Open Access Article
Open Access ArticleCreating magnetic ionic liquid-molecularly imprinted polymers for selective extraction of lysozyme†
Wei Xua,
Qingzhou Daia,
Yuzhi Wang *a,
Xiaojian Hu*b,
Panli Xua,
Rui Nia and
Jiaojiao Menga
*a,
Xiaojian Hu*b,
Panli Xua,
Rui Nia and
Jiaojiao Menga
aState Key Laboratory of Chemo/Biosensing and Chemometrics, College of Chemistry and Chemical Engineering, Hunan University, Changsha, 410082, P. R. China. E-mail: wyzss@hnu.edu.cn; Fax: +86-731-88821848; Tel: +86-731-88821903
bDepartment of Chemistry, School of Basic Medicine, Changsha Medical University, Changsha, 410219, P. R. China
First published on 13th June 2018
Abstract
A novel magnetic (Fe3O4) surface molecularly imprinted polymer (MIP) based on ionic liquid (IL) (Fe3O4@VTEO@IL-MIPs) was prepared for the selective extraction of lysozyme (Lys). As the functional monomer of the MIPs, an imidazolium-based IL with vinyl groups was prepared. It can provide multiple interactions with template molecules. The amount of IL was optimized (200 mg). Fourier transform infrared spectrometry (FT-IR), transmission electron microscopy (TEM), dynamic light scattering (DLS), thermogravimetric analysis (TGA) and a vibrating sample magnetometer (VSM) were used to characterize the MIP. The results indicate the successful formation of an imprinting polymer layer. The concentration of Lys in the supernatant was determined by UV-vis spectrophotometry at a wavelength of 280 nm. The maximum adsorption capability of the MIP is 213.7 mg g−1 and the imprinting factor (IF) is 2.02. It took 2.5 h for the MIP to attain adsorption equilibrium. The structure of the protein was evaluated using circular dichroism (CD) spectra and UV-visible spectra. The adsorption performance was further investigated in detail by selective adsorption experiments, competitive rebinding tests, and reusability and stability experiments. Furthermore, it was utilized to separate the template protein from a mixture of proteins and real samples successfully because of the high adsorption capacity for Lys.
1. Introduction
Molecular imprinting technology (MIT) offers a way to design and prepare functional materials that have unique features of structural predictability and selective extraction. The functional materials are called molecularly imprinted polymers (MIPs). MIPs have tailor-made imprinted cavities, which are complementary to the template molecules in size, shape and functional groups and can interact with template molecules.1 Conceptually, templates, cross-linkers, functional monomers, a polymerization initiator and a solvent (porogen) are used to prepare typical MIPs.2 After removal of the template molecules, the artificial affinity binding cavities remain. Different methods have been employed to synthesize MIPs, such as surface imprinting, epitope imprinting and metal-chelating imprinting.3 Surface imprinting has some fascinating features. For example, template molecules can lightly access to the imprinted cavities in the surface of MIPs. Furthermore, it is easy to elute the template molecules. Owing to unique features of structure predictability and selective extraction, MIPs have achieved lots of successful applications involving solid-phase extraction, drug delivery applications, biosensors and so on.4–6Magnetic molecularly imprinted polymers have attracted increasing attention in recent years.7–10 Fe3O4 magnetic nanoparticles have large surface areas, good biocompatibility and high chemical stability. After coating with functional layers, other good properties can be introduced into magnetic materials, which makes it become an important role in molecular imprinting technology. More importantly, compared with the traditional operation steps of solid phase separation, the magnetic MIPs particles can be fast separated by an external magnetic field. And complicated centrifugation steps can be avoided. Last but not least, Fe3O4 nanoparticles can provide the support material for the MIPs, which is important to synthesis core–shell structure. Magnetic materials was used widely in chemical, medical science and biological.8 Obviously, the polymer particles with magnetism is a must.
Proteins have complex conformation, flexible structure and large size. The activities of enzymes are affected easily, such as immobilization process, operation temperature, pH and humidity.11 Unlike some small molecule compounds, the synthesis process of imprinting enzymes needs to be carried out under mild conditions. Furthermore, another challenge faced by bio-macromolecules for imprinting applications is diffusion limitation. Removing and rebinding template are difficult. It is pretty important to remove template molecules completely to obtain the effective imprinted sites.12 So that the preparation of molecularly imprinted polymer for the bio-macromolecules is difficult.13 Lysozyme (Lys) is considered as an important index in the diagnosis of various diseases involving bronchopulmonary dysplasia in newborns, kidney problems, conjunctivitis and leukemia.14,15 Meanwhile, Lys has specific hydrolytic activity against bacterial cell walls and it is nontoxic to humans, which has been widely used as an antimicrobial agent in the production of wine, cheese and so on.15 So it is necessary to enrich Lys with high selectivity.
Ionic liquids (ILs) are regarded as “green solvents”.16 ILs have interesting physicochemical properties such as high chemical stability, negligible vapour pressure, non-flammability, high ionic conductivity. In addition, it has the capability to attract dissolved molecules by a variety of interactions.17,18 The multiple interactions involve the electrostatic interaction, hydrogen bonding, π–π stacking and ion exchange. In order to form task-specific ionic liquids (TSILs) interacting with analytes or substrates in specific ways, functional groups can be introduced to either component.19 In MIPs, the selection of functional monomers is important. It can form a pre-polymerization complex with the template and strongly interact with the templates by providing functional groups.2 The number of functional monomers is limited, but more and more ILs have achieved successful application as functional monomers in MIPs.20–22 A kind of task-specific ionic liquid can be designed to meet the needs of being a functional monomer. Imidazolium-based IL with vinyl groups was prepared as the functional monomer in this work, which can provide multiple interactions with template molecules.
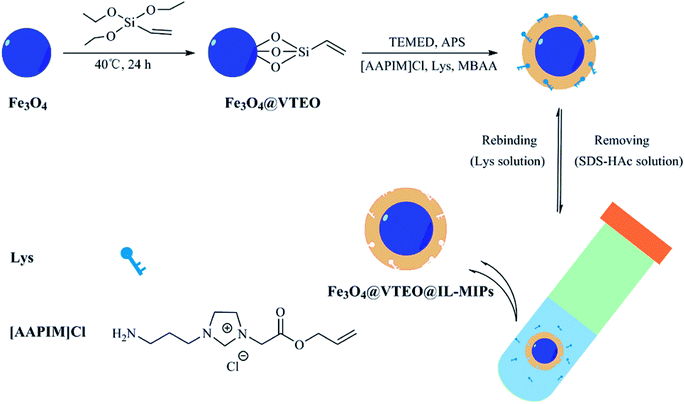
In this manuscript, we designed and fabricated magnetic surface imprinted polymers using ionic liquid as the functional monomer for selective extraction of lysozyme (as indicated in Scheme 1). Magnetic Fe3O4 nanoparticles modified with vinyltriethoxysilane (VTEO) were adopted as the support material. The prepared MIPs (Fe3O4@VTEO@IL-MIPs) were characterized by transmission electron microscope (TEM), dynamic light scattering (DLS), thermogravimetric analysis (TGA), X-ray diffraction (XRD), vibrating sample magnetometer (VSM) and fourier transform infrared spectrometry (FT-IR). The secondary structure of the protein was evaluated by the circular dichroism (CD) spectra.23 Furthermore, UV-visible spectra was used to investigate the structure of the protein. Meanwhile, the adsorption performance were further investigated in detail by selective adsorption experiments, competitive rebinding tests, reusability and stability experiments and real sample adsorption experiments.
2. Experimental section
2.1. Apparatus
A UV-2450 UV-vis spectrophotometer (Shimadzu, Japan) was used for determination of proteins. A FT-IR spectrometer (PerkinElmer, USA) was used to recorded infrared spectra. The morphologies nanomaterials were examined over a HT-7700 transmission electron microscope (TEM, Hitachi, Japan). Thermo-gravimetric analysis (TAG) was investigated by a STA 409 thermal gravimetric analyzer (Netzsch, Germany) under nitrogen atmosphere. X-ray diffraction pattern (XRD) was collected on a D/Max 2500 X-ray diffraction (Rigaku, Japan). An EV11 Vibrating Sample Magnetometer (MicroSense, USA) was employed to research the magnetism of samples. A Mos-500 circular dichroism (CD) spectrometer (Biologic, France) was applied to determine the secondary structure of Lys. Zetasizer Nano-ZS90 (Malvern Instruments, U.K.) was employed to research dynamic light scattering (DLS) study. A JY300C Gel electrophoresis (Beijing Junyi Eastern Electrophoresis Equipment Co., Ltd., China) was used to separate proteins.2.2. Reagents and materials
Ammonium hydroxide solution (27%, W/V), FeCl3·6H2O, FeSO4·7H2O, N,N,N′,N′-tetramethylenediamine (TEMED), absolute ethanol, acetic acid (HAc) were bought from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Ovalbumin (OVA), bovine serum albumin (BSA), bovine hemoglobin (BHb), cytochrome C (Cyt C) and lysozyme (Lys) were purchased from Shanghai yuanye Bio-Technology Co., Ltd. (Shanghai, China). Allyl chloroacetate and N,N-methylenebisacrylamide (MBAA) were purchased from Aladdin chemistry Co., Ltd. (Shanghai, China). Vinyltriethoxysilane (VTEO) was obtained from Adamas Reagent Co., Ltd. (Shanghai, China). N-(3-aminopropyl)-imidazole was gained from TCI Development Co., Ltd. (Shanghai, China). Hydrazine hydrate and ammonium persulfate (APS) were bought from the Institute of Fucheng Chemicals (Tianjin, China). Sodium dodecyl sulfate (SDS) was supplied by Xilong chemistry Co., Ltd. (Shantou, China). All reagents were of analytical grade. All solutions were prepared using deionized water (18.25 MΩ cm, 25 °C). Phosphate buffer solutions (20 mmol L−1, pH = 7.1) were used in this work for most experiments.2.3. Preparation of magnetic ionic liquid-molecularly imprinted polymers (Fe3O4@VTEO@IL-MIPs)
Similarly, the magnetic ionic liquid non-imprinted polymers (Fe3O4@VTEO@IL-NIPs) were synthesised in the same procedure but without adding template protein.
2.4. Protein adsorption experiments
Batch rebinding studies have been carried out to investigate the template binding. The solvent of protein adsorption experiments is same as the solvent used during synthesis. Fe3O4@VTEO@IL-MIPs or Fe3O4@VTEO@IL-NIPs (5 mg) were mixed in Lys solution (1 mL) with different initial concentrations (0.8–2.0 mg mL−1) in phosphate buffer solution. The mixture was then shaken at 25 °C for a period of time. The adsorbent was collected with the help of an external magnet, and the concentration of Lys in the supernatant was measured by UV-vis at wavelength of 280 nm. The adsorption capacity of Fe3O4@VTEO@IL-MIPs or Fe3O4@VTEO@IL-NIPs for Lys was calculated according to the following formula:
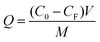 | (1) |
For the kinetics adsorption study, Fe3O4@VTEO@IL-MIPs or Fe3O4@VTEO@IL-NIPs (5 mg) were dispersed in 1 mL of Lys solution with same initial concentration and incubated at designated time intervals.
2.5. Selectivity experiments
The selectivity of imprinted polymers was tested for binding of Lys, OVA, Cyt C, BSA and BHb. All the initial concentration of protein solution is same. After reaching adsorption equilibrium, the concentrations of five proteins in the supernatant were determined by UV-vis respectively at wavelength of 280 nm, 278 nm, 406 nm, 278 nm, 404 nm, respectively. The imprinting factor (IF) was used to evaluate the selective extraction property of MIPs, which is defined as:
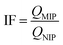 | (2) |
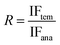 | (3) |
2.6. Real sample adsorption experiments
In the real sample tests, the chicken egg white sample was collected from fresh eggs and diluted 10-fold with phosphate buffer (20 mM, pH 7.1). Fe3O4@VTEO@IL-MIPs or Fe3O4@VTEO@IL-NIPs was added and shaken at room temperature for 12 h. The microspheres were treated with 2% (w/v) SDS-2% (v/v) HAc solution to elute the strongly adsorbed Lys. Finally, the diluted, adsorbed samples were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) with 12% polyacrylamide separating gel.3. Results and discussion
3.1. Preparation of Fe3O4@VTEO@IL-MIPs for Lys
To obtain a kind of material with surface hydrophilic and multiple binding sites for adsorption of Lys, magnetic ionic liquid imprinted polymers were designed through 3 different steps. In the first step, Fe3O4 nanoparticles were synthesized according to the chemical coprecipitation method, and then functionalized with polymerizable vinyl groups by reacting with VTEO. The silica coated on the surface of magnetic Fe3O4 nanoparticles prevents them from oxidation and agglomeration and make them more easily coated by polymers.27 Subsequently, the imprinted layer forming on the surface of Fe3O4@VTEO can rebind template selectively through multiple interactions between Lys and IL, such as electrostatic interaction, hydrogen bonding and π–π stacking. MBAA, as a hydrophilic cross-linking agent, was employed to form the template–monomer interaction network. Better aqueous dispersibility can be brought by introduction of water soluble additives.28 Polymerization was initiated by adding APS and TEMED for 24 h. Lys was eluted from the MIPs layer using a mixture of SDS and HAc (2% w/v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2% v/v). The solid product and liquid phases were separated by an external magnetic field quickly. After Lys was eluted successfully, the product was washed with distilled water, and dried by freeze-drying for 12 h successively for further use.
2% v/v). The solid product and liquid phases were separated by an external magnetic field quickly. After Lys was eluted successfully, the product was washed with distilled water, and dried by freeze-drying for 12 h successively for further use.
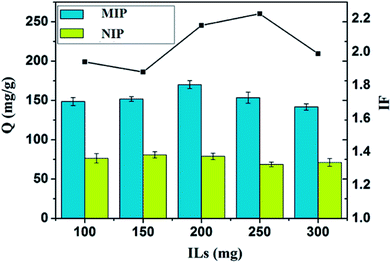
The selective extraction capability of Lys-imprinted polymers is mainly dependent on monomers. The amount of IL during imprinting was investigated in the range of 100–300 mg (as shown in ESI Table S1†). The corresponding rebinding capacities and IF were shown in Fig. 2. When the mass of [AAPIM]Cl (IL) increased to 200 mg, Fe3O4@VTEO@IL-MIPs exhibits increased adsorption for Lys, and the best value of Q was obtained. The maximum adsorption capability of the Fe3O4@VTEO@IL-MIPs is 170.1 mg g−1 and the IF is 2.16. The reason why Q increased is that higher amount of IL could facilitate the interaction between monomer and Lys, thus leading to more imprinted sites on the surface of Fe3O4@VTEO@IL-MIPs. However, it is noteworthy that MIP4 and MIP5 suffered from decreased adsorption capacity with increasing amount of IL. This is because the thicker imprinted polymer layer blocked the imprinted cavities. Moreover, the optimized monomer amount (200 mg) was selected for the following investigation.
3.2. Characterization of Fe3O4@VTEO@IL-MIPs
All details and figures about FT-IR spectra, TEM, DLS, VSM, TGA, and XRD analyses are mentioned in ESI Fig. S1–S6.†3.3. Adsorption isotherms
Lys concentration has a significant effect on the adsorption capacities of the imprinted and non-imprinted magnetic ionic liquid polymers. The results were shown in Fig. 3. The adsorption capacity of Fe3O4@VTEO@IL-MIPs (NIPs) increased obviously when the concentration of Lys vary from 0.8 to 1.2 mg mL−1. The adsorption capacity of Fe3O4@VTEO@IL-MIPs increased continuously but the adsorption capacity of Fe3O4@VTEO@IL-NIPs decreased when the concentration of Lys increased to 1.4 mg mL−1. When the concentration of Lys is 1.4 mg mL−1, the maximum adsorption capacity of Fe3O4@VTEO@IL-MIPs (217.39 mg g−1) was obtained, which is higher than that of Fe3O4@VTEO@IL-NIPs (120.42 mg g−1). As the concentration of Lys increased further, the adsorption capacity of Fe3O4@VTEO@IL-MIPs reduced and the adsorption capacity of Fe3O4@VTEO@IL-NIPs did not change too much. So it suggests that 1.4 mg mL−1 is the suitable concentration of Lys. The result indicates there are affinity binding sites generated on the surface of Fe3O4@IL-MIPs.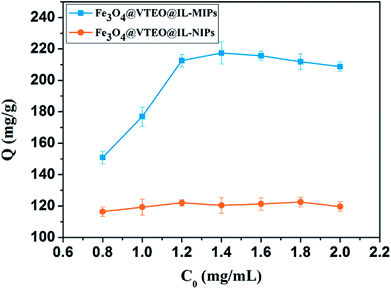 | ||
| Fig. 3 The adsorption isotherm of Lys on Fe3O4@VTEO@IL-MIPs and Fe3O4@VTEO@IL-NIPs. Adsorption conditions: V = 1 mL, mMIPs = mNIPs = 5 mg, CLys = 0.8–2.0 mg mL−1, T = 25 °C, t = 12 h. | ||
The experimental data were then fitted for the Langmuir model. The details and figure and table were shown in ESI Fig. S7 and Table S2.†
3.4. Adsorption kinetics
Adsorption time was investigated in the range of 0.5–5 h. The rebinding kinetics was studied with an initial Lys concentration of 1.4 mg mL−1. The relationship between the adsorption capacity of Fe3O4@VTEO@IL-MIPs (NIPs) and adsorption time was indicated in Fig. 4. Adsorption capacity of Fe3O4@VTEO@IL-MIPs increased rapidly in the first hour and continued to increase until Fe3O4@VTEO@IL-MIPs attained adsorption equilibrium after 2.5 h. When the adsorption time is 2.5 h, the adsorption capacity of Fe3O4@VTEO@IL-MIPs is 213.7 mg g−1, but the adsorption capacity of Fe3O4@VTEO@IL-NIPs is 105.96 mg g−1. Ultimately, 2.5 h was selected as the optimum adsorption time. The high adsorption rate of the Fe3O4@VTEO@IL-MIPs proves once again that the surface of the Fe3O4@VTEO@IL-MIPs produces imprinted cavities during the imprinting process. The imprinted sites locating at the surface of polymers or near the surface make template molecules easily access to the cavities. | ||
| Fig. 4 The adsorption kinetics of Lys on Fe3O4@VTEO@IL-MIPs and Fe3O4@VTEO@IL-NIPs. Adsorption conditions: V = 1 mL, mMIPs = mNIPs = 5 mg, CLys = 1.4 mg mL−1, T = 25 °C. | ||
3.5. Selective adsorption experiments
In order to examine selective extraction ability of the imprinted particles for Lys, we selected four kinds of reference proteins for the selective adsorption experiments. The isoelectric points (pI) and molecular weight (Mw) of these proteins are different. The reference proteins include BHb (64.5 kDa, pI 6.8), BSA (66.4 kDa, pI 4.7), Cyt C (12.4 kDa, 10.0), and OVA (43 kDa, pI 4.7). The Mw and pI of Lys are 14.4 kDa and 10.8, respectively. The template protein has matched size, shape and the placement of functional groups with MIPs. As a result, the adsorption capacity of Fe3O4@VTEO@IL-MIPs to Lys is larger than others reference proteins obviously, which was shown in Fig. 5. BHb and BSA have similar molecular size which is larger than Lys too much. Owing to the strong steric hindrance, it is difficult for BHb and BSA to access to the imprinted cavities. Or, from another perspective, BHb was negatively charged while Lys was positively charged at pH 7.1, so that electrostatic repulsion between the BHb and MIPs is strong, which is similar to BSA and OVA. Although the size and pI of Cyt C is similar to Lys, the adsorption capacity of MIPs to Cyt C is only 37.2 mg g−1, which is due to the mismatched placement of functional groups. All the results investigate that the imprinted particles have selective extraction ability of Lys. The calculated imprinting factor and selectivity factor were given in Table 1. It can be seen that the magnetic imprinted particles show the highest selectivity to the template Lys than others four reference proteins, which confirms Fe3O4@VTEO@IL-MIPs can extract template proteins selectively. | ||
| Fig. 5 Adsorption capacities of different proteins on Fe3O4@VTEO@IL-MIPs and Fe3O4@VTEO@IL-NIPs. Adsorption conditions: V = 1 mL, mMIPs = mNIPs = 5 mg, C0 = 1.4 mg mL−1, T = 25 °C, t = 2.5 h. | ||
| Parameter | IFa | Rb | (QMIP)tem/(QMIP)anac |
|---|---|---|---|
a 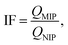 where IF is the imprinting factor for the template molecules.b where IF is the imprinting factor for the template molecules.b 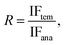 where R is the separation factor.c (QMIP)tem is the adsorption capacity of the MIP for the extraction of template, and (QMIP)ana is the adsorption capacity of the MIP for the extraction of the analogs. where R is the separation factor.c (QMIP)tem is the adsorption capacity of the MIP for the extraction of template, and (QMIP)ana is the adsorption capacity of the MIP for the extraction of the analogs. |
|||
| Lys | 2.23 | — | — |
| BHb | 1.86 | 1.20 | 2.97 |
| BSA | 0.75 | 2.97 | 11.87 |
| Cyt C | 1.05 | 2.12 | 5.39 |
| OVA | 0.60 | 3.72 | 6.81 |
3.6. Competitive batch rebinding tests and real sample adsorption experiments
To better compare the selective extraction ability of Fe3O4@VTEO@IL-MIPs to Lys, competitive adsorption experiments were carried out in binary protein mixture of Lys and BHb. Lys and BHb have same initial concentration. BHb was chosen as a competitive protein, which is because the highest adsorption capacity than others proteins in selective adsorption experiments. The results of SDS-PAGE analysis for the Lys and BHb binary solution were shown in ESI Fig. S8.† The corresponding band of Lys appeared at around 10–15 kDa. Though the molecular weight of BHb is 64.5 kDa, the band of BHb appeared at 10–15 kDa also owing to depolymerization in SDS. Both the BHb and Lys bands of the binary solution after adsorption by Fe3O4@VTEO@IL-MIPs (lane 3) became shallow compared with the initial binary protein mixture (lane 4). It is clear that there is a band at ca. 32 KD, which maybe because BHb depolymerized in SDS incompletely. Though BHb was absorbed to some extent in the competitive batch rebinding tests, the color of Lys band became shallow obviously. It is an effective strategy to imprint and separate Lys.Chicken egg white was taken as the real sample so as to demonstrate the practical applicability of Fe3O4@VTEO@IL-MIPs. As indicated in ESI Fig. S9,† chicken egg white after adsorption by the Fe3O4@VTEO@IL-MIPs exhibited a lighter blue (lane 3) band of Lys, but the band of Lys was no significant change after adsorption by the Fe3O4@VTEO@IL-NIPs (lane 4). The obtained results indicate that the Fe3O4@VTEO@IL-MIPs have potential to separate Lys from chicken egg white.
3.7. Reusability and stability of magnetic ionic liquid imprinted polymers
The reusability of adsorbents is important, which is considered to have a great cost benefit.29 The results were shown in Fig. 6. The adsorption capacity of Fe3O4@VTEO@IL-MIPs is 205.57 mg g−1 in the first time, and it reduced to 170.65 mg g−1 after four adsorption cycles. The results exhibits that the reusability of the measurement is charming which is still above 83% in the final recycling. Some imprinted cavities were destroyed were not eluted entirely when rewashing, which led to slight loss of adsorption capacity. The phenomenon indicates that the magnetic ionic liquid imprinted polymers have good reusability and stability. Furthermore, The CD spectra and the UV-visible spectra of Lys were mentioned in Fig. S10 and S11† to prove the structures of Lys doesn't be changed.3.8. Comparison with other reported methods
Fe3O4@VTEO@IL-MIPs in this work was compared with some other reported Lys imprinted polymers.30–33 It can be seen from Table 2 that the adsorption capacity of Fe3O4@VTEO@IL-MIPs is higher than some imprinted polymers for Lys. The adsorption capacity of the MIPs for Lys in this paper can attain 213.7 mg g−1 and the imprinting factor is 2.02. The SD and RSD obtained is 0.9609 and 0.433%, respectively (n = 6), demonstrating the precision of the UV-vis spectrometer is excellent. Although the adsorption capacity and IF is not the best, a new functional monomer was put forward.| Imprinting method | Qa (mg g−1) | IF | References |
|---|---|---|---|
a  where Q is the adsorption capacity of the MIP for the extraction of template molecules. where Q is the adsorption capacity of the MIP for the extraction of template molecules. |
|||
| Entrapment imprinting | — | 8.6 | 12 |
| Surface imprinting | ∼150 | ∼1.1 | 30 |
| Surface imprinting | 108 | 2.82 | 31 |
| Surface imprinting | 101 | ∼4 | 32 |
| Surface imprinting | 700 | >4 | 33 |
| Surface imprinting | 213.7 | 2.02 | This method |
4. Conclusions
In summary, a new core–shell IL molecularly imprinted polymers was synthesized for highly selective extraction of Lys based on double bond-functionalized Fe3O4 nanoparticles. Fe3O4 nanoparticles were protected by polymers, which can prevent Fe3O4 nanoparticles oxidized and conglomerated. [AAPIM]Cl, the functional monomer of the MIPs, can form strong interactions with template and remarkably improved the imprinting effect. TEM, DLS, TGA, XRD, VSM and FT-IR were used to characterize the composition and morphology of the prepared MIPs. The adsorption capacity of the MIPs for Lys can attain 213.7 mg g−1 and the IF is 2.02. In addition, 2.5 h was chosen as the suitable adsorption time. The high adsorption performance of the MIPs is attractive. From the reusability experiments, it can be seen that the magnetic MIPs microspheres can be used four times at least. Furthermore, Fe3O4@VTEO@IL-MIPs can separate Lys from chicken egg white real sample successfully. In a word, this paper provides an effective strategy to imprint and separate Lys. And our future work is to synthesis better MIPs for selective extraction to Lys.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors greatly appreciate the financial supports by the National Natural Science Foundation of China (No. 21675048) and the Foundation for Innovative Research Groups of NSFC (Grant 21521063).References
- H. Ding, R. Chen, M. Liu, R. Huang, Y. Du, C. Huang, X. Yu, X. Feng and F. Liu, RSC Adv., 2016, 6, 43526–43538 RSC.
- L. Chen, X. Wang, W. Lu, X. Wu and J. Li, Chem. Soc. Rev., 2016, 45, 2137–2211 RSC.
- X. M. Zhang, Y. P. Qin, H. L. Ye, X. T. Ma, X. W. He, W. Y. Li and Y. K. Zhang, Microchim. Acta, 2018, 185, 173 CrossRef PubMed.
- X. Chen and N. Ye, RSC Adv., 2017, 7, 34077–34085 RSC.
- B. Singh and N. Chauhan, Acta Biomater., 2008, 4, 1244–1254 CrossRef PubMed.
- H. Duan, L. Li, X. Wang, Y. Wang, J. Li and C. Luo, RSC Adv., 2015, 5, 18850–18857 RSC.
- J. Guo, Y. Wang, Y. Liu and Y. Zhou, Anal. Methods, 2015, 7, 10018–10025 RSC.
- Y. Liu, Y. Wang, Q. Dai and Y. Zhou, Anal. Chim. Acta, 2016, 936, 168–178 CrossRef PubMed.
- S. Xu, H. Lu, L. Chen and X. Wang, RSC Adv., 2014, 4, 45266–45274 RSC.
- X. Wang, P. Huang, X. Ma, X. Du and X. Lu, J. Chromatogr. A, 2018, 1537, 35–42 CrossRef PubMed.
- S. J. Cho, H. B. Noh, M. S. Won, C. H. Cho, K. B. Kim and Y. B. Shim, Biosens. Bioelectron., 2018, 99, 471–478 CrossRef PubMed.
- T. Kubo, S. Arimura, Y. Tominaga, T. Naito, K. Hosoya and K. Otsuka, Macromolecules, 2015, 48, 4081–4087 CrossRef.
- E. Shoghi, S. Z. Mirahmadi-Zare, R. Ghasemi, M. Asghari, M. Poorebrahim and M. H. Nasr-Esfahani, Microchim. Acta, 2018, 185, 241 CrossRef PubMed.
- S. H. Ou, M. C. Wu, T. C. Chou and C. C. Liu, Anal. Chim. Acta, 2004, 504, 163–166 CrossRef.
- C. Ocaña, A. Hayat, R. Mishra, A. Vasilescu, M. D. Valle and J. L. Marty, Analyst, 2015, 140, 4148–4153 RSC.
- Q. Yang, Y. Wang, H. Zhang, K. Xu, X. Wei, J. Chen and P. Xu, RSC Adv., 2017, 7, 53203–53209 RSC.
- Q. Wen, Y. Wang, K. Xu, N. Li, H. Zhang and Q. Yang, Anal. Chim. Acta, 2016, 939, 54–63 CrossRef PubMed.
- X. Wei, Y. Wang, J. Chen, P. Xu and Y. Zhou, Talanta, 2018, 182, 484–491 CrossRef PubMed.
- Q. Zhao, J. C. Wajert and J. L. Anderson, Anal. Chem., 2010, 82, 707–713 CrossRef PubMed.
- H. Xiang, M. Peng, H. Li, S. Peng and S. Shi, J. Pharm. Biomed. Anal., 2017, 133, 75–81 CrossRef PubMed.
- L. Zhao, J. Yang, H. Ye, F. Zhao and B. Zeng, RSC Adv., 2017, 7, 4704–4709 RSC.
- M. Liu, J. Pi, X. Wang, R. Huang, Y. Du, X. Yu, W. Tan, F. Liu and K. J. Shea, Anal. Chim. Acta, 2016, 932, 29–40 CrossRef PubMed.
- J. Chen, Y. Wang, Q. Zeng, X. Ding and Y. Huang, Anal. Methods, 2014, 6, 4067–4076 RSC.
- Q. Dai, Y. Wang, W. Xu, Y. Liu and Y. Zhou, Microchim. Acta, 2017, 184, 4433–4441 CrossRef.
- J. Xie, G. Zhong, C. Cai, C. Chen and X. Chen, Talanta, 2017, 169, 98–103 CrossRef PubMed.
- Q. Gai, F. Qu, Z. J. Liu, R. J. Dai and Y. K. Zhang, J. Chromatogr. A, 2010, 1217, 5035–5042 CrossRef PubMed.
- N. Li, L. Qi, Y. Shen, J. Qiao and Y. Chen, ACS Appl. Mater. Interfaces, 2014, 6, 17289–17295 Search PubMed.
- J. Xu, Y. Wang and S. Hu, Microchim. Acta, 2017, 184, 1–44 CrossRef.
- R. Gao, Y. Hao, L. Zhang, X. Cui, D. Liu, M. Zhang, Y. Tang and Y. Zheng, Chem. Eng. J., 2016, 284, 139–148 CrossRef.
- S. Ji, N. Li, Y. Shen, Q. Li, J. Qiao and Z. Li, Anal. Chim. Acta, 2016, 909, 60–66 CrossRef PubMed.
- K. Xu, Y. Wang, X. Wei, J. Chen, P. Xu and Y. Zhou, Microchim. Acta, 2018, 185, 146 CrossRef PubMed.
- H. Duan, X. Wang, Y. Wang, Y. Sun, J. Li and C. Luo, Anal. Chim. Acta, 2016, 918, 89–96 CrossRef PubMed.
- J. Chen, S. Lei, Y. Xie, M. Wang, J. Yang and X. Ge, ACS Appl. Mater. Interfaces, 2015, 7, 28606–28615 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra03818j |
| This journal is © The Royal Society of Chemistry 2018 |