Open Access Article
Open Access ArticleA biomimetic fluorescent chemosensor for highly sensitive zinc(II) detection and its application for cell imaging†
Rui Yan a,
Zhi Wang
a,
Zhi Wang c,
Zongliang Dua,
Haibo Wang
c,
Zongliang Dua,
Haibo Wang a,
Xu Cheng*a and
Junjie Xiong*b
a,
Xu Cheng*a and
Junjie Xiong*b
aTextile Institute, College of Light Industry, Textile and Food Engineering, Sichuan University, Chengdu, 610065, China. E-mail: scuchx@163.com
bDepartment of Pancreatic Surgery, West China Hospital, Sichuan University, Chengdu 610041, China. E-mail: junjiex2011@126.com
cState Key Laboratory of Biotherapy, Sichuan University, Chengdu 610041, China
First published on 26th September 2018
Abstract
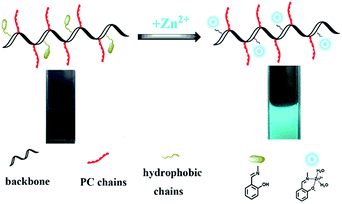
To fabricate a novel biomimetic fluorescent chemosensor, PSaAEMA-co-PMPC was synthesized via atom transfer radical polymerization, and this copolymer could be used for the detection of zinc(II) and cell imaging. A series tests with various metal ions verified the specific fluorescence response behavior. This novel biomimetic fluorescent chemosensor exhibits excellent selectivity for Zn2+ ions over a wide range of tested metal ions in an aqueous solution. Moreover, cytotoxicity and bio-imaging tests were conducted to study the potential bio-application of the chemosensor. Owing to the biomimetic portion (phosphorylcholine), this copolymer possesses outstanding biocompatibility and could clearly image cells. The results indicated that PSaAEMA-co-PMPC has great potential for application in zinc(II) detection and cell imaging.
Introduction
Zinc, the second most abundant transition metal ion in the human body, is often present in a tightly bound form in proteins. It plays several significant roles in various fundamental biological processes. The disruption of Zn(II) homeostasis could lead to Parkinson’s disease, Alzheimer’s disease, immune dysfunction, and infantile diarrhea.1–5 As a consequence, the development of probes to effectively quantify and visualize Zn(II) concentration in biological systems is key to understanding the biological processes of Zn(II) in vivo.Recently, there have been many studies on fluorescence probing methods and fluorescent chemosensors, such as the AIE probe technique,6–13 and fluorescent inorganic nanoparticles.14–18 Currently, there is great interest in the development of new methods with high sensitivity and the exploration of the role of Zn(II) in medicine and biology as well as the environment. Among the various types of methods, fluorescent chemosensors have attracted much attention due to their convenient use and high sensitivity, and could be employed to clarify the various biological functions of targeted metal cations in living cells.19–25 A few Zn(II) fluorescent chemosensors have been fabricated for physiological applications and some are now commercially available. Salicylaldimine Schiff bases, one of the common structures, have aroused great interest for fluorescent chemosensors due to their facile synthesis and high binding affinity towards many metal ions. Salicylaldimine Schiff bases have poor fluorescence, but can display strong fluorescence once they coordinate with special metal ions such as zinc ions via the phenol oxygen atom and the imine nitrogen atom, which results in a rigid structure.26 Until now, a large amount of small molecule fluorescent metal ion probes have been developed based on salicylaldimine Schiff bases in past decades.26–37 However, for the majority of them, their water solubility is very limited since these chemosensors are hydrophobic, which induces aggregation between them. Thus, detection could not be performed in pure water, and is often performed in mixed aqueous solutions with a certain portion of organic solvent. Furthermore, the accuracy and sensitivity of small molecule detection are not satisfactory. To this end, several elegant strategies have been developed to overcome the flaws of small molecule probes, for example conjugating or loading them into inorganic nanoparticles, micelles, polymers, and so forth.38–43 Of all of the above, polymer-based fluorescent chemosensors have several advantages over the others, such as water solubility, exceptional processability, and signal amplification. Hence, efforts have been dedicated to the fabrication of polymeric chemosensors, which contain salicylaldimine Schiff bases. Theato et al. prepared thermo- and light-responsive copolymers by grafting salicylaldehyde to amine pendant-functionalized polymers.44 Cai et al. synthesized block copolymers and constructed a variety of coordinated polymeric aggregates with different metal ions.45–47 Zhang et al. constructed well-defined polymeric salicylaldimine Schiff bases via RAFT alternating copolymerization and they further confirmed the superb accuracy and sensitivity of copolymers compared with small molecule detection.40 However, almost all of the reported studies on polymer-based fluorescent chemosensors primarily focus on easy fabrication processes, high solubility, accuracy and sensitivity, but cytocompatibility has not been taken seriously.
To be used safely, it is absolutely necessary for probes to have excellent biocompatibility properties. Phosphorylcholine (PC) groups, a major headgroup in phospholipids, which are the main constituents of plasma membranes, have been often conjugated into different polymers to mimic membrane structure and have made quite good progress.48 2-Methacryloyloxyethyl phosphorylcholine (MPC) is a methacrylate monomer with a phosphorylcholine group, which has been extensively synthesized to mimic cell membranes and is considered as a biocompatible material. On account of their simple molecular design and synthesis process, excellent biocompatibility, water solubility, and transparency in visible light, MPC polymers are considered promising in materials science and biomedical applications. In our previous studies,49,50 these polymers with PC shells exhibited great biostability and biocompatibility, which supported the polymers’ normal functions in complex environments. Additionally, MPC polymers were also developed by Ji’s group as a biocompatible imaging agent.51–54 It turned out that these biomimetic polymers were suitable for biological materials and imaging agents. Thus, the combination of salicylaldimine Schiff bases with MPC polymers would utilize well the advantages of both of their merits.
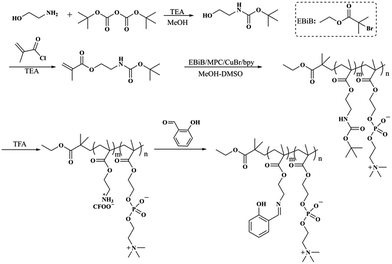
Herein, a biomimetic fluorescent chemosensor was designed and used for the delicate detection of zinc(II). To synthesize this copolymer, a three-step process was required. Firstly, ATRP polymerization was conducted with N-Boc-aminoethyl methacrylate and MPC using ethyl α-bromoisobutyrate as the initiator to produce PNBAEMA-co-PMPC. Then, PNBAEMA-co-PMPC was deprotected to obtain PAEMA-co-PMPC. Lastly, salicylaldehyde and PAEMA-co-PMPC were connected by Schiff base bonds (PSaAEMA-co-PMPC). The polymer chemosensor could efficiently bind Zn2+ ions, which specifically led to a change in fluorescence intensity. Furthermore, the cytotoxicity and cell uptake behaviors of PSaAEMA-co-PMPC were investigated to evaluate the biological performance of this material (Scheme 1).
Experimental
Materials
Ethanolamine, N-Boc-aminoethyl, methacryloyl chloride and ethyl α-bromoisobutyrate were purchased from Aladdin. MPC was synthesized according to our previous studies. Chloride salts, MgSO4, and nitrate salts were used for all of the sensing experiments. Water was prepared by our own filtration system. The human umbilical vein endothelial cell line (HUVEC) and HeLa cells were obtained from the Cell Bank of the Chinese Science Academy, Shanghai, China. All of the reagents obtained from commercial sources were used as received without further purification.Instrumentations
Absorption spectra were recorded on an Analytic-jena Specord S600 (Germany) spectrophotometer in absorbance mode using quartz cuvettes of 1 cm path length. 1H NMR measurements were carried out on a Bruker AV400 spectrometer. A Perkin Elmer LS-55 spectrofluorometer was used to measure the fluorescence spectra. Fluorescent pictures were taken on a ZEISS Axio Scope Fluorescence Microscope.Synthesis of N-Boc-ethanolamine
Ethanolamine (1.10 g, 18.3 mmol) was added into 200 mL methanol and a solution of di-tert-butyl dicarbonate (0.01 mol) was added dropwise over a period of about 2 h at 0 °C. After stirring for 24 h, the solution was concentrated under reduced pressure. Then, the crude produce was extracted with methylene chloride three times. The extract was dried with anhydrous Na2SO4 and concentrated under vacuum. Yield: 80%. 1H NMR (500 MHz, CDCl3): d 1.42 (9H, –C(CH3)3), 3.15 (2H, HOCH2CH2CO–), 3.67 (2H, HOCH2CH2CO–).Synthesis of N-Boc-aminoethyl methacrylate
N-Boc-aminoethyl methacrylate was synthesized by a condensation reaction between acyl chloride and hydroxyl groups. N-Boc-aminoethyl (20.2 g, 0.1 mol), triethylamine (10.1 g 0.1 mol) and 300 mL of methylene chloride were added into a flask at room temperature, and a methylene chloride solution (15 mL) of methacryloyl chloride (8.32 g, 0.08 mol) was added dropwise. After 12 h stirring, deionized water was added and extracted with DCM three times. The extract was dried with anhydrous Na2SO4 and concentrated under vacuum. Yield: 79%. 1H NMR (500 MHz, CDCl3): d 1.42 (9H, –C(CH3)3), 1,91 (3H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH3)–) 3.15 (2H, HOCH2CH2CO–), 4.36 (2H, –OCH2CH2CO–), 5.56, 6.12 (2H, CH2
CH(CH3)–) 3.15 (2H, HOCH2CH2CO–), 4.36 (2H, –OCH2CH2CO–), 5.56, 6.12 (2H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(CH3)–).
CH(CH3)–).
Synthesis of P(N-Boc-aminoethyl methacrylate)-co-P(2-methacryloyloxyethyl phosphorylcholine) (PNBAEMA-co-PMPC)
PNBAEMA-co-PMPC was synthesized by the ATRP polymerization of N-Boc-aminoethyl methacrylate and MPC using ethyl α-bromoisobutyrate as the initiator. In a typical reaction, N-Boc-aminoethyl methacrylate (4 g, 25 mmol), MPC (4 g, 25 mmol), ethyl α-bromoisobutyrate (124 mg, 0.625 mmol), CuBr (89 mg, 0.625 mmol), PMDETA (216 mg, 1.25 mmol) and 10 mL DMSO/MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were added into a 25 mL flask under a nitrogen atmosphere. The reaction mixture was degassed by three freeze-pump-thaw cycles and stirred at 30 °C for 12 h. Then, the reaction mixture was exposed to air and the solution was diluted with MeOH and passed through silica gel column chromatography to remove the catalyst. The filtrate was concentrated and precipitated into ethyl ether and the precipitate was dried under vacuum. Yield: 65%.
1) were added into a 25 mL flask under a nitrogen atmosphere. The reaction mixture was degassed by three freeze-pump-thaw cycles and stirred at 30 °C for 12 h. Then, the reaction mixture was exposed to air and the solution was diluted with MeOH and passed through silica gel column chromatography to remove the catalyst. The filtrate was concentrated and precipitated into ethyl ether and the precipitate was dried under vacuum. Yield: 65%.
Synthesis of P(aminoethyl methacrylate)-co-P(2-methacryloyloxyethyl phosphorylcholine)trifluoroacetate salt (PAEMA-co-PMPC)
0.15 g PNBAEMA-co-PMPC was dissolved in 3 mL methanol and 3 mL DMSO in a 25 mL flask. Then, 6 mL TFA was added and stirred at room temperature for 5 h. The reaction solution was concentrated under reduced pressure. Finally, the crude product was dialyzed against distilled water (MWCO 3500) for 2 days and lyophilized. Yield: 70%.Synthesis of P(2-salicylaldehyde-aminoethyl ethanolamine methacrylate)-co-P(2-methacryloyloxyethyl phosphorylcholine) (PSaAEMA-co-PMPC)
PAEMA-co-PMPC (508 mg, 2 mmol) and anhydrous TEA (0.55 mL, 4 mmol) were dissolved in 10 mL anhydrous methanol, and then salicylaldehyde (0.23 mL, 2.2 mmol) was added. The reaction mixture was stirred at room temperature for 12 h, and the solution was then concentrated under reduced pressure. The crude product was precipitated into ethyl ether. After drying for two days in a vacuum, a yellow powder was obtained. Yield: 70%.Absorption and fluorescence measurements
The copolymers (PSaAEMA-co-PMPC) were prepared and dissolved in PBS (20 mM; pH = 7.4) before use. Cationic solutions were prepared by dissolving metal salts in deionized water with a concentration of 0.1 M and were diluted as required before use. A solution of the test sample (4.0 mL, 20 μM for UV-Vis and 40 μM for fluorescence) was prepared, and UV-Vis and fluorescence spectra were recorded at room temperature. The total volume of each ionic solution introduced to the test solution was no more than 25 μL, and the changes in fluorescence intensity were recorded after the solution was mixed for 10 min (λex = 368 nm).Cell cytotoxicity assay
The cytotoxicity of PSaAEMA-co-PMPC was evaluated by an in vitro MTT assay. HUVEC cells and HeLa cells were seeded in 96-well plates with 6 × 103 cells per well. After incubation for 24 h, the culture medium was replaced by fresh medium with PSaAEMA-co-PMPC at different concentrations (10 to 1000 μg mL−1). After the cells were incubated for 48 h, the cells were subjected to an MTT assay.Cell imaging
HeLa cells were used for cell imaging in this study. HeLa cells were seeded in 24-well plates with 2 × 104 cells per well. After incubation for 24 h, the culture medium was replaced by fresh medium with PSaAEMA-co-PMPC (20 μM). After incubation for a further 1 h, the cells were washed with cold PBS three times. Then the cells were further treated with ZnCl2 (25 mM) plus sodium pyrithione (5 mM). After this, the cells were washed with cold PBS three times and fixed with 4% formaldehyde for 15 min. Finally, the cells were observed using a fluorescence microscope.Results and discussion
Synthesis of Zn2+ probe
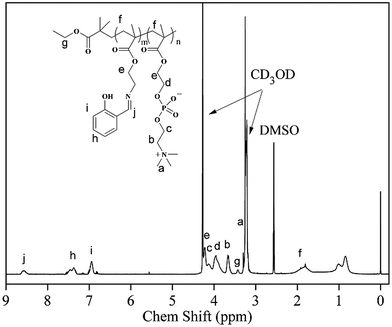
The synthetic route for the preparation of the PSaAEMA-co-PMPC polymer is illustrated in Scheme 2. This copolymer was prepared in three steps. First, N-Boc-aminoethyl methacrylate was synthesized by N(Boc) protected ethanolamine and methacryloylchloride, and the product was sequentially polymerized with MPC via ATRP using EBIB as the initiator. Then, deprotection of the N-Boc of the copolymer using TFA exposed a free amine group which could react with salicylaldehyde. Finally, salicylaldehyde was grafted to the copolymer through Schiff base bonds. Fig. S1 and S2† show the 1H NMR spectra of N(Boc) protected ethanolamine and N-Boc-aminoethyl methacrylate, respectively. The results indicated that the monomer was prepared successfully. Moreover, the 13C NMR spectrum (Fig. S3†) further confirmed the correctness of the structure. Fig. 1 shows the 1H NMR spectrum of the polymer probe. The signal at δ = 8.5 ppm (j) was assigned to the characteristic peaks of the Schiff bases, and the signal at δ = 3.6 ppm (b) was assigned to the methylene protons of the choline units. The ratio of j and b is roughly equal to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, which suggested that the two units were equivalent in PSaAEMA-co-PMPC. As the Mn content of PMPC-co-PMEMA could not be determined by GPC, the Mn content was estimated from the 1H NMR spectrum by comparing the proportion of the signals at δ = 3.40 (EBiB) and δ = 3.6 ppm (b); the Mn content of PMPC-b-PMEMA was about 6494 g mol−1.
2, which suggested that the two units were equivalent in PSaAEMA-co-PMPC. As the Mn content of PMPC-co-PMEMA could not be determined by GPC, the Mn content was estimated from the 1H NMR spectrum by comparing the proportion of the signals at δ = 3.40 (EBiB) and δ = 3.6 ppm (b); the Mn content of PMPC-b-PMEMA was about 6494 g mol−1.
Zn2+ ion recognition
It is known that salicylaldimine Schiff bases can coordinate with various metal ions, especially zinc complexes, which often leads to strong fluorescence emission. Therefore, we designed a monomer that possessed an amine group to form a Schiff base with salicylaldehyde and speculated that this part of the copolymer would show no fluorescence prior to the addition of zinc ions, but once the zinc ions were added, the copolymer would emit intense fluorescence. To verify our presumption, we examined the UV-Vis spectra of the copolymer with a gradual addition of Zn2+. Fig. 2 shows the absorption changes of the copolymer upon a gradual addition of 0–1.0 equiv. Zn2+. It can be seen that with the addition of Zn2+ into the copolymer solution, the absorption bands at 254 and 316 nm decreased gradually, and new absorption bands at 363 nm emerged simultaneously. Three isosbestic points could be clearly seen (Fig. 2). The absorption bands at 316 nm gradually decrease which was attributed to the n–π* transition of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N. The above results indicated a conversion of the Schiff bases to Schiff base–Zn2+ complexes. No further changes were observed in the absorbance spectra with up to 1 equiv. Zn2+ addition, revealing that the binding ratio between Zn2+ and the copolymer should be 1
N. The above results indicated a conversion of the Schiff bases to Schiff base–Zn2+ complexes. No further changes were observed in the absorbance spectra with up to 1 equiv. Zn2+ addition, revealing that the binding ratio between Zn2+ and the copolymer should be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
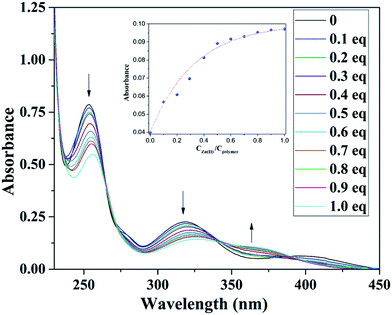 | ||
| Fig. 2 Absorption spectra of 10 mol L−1 copolymer with the addition of 0–15 mol L−1 Zn2+. Inset: The absorbance ratio (A383/A346) as a function of Zn2+ concentration. | ||
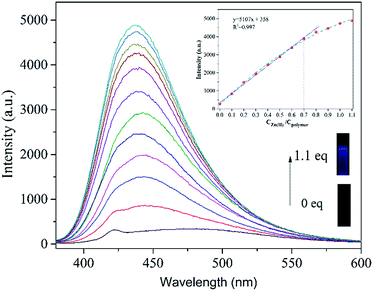
To test the sensing ability of PSaAEMA-co-PMPC for Zn2+ in aqueous solutions, fluorescence titration of this copolymer with a gradual addition of Zn2+ was investigated and the results are shown in Fig. 3. The addition of Zn2+ leads to an increase in 450 nm emission intensity, which is ascribed to the formation of zinc complexes, which inhibit C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N isomerization and cause a chelation-enhanced fluorescence effect. In the fluorescence spectra under the same test conditions, more than an 800-fold fluorescence enhancement at around 450 nm occurred upon the addition of Zn2+ (0.1 equiv., Fig. 3). This increase is similar to that with the addition of 1 equiv. of Zn2+. As shown in the inset, a linear relationship is observed between the intensity and the amount of Zn2+ in the range of 0–0.7 equiv., which corresponds to 0–14 mM Zn2+ (linearly dependent coefficient: R2 = 0.997). The linear fitting of the titration curve further confirmed that the binding of the copolymer and Zn2+ was most probably a 1
N isomerization and cause a chelation-enhanced fluorescence effect. In the fluorescence spectra under the same test conditions, more than an 800-fold fluorescence enhancement at around 450 nm occurred upon the addition of Zn2+ (0.1 equiv., Fig. 3). This increase is similar to that with the addition of 1 equiv. of Zn2+. As shown in the inset, a linear relationship is observed between the intensity and the amount of Zn2+ in the range of 0–0.7 equiv., which corresponds to 0–14 mM Zn2+ (linearly dependent coefficient: R2 = 0.997). The linear fitting of the titration curve further confirmed that the binding of the copolymer and Zn2+ was most probably a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 metal-to-ligand ratio. This indicates that the copolymer has potential for application in the quantitative determination of Zn2+ in aqueous solutions.
1 metal-to-ligand ratio. This indicates that the copolymer has potential for application in the quantitative determination of Zn2+ in aqueous solutions.
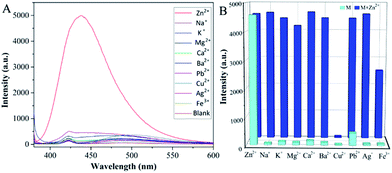
Then, the PSaAEMA-co-PMPC solution was treated with various metal ions to verify the fluorescence response behavior. The measurements were conducted with excitation at 368 nm to examine the ion selective ability of the copolymer in aqueous solutions. As shown in Fig. 4A, the addition of Zn2+ causes an obvious 5000-fold emission enhancement at around 450 nm. However, the emission intensities at around 450 nm were barely enhanced with the addition of Na+, K+, Mg2+, Ca2+, Ba2+, Pb2+, Cu2+, Ag2+ and Fe3+. Therefore, the copolymer exhibits an excellent selectivity toward Zn2+ binding. Fig. 4B shows the fluorescence response of PSaAEMA-co-PMPC with different metal ions in the absence and presence of Zn2+. Most of the representative ions like Na+, K+, Ba2+, Pb2+ and Ag2+ cause a slight interference in Zn2+ detection. The addition of Ca2+ causes a small increase in the emission intensity, whereas Mg2+ and Fe3+ partially quench the fluorescence, which is due to their competitive coordination to Zn2+, as verified by the previous reports. Besides, the presence of Cu2+ induces absolute quenching, since Cu2+ has a higher binding constant with the salicylaldimine Schiff bases as well as a heavy atom quenching effect.
Living cell imaging
Before PSaAEMA-co-PMPC can be used as a bio-imaging probe, it is necessary to evaluate its cytotoxicity. For this purpose, cultured HeLa cells and HUVEC cells were incubated with different concentrations of copolymer solution (from 10 to 1000 μg mL−1) for 48 h. Subsequently, an MTT assay was carried out. Fig. 5 demonstrated that, even when treated with a certain high concentration of this copolymer (1000 μg mL−1), 80% of cells were still alive. This result indicates that PSaAEMA-co-PMPC has a low cytotoxicity to cells and is suitable for intracellular imaging.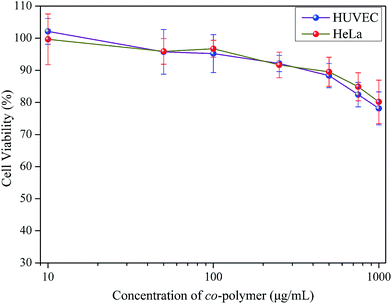 | ||
| Fig. 5 Cell cytotoxicity of HeLa and HUVEC cells incubated with various concentrations of PSaAEMA-co-PMPC. | ||
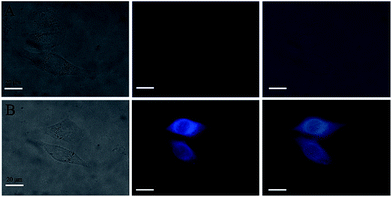
Then, the application of PSaAEMA-co-PMPC for the fluorescence imaging of Zn2+ in live HeLa cells was investigated. As observed by laser scanning confocal microscopy, HeLa cells incubated with 20 μg mL−1 PSaAEMA-co-PMPC for 20 min at 37 °C showed no intracellular fluorescence. Then, the cells were cultured with 10 μmol L−1 Zn2+ and 10 μmol L−1 pyrithione, which is a zinc selective ionophore, for 20 min at 37 °C and then washed with PBS three times. A significant fluorescence increase from the intracellular area was shown (Fig. 6), and we could clearly observe the entire cellular outline. These results suggested that PSaAEMA-co-PMPC could be used as an effective chemosensor for Zn2+ imaging in living cells.
Conclusion
In summary, we have developed a novel salicylaldehyde based chemosensor PSaAEMA-co-PMPC, which displays strong fluorescence with high selectivity and sensitivity for Zn2+ compared to other competing metal ions. The sensor gives a fluorescence response in an aqueous solution upon binding with zinc(II) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Moreover, due to the structure of PC, the cytocompatibility of the copolymer was excellent, which is perfect for biological applications. Hence, the biological application of PSaAEMA-co-PMPC was evaluated for the detection of Zn2+ in living cells. The results showed that it had low cytotoxicity and excellent cell fluorescence illumination and suggested that PSaAEMA-co-PMPC was cell-permeable and capable of the intracellular detection of Zn2+.
1 ratio. Moreover, due to the structure of PC, the cytocompatibility of the copolymer was excellent, which is perfect for biological applications. Hence, the biological application of PSaAEMA-co-PMPC was evaluated for the detection of Zn2+ in living cells. The results showed that it had low cytotoxicity and excellent cell fluorescence illumination and suggested that PSaAEMA-co-PMPC was cell-permeable and capable of the intracellular detection of Zn2+.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was funded by the National Natural Science Foundation of China (No. 51773129, 51503130), the Support Plan of Science and Technology Department of Sichuan Province, China (2017GZ0129, 2018SZ0174), the International Science and Technology Cooperation Program of Chengdu (2017-GH02-00068-HZ), the Postdoctoral Research Foundation of Sichuan University (2018SCU12049) and was supported by the Graduate Student’s Research and Innovation Fund of Sichuan University (2018YJSY084).Notes and references
- J. R. Burdo and J. R. Connor, BioMetals, 2003, 16, 63–75 CrossRef CAS PubMed.
- E. J. Martinez-Finley, S. Chakraborty, S. J. B. Fretham and M. Aschner, Metallomics, 2012, 4, 593–605 RSC.
- D. J. Bonda, H. G. Lee, J. A. Blair, X. W. Zhu, G. Perry and M. A. Smith, Metallomics, 2011, 3, 267–270 RSC.
- M. Tyszka-Czochara, A. Grzywacz, J. Gdula-Argasinska, T. Librowski, B. Wilinski and W. Opoka, Acta Pol. Pharm., 2014, 71, 369–377 Search PubMed.
- S. L. Sensi, P. Paoletti, J. Y. Koh, E. Aizenman, A. I. Bush and M. Hershfinkel, J. Neurosci., 2011, 31, 16076–16085 CrossRef CAS PubMed.
- Q. Y. Cao, R. Jiang, M. Liu, Q. Wan, D. Xu, J. Tian, H. Huang, Y. Wen, X. Zhang and Y. Wei, Mater. Sci. Eng., C, 2017, 80, 578–583 CrossRef CAS PubMed.
- Q. Y. Cao, R. Jiang, M. Liu, Q. Wan, D. Xu, J. Tian, H. Huang, Y. Wen, X. Zhang and Y. Wei, Mater. Sci. Eng., C, 2017, 80, 411–416 CrossRef CAS PubMed.
- H. Huang, D. Xu, M. Liu, R. Jiang, L. Mao, Q. Huang, Q. Wan, Y. Wen, X. Zhang and Y. Wei, Mater. Sci. Eng., C, 2017, 78, 862–867 CrossRef CAS PubMed.
- R. Jiang, L. Huang, M. Liu, F. Deng, H. Huang, J. Tian, Y. Wen, Q. Y. Cao, X. Zhang and Y. Wei, Mater. Sci. Eng., C, 2018, 83, 115–120 CrossRef CAS PubMed.
- R. Jiang, M. Liu, H. Huang, L. Huang, Q. Huang, Y. Wen, Q.-y. Cao, J. Tian, X. Zhang and Y. Wei, Dyes Pigm., 2018, 149, 581–587 CrossRef CAS.
- R. Jiang, M. Liu, C. Li, Q. Huang, H. Huang, Q. Wan, Y. Wen, Q. Y. Cao, X. Zhang and Y. Wei, Mater. Sci. Eng., C, 2017, 80, 708–714 CrossRef CAS PubMed.
- M. Liu, G. Zeng, K. Wang, Q. Wan, L. Tao, X. Zhang and Y. Wei, Nanoscale, 2016, 8, 16819–16840 RSC.
- Y. Liu, L. Mao, X. Liu, M. Liu, D. Xu, R. Jiang, F. Deng, Y. Li, X. Zhang and Y. Wei, Mater. Sci. Eng., C, 2017, 79, 590–595 CrossRef CAS PubMed.
- L. Mao, M. Liu, L. Huang, D. Xu, Q. Wan, G. Zeng, Y. Dai, Y. Wen, X. Zhang and Y. Wei, Mater. Sci. Eng., C, 2017, 79, 596–604 CrossRef CAS PubMed.
- Y. Q. Niu, T. He, J. Song, S. P. Chen, X. Y. Liu, Z. G. Chen, Y. J. Yu and S. G. Chen, Chem. Commun., 2017, 53, 7541–7544 RSC.
- S. Yu, D. Xu, Q. Wan, M. Liu, J. Tian, Q. Huang, F. Deng, Y. Wen, X. Zhang and Y. Wei, Mater. Sci. Eng., C, 2017, 78, 191–197 CrossRef CAS PubMed.
- X. Zhang, K. Wang, M. Liu, X. Zhang, L. Tao, Y. Chen and Y. Wei, Nanoscale, 2015, 7, 11486–11508 RSC.
- X. Zhang, S. Wang, L. Xu, L. Feng, Y. Ji, L. Tao, S. Li and Y. Wei, Nanoscale, 2012, 4, 5581–5584 RSC.
- P. Chabosseau, J. Woodier, R. Cheung and G. A. Rutter, Metallomics, 2018, 10, 229–239 RSC.
- M. J. Chang and M. H. Lee, Dyes Pigm., 2018, 149, 915–920 CrossRef CAS.
- K. Du, S. Z. Niu, X. Z. Chen and P. F. Zhang, Tetrahedron Lett., 2018, 59, 356–360 CrossRef CAS.
- J. Kim, E. G. Camemolla, C. DeVaul, A. M. Shaltout, D. Faccio, V. M. Shalaev, A. V. Kildishev, M. Ferrera and A. Boltasseva, Nano Lett., 2018, 18, 740–746 CrossRef CAS PubMed.
- S.-R. Liu and S.-P. Wu, Sens. Actuators, B, 2012, 171–172, 1110–1116 CrossRef CAS.
- R. Mehta, M. H. Qureshi, M. K. Purchal, S. M. Greer, S. Z. Gong, C. Ngo and E. L. Que, Chem. Commun., 2018, 54, 5442–5445 RSC.
- V. C. Pierre, S. M. Harris and S. L. Pailloux, Acc. Chem. Res., 2018, 51, 342–351 CrossRef CAS PubMed.
- D. Chen, K. P. Taylor, Q. Hall and J. M. Kaplan, Genetics, 2016, 204, 1151–1159 CrossRef CAS PubMed.
- W. H. Hsieh, C.-F. Wan, D.-J. Liao and A.-T. Wu, Tetrahedron Lett., 2012, 53, 5848–5851 CrossRef CAS.
- M. Kumar, A. Kumar, M. K. Singh, S. K. Sahu and R. P. John, Sens. Actuators, B, 2017, 241, 1218–1223 CrossRef CAS.
- Z. Liu, C. Zhang, Y. Chen, W. He and Z. Guo, Chem. Commun., 2012, 48, 8365–8367 RSC.
- Z. Liu, C. Zhang, Y. Chen, F. Qian, Y. Bai, W. He and Z. Guo, Chem. Commun., 2014, 50, 1253–1255 RSC.
- X. Wu, N. Xu, Z. Zhu, Y. Cai, Y. Zhao and D. Wang, Polym. Chem., 2014, 5, 1202–1209 RSC.
- Y. Zhou, Z. X. Li, S. Q. Zang, Y. Y. Zhu, H. Y. Zhang, H. W. Hou and T. C. Mak, Org. Lett., 2012, 14, 1214–1217 CrossRef CAS PubMed.
- J. Zhu, Y. Zhang, Y. Chen, T. Sun, Y. Tang, Y. Huang, Q. Yang, D. Ma, Y. Wang and M. Wang, Tetrahedron Lett., 2017, 58, 365–370 CrossRef CAS.
- S. Erdemir and B. Tabakci, Dyes Pigm., 2018, 151, 116–122 CrossRef CAS.
- Z. Y. Li, H. K. Su, K. Zhou, B. Z. Yang, T. X. Xiao, X. Q. Sun, J. L. Jiang and L. Y. Wang, Dyes Pigm., 2018, 149, 921–926 CrossRef CAS.
- Y. Y. Liu, Y. Y. Li, Q. Feng, N. Li, K. Li, H. W. Hou and B. Zhang, Luminescence, 2018, 33, 29–33 CrossRef CAS PubMed.
- S. Sakunkaewkasem, A. Petdum, W. Panchan, J. Sirirak, A. Charoenpanich, T. Sooksimuang and N. Wanichacheva, ACS Sens., 2018, 3, 1016–1023 CrossRef CAS PubMed.
- X. Chen, N. Xu, N. Li, L. Lu, Y. Cai, Y. Zhao and D. Wang, Soft Matter, 2013, 9, 1885–1894 RSC.
- K. Johmoto, A. Sekine and H. Uekusa, Cryst. Growth Des., 2012, 12, 4779–4786 CrossRef CAS.
- S. Wang, B. Wu, F. Liu, Y. Gao and W. Zhang, Polym. Chem., 2015, 6, 1127–1136 RSC.
- B. Wu, L. Xu, S. Wang, Y. Wang and W. Zhang, Polym. Chem., 2015, 6, 4279–4289 RSC.
- J. Xu, R. Yan, H. Wang, Z. Du, J. Gu, X. Cheng and J. Xiong, RSC Adv., 2018, 8, 6798–6804 RSC.
- H. Wang, Y. Wu, G. Liu, Z. Du and X. Cheng, Macromol. Chem. Phys., 2016, 217, 2004–2012 CrossRef CAS.
- F. D. Jochum and P. Theato, Macromolecules, 2009, 42, 5941–5945 CrossRef CAS.
- X. W. Wu, N. Xu, Z. G. Zhu, Y. L. Cai, Y. Zhao and D. J. Wang, Polym. Chem., 2014, 5, 1202–1209 RSC.
- H. A. Gao, G. H. Liu, X. J. Chen, Z. H. Hao, J. Y. Tong, L. C. Lu, Y. L. Cai, F. Long and M. Q. Zhu, Macromolecules, 2010, 43, 6156–6165 CrossRef CAS.
- X. J. Chen, N. Xu, N. Li, L. C. Lu, Y. L. Cai, Y. Zhao and D. J. Wang, Soft Matter, 2013, 9, 1885–1894 RSC.
- Y. Iwasaki and K. Ishihara, Sci. Technol. Adv. Mater., 2012, 13, 064101 CrossRef PubMed.
- H. Wang, J. Xiong, G. Liu and Y. Wang, Macromol. Chem. Phys., 2016, 217, 2049–2055 CrossRef CAS.
- H. B. Wang, G. Y. Liu, S. H. Dong, J. J. Xiong, Z. L. Dua and X. Cheng, J. Mater. Chem. B, 2015, 3, 7401–7407 RSC.
- D. D. Chen, M. D. Wu, B. C. Li, K. F. Ren, Z. K. Cheng, J. Ji, Y. Li and J. Q. Sun, Adv. Mater., 2015, 27, 5882–5888 CrossRef CAS PubMed.
- X. S. Liu, H. Y. Huang, Q. Jin and J. Ji, Langmuir, 2011, 27, 5242–5251 CrossRef CAS PubMed.
- G. Y. Liu, L. P. Lv, C. J. Chen, X. S. Liu, X. F. Hu and J. Ji, Soft Matter, 2011, 7, 6629–6636 RSC.
- Y. J. Chen, Y. Wang, H. B. Wang, F. Jia, T. J. Cai, J. Ji and Q. Jin, Polymer, 2016, 97, 449–455 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra06501b |
| This journal is © The Royal Society of Chemistry 2018 |