Open Access Article
Open Access ArticleSpecific and sensitive imaging of basal cysteine over homocysteine in living cells†
Longxue Nie,
Bingpeng Guo,
Congcong Gao,
Shaowen Zhang*,
Jing Jing * and
Xiaoling Zhang
* and
Xiaoling Zhang *
*
Key Laboratory of Cluster Science of Ministry of Education, Beijing Key Laboratory of Photo-electronic/Electro-photonic Conversion Materials, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, Beijing 100081, P. R. China. E-mail: zhangxl@bit.edu.cn; hellojane@bit.edu.cn; swzhang@bit.edu.cn
First published on 6th November 2018
Abstract
Biological thiols play important roles in maintaining appropriate redox status of organisms. Accepting the challenge to differentiate structurally similar cysteine (Cys) and homocysteine (Hcy), we have successfully developed a miniature synthetic turn-on fluorescent probe based on 6-(2-benzothiazolyl)-2-naphthalenol for Cys. This probe is able to specifically react with Cys to yield its naphthalenol derivative, accompanied by remarkable green fluorescence enhancement with a detection limit of 14.8 nM. Besides, this probe displays much greater selectivity for Cys over other biological thiols, including homocysteine (Hcy) and glutathione (GSH). Practically, good cell permeability and low cytotoxicity make it suitable for monitoring basal Cys in living cells.
1. Introduction
Biological thiols including cysteine (Cys), homocysteine (Hcy) and glutathione have received a great deal of attention due to their diverse biological functions in living organisms.1–5 Among them, Cys is an enriched amino acid that is involved in various physiological and pathological processes. Intracellular Cys is generally generated from methionine by methionine-adenosyl-transferase, adenosyl-homocysteinase, cystathionine-β-synthase, or cystathionine-γ-lyase.6 At normal levels, Cys maintains the synthesis of proteins, and acts as a source of sulfide in human metabolism.7,8 A deficiency of Cys induces diseases such as decreased hematopoiesis, leucocyte loss, psoriasis, neurotoxicity, edema, liver damage and Parkinson's disease.9–11 On the other hand, excessive Cys causes rheumatoid arthritis and Alzheimer's disease.12–17 In order to understand the biological properties of Cys better, it is important to develop sensitive and selective methods for monitoring extracellular Cys.As a powerful tool, fluorescence spectroscopy has been widely applied for detection of varied cellular species due to its high sensitivity, fast-response, simplicity of implementation, cost-effectiveness, real-time detection, noninvasiveness, and good compatibility with biosystems.18–23 For thiol-containing biomolecules fluorescence probes based on different response mechanisms have been well developed, including Michael addition,24–26 cyclization reaction with aldehyde,27–29 cleavage reaction,30–33 nucleophilic substitution,34–39 disulfide exchange reaction40–42 and other mechanisms.43,44 To achieve better selectivity of Cys over Hcy and GSH, the Michael addition reaction is among the most widely used strategies for fluorescent sensing of Cys. Strongin and co-workers creatively developed a combination of Michael addition and cyclization reaction to improve the selectivity of Cys/Hcy over GSH.45 Yoon and co-workers reported a fluorescent probe based on fluorescein with excellent selectivity and sensitivity for thiols, and it was successfully applied for bio imaging.46 Sun and co-workers reported a novel fluorescent probe based on a,b-unsaturatedacyl sulfonamide to detect thiols. It reacted selectively with cysteine but not with the other natural amino acids, and subsequently applied to detect intracellular thiols.47 Though these probes employ high sensitivity toward thiol-containing compounds, the selective detection of Cys is still challenging because of disturbance of other biological thiols, especially Hcy. This challenge arises from the structural and reactive similarity between Cys and Hcy which differ by a single methylene of their side chains. Currently claimed fluorescent probes for Cys over Hcy often utilize the different relative kinetical rates which may still cause interference from Hcy after a certain reaction time. Optimal solutions without any kinetical control are in great demand.
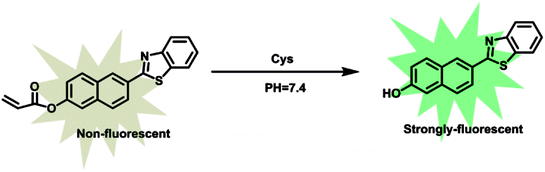
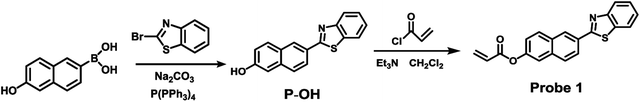
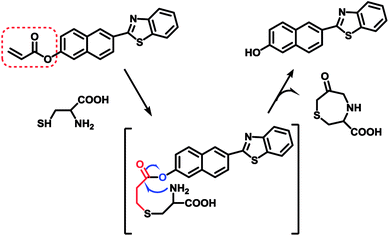
As our continuous interests in monitoring cellular redox status changes,48–51 we designed and synthesized a simple fluorescent probe 1 to detect cellular Cys in living cells. As demonstrated in Scheme 1, probe 1 carrying an acrylates group, which acts as a Michael addition acceptor is able to react with Cys through thioether formation and followed by cyclization to release emissive fluorophore. Probe 1 is easily accessible by two-step synthesis method, which is weakly-fluorescent with an absolute quantum yield of 0.08 due to the photo induced electron transfer (PET) process. After the reaction with Cys, the product emits strong green fluorescence and gives an absolute quantum yield of 0.78. Experimental results show that probe 1 exhibits high sensitivity (the detection limit of 14.8 nM) against Cys, and high selectivity over Hcy and other biological thiols. More importantly, probe 1 showed good cell permeability, low cytotoxicity and was successfully applied to imaging basal Cys in living cells.
2. Experimental
2.1. Materials and instrumentals
All other chemicals used in this paper were obtained from commercial suppliers and used without further purification. Silica gel (200–300 mesh, Qingdao Haiyang Chemical Co.) was used for column chromatography. NMR spectra were recorded on a Bruker Avance III at 400 MHz for 1H NMR and at 100 MHz for 13C NMR with chemical shifts reported as ppm (in DMSO-d6 TMS as internal standard). Mass spectra (MS) were measured with Bruker Apex IV FTMS using electrospray ionization (ESI). Absorption spectra were recorded on a Purkinje TU-1901 spectrophotometer. Fluorescence measurements were taken on a Hitachi F-7000 fluorescence spectrometer with a 10 mm quartz cuvette. pH measurements were carried out with a pH acidometer (Mettler Toledo FE-30). Fluorescence imaging was observed under an Olympus IX81 confocal fluorescence microscope. The absolute fluorescence quantum yield values were measured using Hamamatsu Photonic Multi-Channel Analyzer PMA-12.2.2. General procedure for analysis
Parent stock solution of fluorescent probe 1 (1.0 mM) was prepared in dimethyl sulfoxide (DMSO). The solution of test was prepared by placing 50 μL of parent stock solution and appropriate volume of other solution into the test tube, then diluting the solution to 10 mL with the mixture of ethanol and ultrapure water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8, v/v) containing phosphate buffered saline (PBS, 10 mM, pH = 7.4). All spectra were obtained in a quartz cuvette (path length = 1 cm).
8, v/v) containing phosphate buffered saline (PBS, 10 mM, pH = 7.4). All spectra were obtained in a quartz cuvette (path length = 1 cm).
All the solutions (10.0 mM) were prepared in deionized water. All the amino acids were purchased from Sigma-Aldrich Chemical. PBS solution was prepared with Na2HPO4 and KH2PO4, and adjusted to pH 7.4.
2.3. Determination of the detection limit
Referring to previous papers, the detection limit was calculated based on fluorescence titration.52 Fluorescence titration was carried out in H2O/ethanol solution (H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol = 8
ethanol = 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, (v/v), 10 mM PBS, pH = 7.4) to determine the detection limit, which was then calculated with the following equation:
2, (v/v), 10 mM PBS, pH = 7.4) to determine the detection limit, which was then calculated with the following equation:| Detection limit = 3σ/k |
2.4. Cytotoxicity assay
HeLa cells were cultured in culture media (DMEM) in an atmosphere of 5% CO2 and 95% air at 37 °C. The cells were seeded into 96-well plates at a density of 3 × 103 cells per well in culture media, then 0, 5, 10 μM probe 1 were added, respectively. Next, the cells were incubated at 37 °C in an atmosphere of 5% CO2 and 95% air for 24 h. Finally, 20 μL MTT was added and cultured for another 4 h, respectively.2.5. Cell culture and imaging
HeLa cells were grown on glass-bottom culture dishes using DMEM supplemented with 10% (v/v) fetal bovine serum (FBS) and 50 μg mL−1 penicillin-streptomycin in a humidified 37 °C, 5% CO2 incubator. Before use, the adherent cells were washed three times with FBS-free DMEM. The cells were incubated with 5 μM probe in culture media for 30 min at 37 °C and then washed with PBS (pH 7.4) twice. Fluorescence imaging of HeLa cells was observed under an Olympus IX81 confocal fluorescence microscope, and the excitation wavelength was 405 nm.2.6. Synthesis of fluorescent probe 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VToluene
VToluene![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) VH2O = 1
VH2O = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). The mixture was refluxed for 3 h then cooled to ambient temperature. Poured into the water, the solution was adjusted by acid and stand for 10 min, and the precipitate was obtained by filtration. The filtrate was evaporated under reduced pressure and purified by silica gel column chromatography affording P-OH as white solid. 1H NMR (400 MHz, DMSO) δ 10.14 (s, 1H), 8.56 (s, 1H), 8.16 (d, J = 7.9 Hz, 1H), 8.12–7.99 (m, 2H), 7.86 (d, J = 8.6 Hz, 1H), 7.56 (t, J = 7.6 Hz, 1H), 7.46 (t, J = 7.5 Hz, 1H), 7.20 (d, J = 13.6 Hz, 1H).
1). The mixture was refluxed for 3 h then cooled to ambient temperature. Poured into the water, the solution was adjusted by acid and stand for 10 min, and the precipitate was obtained by filtration. The filtrate was evaporated under reduced pressure and purified by silica gel column chromatography affording P-OH as white solid. 1H NMR (400 MHz, DMSO) δ 10.14 (s, 1H), 8.56 (s, 1H), 8.16 (d, J = 7.9 Hz, 1H), 8.12–7.99 (m, 2H), 7.86 (d, J = 8.6 Hz, 1H), 7.56 (t, J = 7.6 Hz, 1H), 7.46 (t, J = 7.5 Hz, 1H), 7.20 (d, J = 13.6 Hz, 1H).
3. Results and discussion
3.1. Response of probe 1 to Cys
The absorption and fluorescence spectra of probe 1 were measured in H2O/ethanol solution (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
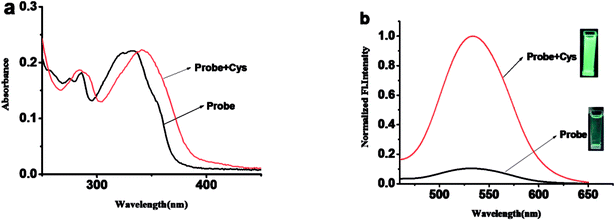
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v, 10 mM PBS, pH = 7.4). As shown in Fig. 1, probe 1 shows a maximum absorption at 331 nm and weak fluorescence with a relatively low quantum yield of 0.08. After the addition of Cys, the maximum absorption red shifted 13 nm to 343 nm (Fig. 1a). Meanwhile, slight blue shift and 24-fold fluorescence enhancement at 525 nm were obtained and the solution emitted strong green fluorescence under a handhold UV lamp (Fig. 1b), just like we expected, given the absolute quantum yield of 0.78 for the final fluorophore P-OH.
2, v/v, 10 mM PBS, pH = 7.4). As shown in Fig. 1, probe 1 shows a maximum absorption at 331 nm and weak fluorescence with a relatively low quantum yield of 0.08. After the addition of Cys, the maximum absorption red shifted 13 nm to 343 nm (Fig. 1a). Meanwhile, slight blue shift and 24-fold fluorescence enhancement at 525 nm were obtained and the solution emitted strong green fluorescence under a handhold UV lamp (Fig. 1b), just like we expected, given the absolute quantum yield of 0.78 for the final fluorophore P-OH.
3.2. Quantification of Cys and detection limit
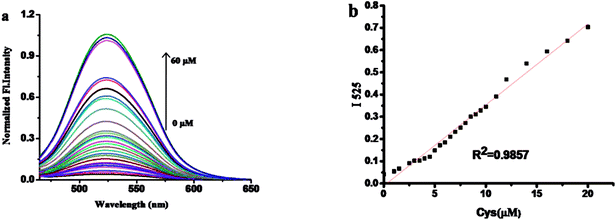
The fluorescence spectra of probe 1 with various concentrations of Cys is shown in Fig. 2. Upon gradually increasing of Cys, the fluorescence band centered at 525 nm increased subsequently. There was a good linearity between the fluorescence intensity at 525 nm and the concentrations of Cys in the range of 1 to 20 μM.The detection limit of Cys is calculated to be 14.8 nM. The results show that probe 1 has better detection limit than those reported fluorescent probes for Cys (Table S1†). These above results demonstrated that probe 1 could detect Cys quantitatively by fluorescence spectrometry methods with an excellent sensitivity (Table S1†).3.3. The kinetic profile of the recognition of probe 1 for Cys
The response time of probe 1 towards Cys is evaluated by fluorescence spectroscope. When Cys (60 μM) was added to the solution of probe 1 (5 μM), the fluorescence intensity at 525 nm levels off after 40 min (Fig. S1†). The result shows that probe 1 could completely react with Cys within 40 min, thus we chose 40 min to verify the quantification and selectivity of Cys.3.4. The selectivity of probe 1
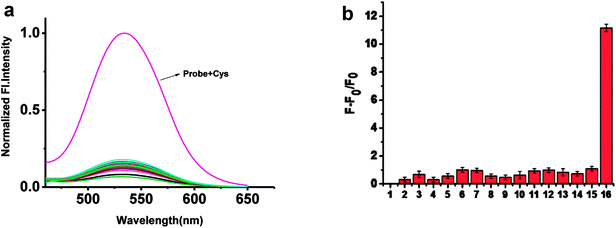
To confirm the selectivity of probe 1 towards Cys, other amino acids and GSH were added into the solution of probe 1. As shown in Fig. 3 and S2,† upon addition of 10 mM GSH, and other amino acids, or cations including K+, Na+, Mg2+, Al3+, Fe2+ into 5 μM probe 1 solution, nearly no fluorescence change were observed except Cys. Only Cys gave12-fold enhancement of fluorescence.3.5. Reaction mechanism
It has been well documented that the acrylate group could be used as an efficient reaction site for Cys.53 The mechanism of probe 1 responding to Cys was based on the Michael addition reaction of Cys with the acrylate group to generate the corresponding thioether, a subsequent intramolecular cyclization and further self-immolation to yield the desired lactam, as shown in Scheme 3. To gain further insight into the reaction mechanism, mass spectrometric analysis was carried out to verify the final product after reaction between probe 1 and Cys. ESI-HRMS in Fig. S9† showed a main peak at m/z 278.0638[M + H]+ corresponding to compound P-OH (calculated at m/z 278.0634[M + H]+), which disclosed the proposed mechanism below.3.6. Bioimaging applications and cytotoxicity
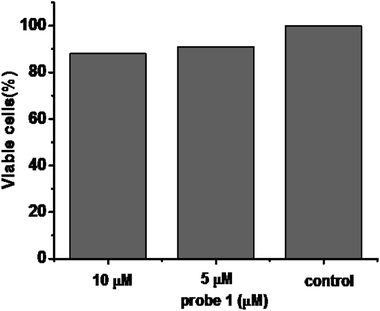
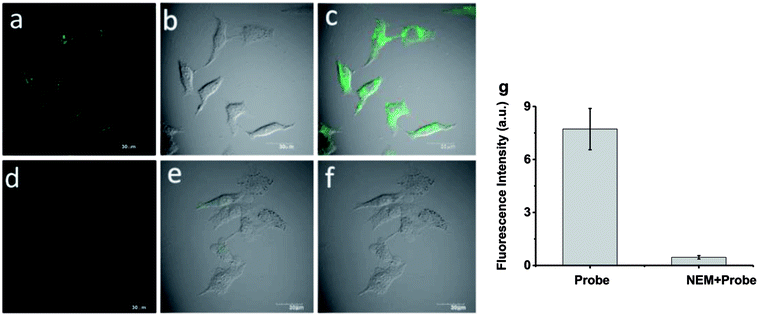
To further demonstrate the biological compatibility before real time imaging in living cells,we evaluated the cytotoxicity of probe 1. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide(MTT) assays were performed in HeLa cells with 0, 5, 10 μM probe 1 for 24 h, respectively. The viability results in Fig. 4 clearly showed that probe 1 was minimal toxic to cultured cells even at the concentration of as high as 10 μM. This illustrates probe 1 is suitable for living cell imaging at its work concentration, 5 μM.Inspired by the experimental results in vitro of the probe 1 and encouraged by the good biocompatibility, we expected that the high sensitive probe could have a good application for imaging endogenous Cys in living cells. In order to verify our conjecture, HeLa cells were incubated with probe 1 (5 μM) for 30 min, and a strong green fluorescence was observed as shown in Fig. 5a–c. When HeLa cells were treated with N-ethylmaleimide (100 ng mL−1)as Cys scavenger for 1 h before they were incubated with probe 1 (5 μM), hardly any intracellular fluorescence was observed inFig. 5d–f. From Fig. 5g, these results clearly demonstrated that probe 1was cell-permeable and could provide a good option for imaging basal Cys in living cells. Moreover, the same experiments were also tested in normal cells apart from cancer cells, like RAW 264.7 and HepG2 and similar cellular fluorescence change was observed (Fig. S3 and S4†).
4. Conclusion
In summary, we have developed a reaction based turn-on fluorescent probe 1 conjugating with a reactive acrylate for visualization basal Cys in living cells. The synthesis of probe 1 is simple, and the probe showed excellent selectivity toward Cys over Hcy. Different from previously reported Cys detection methods, the reactivity between Cys and Hys is significantly different, therefore kinetically disfavorability of Hcy is no longer needed to improve the selectivity. Furthermore, monitoring basal Cys effectively in living cells was easily implemented attributing to its low cytotoxic, good sensitivity and good selectivity towards Cys over Hcy and GSH. This method could became an potential tool to reveal the behaviors of Cys in living organism.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Nature Science Foundation of China (No. 21575015 and 21505004).References
- Y. Zhou and J. Yoon, Recent progress in fluorescent and colorimetric chemosensors for detection of amino acids, Chem. Soc. Rev., 2012, 41(1), 52–67 RSC.
- J. Yoon, L. Cui and Y. Baek, et al., AIE and ESIPT based kinetic resolved fluorescent probe for Biothiols, J. Mater. Chem. C, 2016, 4(14), 2909–2914 RSC.
- H. S. Jung, J. H. Han and T. Pradhan, et al., A cysteine-selective fluorescent probe for the cellular detection of cysteine, Biomaterials, 2012, 33(3), 945–953 CrossRef CAS PubMed.
- Y. Liu, X. Lv and M. Hou, et al., Selective fluorescence detection of cysteine over homocysteine and glutathione based on a cysteine-triggered dual Michael Addition/Retro-aza-aldol cascade reaction, Anal. Chem., 2015, 87(22), 11475–11483 CrossRef CAS PubMed.
- Y. Kim, S. V. Mulay and M. Choi, et al., Exceptional time response, stability and selectivity in doubly-activated phenyl selenium-based glutathione-selective platform, Chem. Sci., 2015, 6(10), 5435–5439 RSC.
- Y. Yue, F. Huo and P. Ning, et al., Dual-site fluorescent probe for visualizing the metabolism of Cys in living cells, J. Am. Chem. Soc., 2017, 139(8), 3181–3185 CrossRef CAS PubMed.
- H. S. Jung, J. H. Han and T. Pradhan, et al., A cysteine-selective fluorescent probe for the cellular detection of cysteine, Biomaterials, 2012, 33(3), 945–953 CrossRef CAS PubMed.
- H. S. Jung, T. Pradhan and J. H. Han, et al., Molecular modulated cysteine-selective fluorescent probe, Biomaterials, 2012, 33(33), 8495–8502 CrossRef CAS PubMed.
- L. M. Lópezsánchez, C. Lópezpedrera and A. Rodríguezariza, Proteomic approaches to evaluate protein S-nitrosylation in disease, Mass Spectrom. Rev., 2014, 33(1), 7–20 CrossRef PubMed.
- R. Janáky, V. Varga and A. Hermann, et al., Mechanisms of L-cysteine neurotoxicity, Neurochem. Res., 2000, 25(9–10), 1397–1405 CrossRef.
- S. Shahrokhian, Lead phthalocyanine as a selective carrier for preparation of a cysteine-selective electrode, Anal. Chem., 2001, 73(24), 5972–5978 CrossRef CAS PubMed.
- J. Liu, Y. Q. Sun and Y. Huo, et al., Simultaneous fluorescence sensing of Cys and GSH from different emission channels, J. Am. Chem. Soc., 2014, 136(2), 574–577 CrossRef CAS PubMed.
- W. Hao, A. Mcbride and S. Mcbride, et al., Colorimetric and near-infrared fluorescence turn-on molecular probe for direct and highly selective detection of cysteine in human plasma, J. Mater. Chem., 2011, 21(4), 1040–1048 RSC.
- Y. W. Wang, S. B. Liu and W. J. Ling, et al., A fluorescent probe for relay recognition of homocysteine and Group IIIA ions including Ga(III), Chem. Commun., 2016, 52(4), 827–830 RSC.
- J. Dorszewska, M. Prendecki and A. Oczkowska, et al., Molecular basis of familial and sporadic Alzheimer's disease, Curr. Alzheimer Res., 2016, 13(9), 952–963 CrossRef CAS PubMed.
- D. W. Jacobsen, Acquired hyperhomocysteinemia in heart transplant recipients, Clin. Chem., 1998, 44(11), 2238–2239 CAS.
- S. Seshadri, A. Beiser, J. Selhub, P. F. Jacques, I. H. Rosenberg, R. B. D'Agostino, P. W. Wilson and P. A. Wolf, Plasma homocysteine as a risk factor for dementia and Alzheimer's disease, N. Engl. J. Med., 2002, 346(7), 476–483 CrossRef CAS PubMed.
- X. Li, X. Gao and W. Shi, et al., Design strategies for water-soluble small molecular chromogenic and fluorogenic probes, Chem. Rev., 2014, 114(1), 590–659 CrossRef CAS PubMed.
- J. F. Zhang, Y. Zhou and J. Yoon, et al., ChemInform Abstract: Recent Progress in Fluorescent and Calorimetric Chemosensors for Detection of Precious Metal Ions (Silver, Gold and Platinum Ions), J. Cheminf., 2011, 42(42), 3416–3429 Search PubMed.
- X. Chen, X. Tian and I. Shin, et al., ChemInform Abstract: Fluorescent and Luminescent Probes for Detection of Reactive Oxygen and Nitrogen Species, Chem. Soc. Rev., 2011, 40(9), 4783–4804 RSC.
- J. Lu, Y. Song and W. Shi, et al., A long-wavelength fluorescent probe for imaging reduced glutathione in live cells, Sens. Actuators, B, 2012, 161(1), 615–620 CrossRef CAS.
- H. Maeda, H. Matsuno and M. Ushida, et al., 2,4-Dinitrobenzenesulfonyl fluoresceins as fluorescent alternatives to Ellman's reagent in thiol-quantification enzyme assays, Angew. Chem., Int. Ed., 2005, 44(19), 2922–2925 CrossRef CAS PubMed.
- C. Yin, F. Huo and J. Zhang, et al., ChemInform Abstract: Thiol-Addition reactions and rheirapplications in thiol recognition, Chem. Soc. Rev., 2013, 42(14), 6032–6059 RSC.
- D. Kand, A. M. Kalle and S. J. Varma, et al., A chromenoquinoline-based fluorescent off-on thiol probe for bioimaging, Chem. Commun., 2012, 48(21), 2722–2724 RSC.
- H. S. Jung, T. Pradhan and J. H. Han, et al., Molecular modulated cysteine-selective fluorescent probe, Biomaterials, 2012, 33(33), 8495–8502 CrossRef CAS PubMed.
- B. K. Mcmahon and T. Gunnlaugsson, Selective detection of the reduced form of glutathione (GSH) over the oxidized (GSSG) form using a combination of glutathione reductase and a Tb(III)-cyclen maleimide based lanthanide luminescent 'switch on' assay, J. Am. Chem. Soc., 2012, 134(26), 10725–10728 CrossRef CAS PubMed.
- P. Wang, J. Liu and X. Lv, et al., A naphthalimide-based glyoxal hydrazone for selective fluorescence turn-on sensing of Cys and Hcy, Org. Lett., 2012, 14(2), 520–523 CrossRef CAS PubMed.
- F. Kong, R. Liu and R. Chu, et al., A highly sensitive near-infrared fluorescent probe for cysteine and homocysteine in living cells, Chem. Commun., 2013, 49(80), 9176–9178 RSC.
- X. Zhou, D. Guo and Y. Jiang, et al., A novel AIEE and EISPT fluorescent probe for selective detection of cysteine[J], Tetrahedron Lett., 2017, 58(33), 3214–3218 CrossRef CAS.
- Y. Lin, W. Lin and Z. Sheng, et al., A unique approach to development of near-infrared fluorescent sensors for in vivo imaging, J. Am. Chem. Soc., 2012, 134(32), 13510–13523 CrossRef PubMed.
- M. Wei, P. Yin and Y. Shen, et al., A new turn-on fluorescent probe for selective detection of glutathione and cysteine in living cells, Chem. Commun., 2013, 49(41), 4640–4642 RSC.
- J. Yin, Y. Kwon and D. Kim, et al., A Cyanine Based Fluorescence Probe for Highly Selective Detection of Glutathione in Cell Cultures and Live Mice Tissues, J. Am. Chem. Soc., 2014, 136(14), 5351–5358 CrossRef CAS PubMed.
- M. Li, X. Wu and Y. Wang, et al., A near-infrared colorimetric fluorescent chemodosimeter for the detection of glutathione in living cells, Chem. Commun., 2014, 50(14), 1751–1753 RSC.
- L. Y. Niu, Y. S. Guan and Y. Z. Chen, et al., BODIPY-based ratiometric fluorescent sensor for highly selective detection of glutathione over cysteine and homocysteine, J. Am. Chem. Soc., 2012, 134(46), 18928–18931 CrossRef CAS PubMed.
- X. F. Yang, Q. Huang and Y. Zhong, et al., A dual emission fluorescent probe enables simultaneous detection of glutathione and cysteine/homocysteine, Chem. Sci., 2014, 5(6), 2177–2183 RSC.
- S. Y. Lim, K. H. Hong and D. I. Kim, et al., Tunable Heptamethine-Azo dye conjugate as an NIR fluorescent probe for the selective detection of mitochondrial glutathione over cysteine and homocysteine, J. Am. Chem. Soc., 2014, 136(19), 7018–7025 CrossRef CAS PubMed.
- Y. S. Guan, L. Y. Niu and Y. Z. Chen, et al., A near-infrared fluorescent sensor for selective detection of cysteine and its application in live cell imaging, RSC Adv., 2014, 4(16), 8360 RSC.
- H. Lv, X. F. Yang and Y. Zhong, et al., Native chemical ligation combined with spirocyclization of benzopyrylium dyes for the ratiometric and selective fluorescence detection of cysteine and homocysteine, Anal. Chem., 2014, 86(3), 1800–1807 CrossRef CAS PubMed.
- J. Liu, Y.-Q. Sun, H. Zhang, Y. Huo, Y. Shi and W. Guo, Simultaneous fluorescent imaging of Cys/Hcy and GSH from different emission channels, Chem. Sci., 2014, 5, 3183–3188 RSC.
- J. H. Lee, C. S. Lim and Y. S. Tian, et al., A two-photon fluorescent probe for thiols in live cells and tissues, J. Am. Chem. Soc., 2010, 132(4), 1216–1217 CrossRef CAS PubMed.
- C. S. Lim, G. Masanta and H. J. Kim, et al., Ratiometric detection of mitochondrial thiols with a two-photon fluorescent probe, J. Am. Chem. Soc., 2011, 133(29), 11132–11135 CrossRef CAS PubMed.
- M. H. Lee, J. H. Han and P. S. Kwon, et al., Hepatocyte-targeting single galactose-appended naphthalimide: a tool for intracellular thiol imaging in vivo, J. Am. Chem. Soc., 2012, 134(2), 1316–1322 CrossRef CAS PubMed.
- H. S. Jung, Ji H. Han and Y. Habata, An iminocoumarin-Cu(II) ensemble-based chemodosimeter toward thiols, Chem. Commun., 2011, 47(18), 5142–5144 RSC.
- Y. Bao, Q. Li and B. Liu, et al., Conjugated polymers containing a 2,2'-biimidazole moiety--a novel fluorescent sensing platform, Chem. Commun., 2012, 48(1), 118–120 RSC.
- X. Yang, Y. Guo and R. M. Strongin, Conjugate Addition/Cyclization sequence enables selective and simultaneous fluorescence detection of cysteine and homocysteine, Angew. Chem., Int. Ed., 2011, 50(45), 10690–10693 CrossRef CAS PubMed.
- X. Chen, S. K. Ko and M. J. Kim, et al., A thiol-specific fluorescent probe and its application for bioimaging, Chem. Commun., 2010, 46(16), 2751 RSC.
- H. Zhang, P. Wang and Y. Yang, et al., A selective fluorescent probe for thiols based on α,β-unsaturated acyl sulfonamide, Chem. Commun., 2012, 48(86), 10672–10674 RSC.
- C. Gao, Y. Tian and R. B. Zhang, et al., Endoplasmic reticulum directed ratiometric fluorescent probe for quantitively detection of basal H2O2, Anal. Chem., 2017, 89(23), 12945–12950 CrossRef CAS PubMed.
- B. Guo, H. Nie and W. Yang, et al., A highly sensitive and rapidly responding fluorescent probe with a large Stokes shift for imaging intracellular hypochlorite, Sens. Actuators, B, 2016, 236, 459–465 CrossRef CAS.
- B. Guo, J. Jing and H. Nie, et al., A lysosome-targetable versatile fluorescent probe for imaging viscosity and peroxynitrite with different fluorescence signals in living cells, J. Mater. Chem. B, 2018, 6, 580–585 RSC.
- Y. Tian, F. Xin and C. Gao, et al., Ratiometric fluorescent imaging for endogenous selenocysteine in cancer cell matrix, J. Mater. Chem. B., 2017, 5, 6890–6896 RSC.
- C. Liu, C. Shao and H. Wu, et al., A fast-response, highly sensitive and selective fluorescent probe for the ratiometric imaging of hydrogen peroxide with a 100 nm red-shifted emission, RSC Adv., 2014, 4(31), 16055–16061 RSC.
- N. J. Leonard and R. Y. Ning, The synthesis and stereochemistry of substituted 1,4-thiazepines related to the penicillins, J. Org. Chem., 1966, 31(12), 3928–3935 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra05908j |
| This journal is © The Royal Society of Chemistry 2018 |