J. Gastrointest. Oncol., 2016, 7, 158–165, DOI:10.3978/j.issn.2078-6891.2015.067.
- D. A. Altomare and J. R. Testa, Perturbations of the AKT signaling pathway in human cancer, Oncogene, 2005, 24, 7455–7464, DOI:10.1038/sj.onc.1209085.
- C. Blanco-Aparicio, O. Renner, J. F. Leal and A. Carnero, PTEN, more than the AKT pathway, Carcinogenesis, 2007, 28, 1379–1386, DOI:10.1093/carcin/bgm052.
- M. Shirzad, E. Heidarian, P. Beshkar and M. Gholami-Arjenaki, Biological Effects of Hesperetin on Interleukin-6/Phosphorylated Signal Transducer and Activator of Transcription 3 Pathway Signaling in Prostate Cancer PC3 Cells, Pharmacogn. Res., 2017, 9, 188–194, DOI:10.4103/0974-8490.204655.
- S. He, X. Wang, Y. Zhong, L. Tang, Y. Zhang, Y. Ling, Z. Tan, P. Yang and A. Chen, Hesperetin post-treatment prevents rat cardiomyocytes from hypoxia/reoxygenation injury in vitro via activating PI3K/Akt signaling pathway, Biomed. Pharmacother., 2017, 91, 1106–1112, DOI:10.1016/j.biopha.2017.05.003.
- G. D. Kim, Hesperetin Inhibits Vascular Formation by Suppressing of the PI3K/AKT, ERK, and p38 MAPK Signaling Pathways, Prev. Nutr. Food Sci., 2014, 19, 299–306, DOI:10.3746/pnf.2014.19.4.299.
- D. B. Longley, D. P. Harkin and P. G. Johnston, 5-Fluorouracil: mechanisms of action and clinical strategies, Nat. Rev. Cancer, 2003, 3, 330–338, DOI:10.1038/nrc1074.
- J. Liu, Z. Wang, K. Wu, J. Li, W. Chen, Y. Shen and S. Guo, Paclitaxel or 5-fluorouracil/esophageal stent combinations as a novel approach for the treatment of esophageal cancer, Biomaterials, 2015, 53, 592–599, DOI:10.1016/j.biomaterials.2015.03.009.
- I. Vivanco and C. L. Sawyers, The phosphatidylinositol 3-kinase AKT pathway in human cancer, Nat. Rev. Cancer, 2002, 2, 489–501, DOI:10.1038/nrc839.
- Y. Guo, H. Chang, J. Li, X. Y. Xu, L. Shen, Z. B. Yu and W. C. Liu, Thymosin alpha 1 suppresses proliferation and induces apoptosis in breast cancer cells through PTEN-mediated inhibition of PI3K/Akt/mTOR signaling pathway, Apoptosis, 2015, 20, 1109–1121, DOI:10.1007/s10495-015-1138-9.
- M. Block, S. Fister, G. Emons, S. Seeber, C. Grundker and A. R. Gunthert, Antiproliferative effects of antiestrogens and inhibitors of growth factor receptor signaling on endometrial cancer cells, Anticancer Res., 2010, 30, 2025–2031 Search PubMed.
- W. G. Jiang, A. J. Sanders, M. Katoh, H. Ungefroren, F. Gieseler, M. Prince, S. K. Thompson, M. Zollo, D. Spano, P. Dhawan, D. Sliva, P. R. Subbarayan and M. Sarkar, et al., Tissue invasion and metastasis: Molecular, biological and clinical perspectives, Semin. Cancer Biol., 2015, 35(suppl.), S244–S275, DOI:10.1016/j.semcancer.2015.03.008.
- H. Kato and M. Nakajima, Treatments for esophageal cancer: a review, Gen. Thorac. Cardiovasc. Surg., 2013, 61, 330–335, DOI:10.1007/s11748-013-0246-0.
- D. Lin and L. Leichman, The current status of neoadjuvant therapy for esophageal cancer, Semin. Thorac. Cardiovasc. Surg., 2014, 26, 102–109, DOI:10.1053/j.semtcvs.2014.07.002.
- J. Uzoigwe and E. R. Sauter, Cancer prevention and treatment using combination therapy with plant- and animal-derived compounds, Expert Rev. Clin. Pharmacol., 2012, 5, 701–709, DOI:10.1586/ecp.12.62.
- J. Zhang, J. Song, D. Wu, J. Wang and W. Dong, Hesperetin induces the apoptosis of hepatocellular carcinoma cells via mitochondrial pathway mediated by the increased intracellular reactive oxygen species, ATP and calcium, Med. Oncol., 2015, 32, 101, DOI:10.1007/s12032-015-0516-z.
- J. Zhang, D. Wu, V. Vikash, J. Song, J. Wang, J. Yi and W. Dong, Hesperetin Induces the Apoptosis of Gastric Cancer Cells via Activating Mitochondrial Pathway by Increasing Reactive Oxygen Species, Dig. Dis. Sci., 2015, 60, 2985–2995, DOI:10.1007/s10620-015-3696-7.
- A. Adan and Y. Baran, Fisetin and hesperetin induced apoptosis and cell cycle arrest in chronic myeloid leukemia cells accompanied by modulation of cellular signaling, Tumour Biol., 2016, 37, 5781–5795, DOI:10.1007/s13277-015-4118-3.
- N. Zhang, Y. Yin, S. J. Xu and W. S. Chen, 5-Fluorouracil: mechanisms of resistance and reversal strategies, Molecules, 2008, 13, 1551–1569 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |