Open Access Article
Open Access ArticleAnion–cation co-operative catalysis by artificial sweetener saccharine-based ionic liquid for sustainable synthesis of 3,4-dihydropyrano[c]chromenes, 4,5-dihydropyrano[4,3-b]pyran and tetrahydrobenzo[b]pyrans in aqueous medium†
Himani Sharma and
Suman Srivastava *
*
Department of Applied Sciences, National Institute of Technology, Delhi, IAMR Campus, Sec A-7, Narela, Delhi 110040, India. E-mail: sumanbhu08@gmail.com
First published on 20th November 2018
Abstract
In this study, a saccharine-based ionic liquid [Bmim]Sac has been found to be a sustainable catalyst for the synthesis of 3,4-dihydropyrano[c]chromenes, 4,5-dihydropyrano[4,3-b]pyran and tetrahydrobenzo[b]pyrans scaffolds through Domino Knoevenagel–Michael reaction. The easy recovery of the catalyst and high yield of the products make the protocol attractive, sustainable and economical. A mechanistic hypothesis is discussed using the concept of cooperative catalysis based on the dual (electrophilic/nucleophilic) activation of reactants by [Bmim]Sac. Furthermore, dual hydrogen bonding of saccharinate anions plays an important role in the activation of nucleophiles.
Introduction
Currently, ionic liquids have attracted significant attention in green organic synthesis owing to their unique properties such as low vapor pressure, wide liquid range, good conductivity and large electrochemical window.1 In addition to these, the gold benchmark for green chemistry is functional ionic liquid-mediated synthesis (FILMs).2 Nowadays, FILMs has become a novel approach representing an attempt to describe “design capacity of ionic liquids”, which makes them an accurate working system rather than simply novel media, and their properties can be altered to suit the requirement of a particular process.3 This unique property of the materials obtained by FILMs gives them ability to serve as catalysts. For example, ILs with acidic groups have been used in Fischer esterification, alcohol dehydrodimerization, pinacol rearrangement,4 and Mannich reactions;5 with basic groups, they have been utilized in Markovnikov addition,6 Michael addition,7 and absorption of CO2 and SO2.8The 3,4-dihydropyrano[c]chromene and tetrahydrobenzo[b]pyran units are privileged, heterocyclic motifs that form the core of a large family of natural products with strong bioactivity profiles.9 Multicomponent methods have been reported for the synthesis of 3,4-dihydropyrano[c]chromenes employing L-proline–melamine,10a magnetic nanoparticle-tagged ionic liquid,10b SiO2/H3PW12O40 nanohybrid material,10c [DBU][Ac],10d ammonium acetate,10e visible light,10f thiourea dioxide,10g silica-grafted ionic liquids,10h crown ether complex cation ionic liquids (CECILs),10i SDS,10j [TETA]TFA,10k and starch solution10l as catalysts. Some studies on the multicomponent entry to tetrahydrobenzo[b]pyran motifs have reported employing H2O/PEG-400,11a sulfonic acid-functionalized magnetic Fe3−xTixO4 nanoparticles,11b L-tyrosine,11c Fe3O4@SiO2-imid-PMAn magnetic nanocatalyst,11d inorganic–organic hybrid magnetic nanocatalyst11e and magnetite-dihydrogen phosphate11f as catalysts.
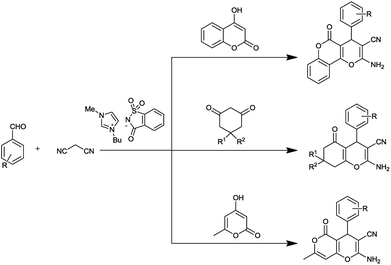
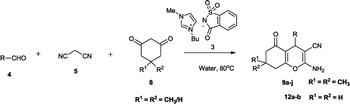
Despite the availability of these methods, ionic liquid-mediated syntheses of 3,4-dihydropyrano[c]chromenes and tetrahydrobenzo[b]pyrans are still less explored and there remains enough scope for an efficient, high yielding, and mild approach to achieve such systems. With increasing concerns about environmental protection, synthesis of ILs from non-toxic materials is desirable. As a part of our attempt to develop synthesis of biologically important heterocycles12 via green methodology,13 we herein report a saccharine-based ionic liquid14-mediated protocol for the synthesis of 3,4-dihydropyrano[c]chromene and tetrahydrobenzo[b]pyran (Fig. 1). The saccharin group was chosen as it is less toxic than other ionic liquids.16
Results and discussion
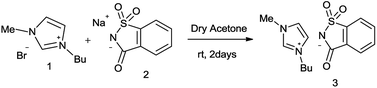
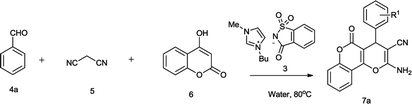
[Bmim]Sac was synthesized by the reported procedure, as shown in Fig. 2.15 Initially, the reaction between benzaldehyde (4a), malononitrile (5) and 4-hydroxycoumarin (6) was employed as the model reaction to screen ILs in water, ethanol and other common solvents to develop appropriate reaction conditions.As evident from the results summarized in Table 1, the [Bmim]-based ionic liquid with different anions could catalyse the reaction. However, the reaction of [Bmim]Sac anions proceeded very well as compared to that with others in neat as well as in water and afforded the product 7a with moderate to excellent yield (82% and 95%), respectively. The results are summarized in Table 1. The use of water as solvent improved the yield of the desired product slightly and also reduced the amount of catalyst from 20 mol% to 5 mol% effectively (Table 1, entries 11 and 14). Higher amount of [Bmim]Sac was needed for proper mixing of reactant only in the absence of water. In the presence of imidazole, saccharine and sod saccharinate as catalysts, no product was observed (Table 1, entries 7, 8, and 9).
| S. no. | Catalyst/ILa | Condition | Time (min) | % yieldc | Ref. |
|---|---|---|---|---|---|
| a Reaction conditions: benzaldehyde (1 mmol), malononitrile (1 mmol), 4-hydroxy coumarin (1 mmol), water (2 ml), catalyst (20 mol%).b Catalyst (5 mol%).c Isolated yield. | |||||
| 1 | [Bmim]Br | 80 °C, water | 75 | 56 | |
| 2 | [Bmim]Cl | 80 °C , water | 80 | 59 | |
| 3 | [Bmim]OH | 80 °C, water | 90 | 65 | |
| 4 | [Bmim]BF4 | 80 °C, water | 120 | 70 | |
| 5 | [Bmim] SO3H | 80 °C, water | 100 | 75 | |
| 6 | [Bmim]PF6 | 80 °C, water | 120 | 62 | |
| 7 | Imidazole | 80 °C, water | 24 h | NR | |
| 8 | Saccharine | 80 °C, water | 24 h | NR | |
| 9 | Sod saccharinate | 80 °C, water | 24 h | NR | |
| 10 | — | Water, 80 °C | 24 h | NR | |
| 11 | [BMim]Sac | 80 °C | 10 | 82 | |
| 12 | [Bmim]Sac | Ethanol, 80 °C | 45 | 75 | |
| 13 | [Bmim]Sac | Methanol, 65 °C | 60 | 50 | |
| 14 | [Bmim]Sacb | Water, 80 °C | 10 | 95 | |
| 15 | [Sipim]HSO4 | 100 °C, 0.08 mmol | 30 | 94 | 10h |
| 16 | [TETA]TFA | Ethanol–water, reflux (5 mol%) | 20 | 86 | 10k |
| 17 | Starch solution | 50 °C, 4 ml | 25 | 95 | 10l |
| 18 | [18-C-6K][OAc] | EtOH, reflux, (30 mol%) | 15 | 90 | 10i |
| 19 | NH4OAc | EtOH, reflux (15 mol%) | 3 | 94 | 10e |
| 20 | Thiourea dioxide | Water, 70 °C (10 mol%) | 13 | 93 | 10g |
| 21 | SDS | Water, 60 °C (20 mol%) | 120 | 85 | 10j |
The influence of the reaction temperature and the amount of the ionic liquid on the catalysis performance was also studied. The reaction proceeded slowly at room temperature, and the reaction yield increased with increasing temperature to 80 °C. To show the merit of our procedure, we have compared our result for the synthesis of 3,4-dihydropyrano[c]chromenes using [Bmim]Sac with the result of some other ionic liquids reported in literature for the same transformation. The results are summarized in Table 1 (entry 15–21). As can be clearly seen from Table 1, the best result was obtained at 80 °C in the presence of 5 mol% of catalyst. Similar optimizations were performed for products 7b and 9a; in all cases, 80 °C and 5 mol% of catalyst were the optimum conditions.
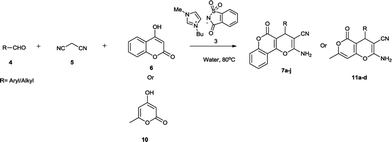
Using these optimized conditions and to show the generality and scope of this methodology, reactions were explored for the synthesis of a wide variety of 3,4-dihydropyrano[c]chromene and tetrahydrobenzo[b]pyran derivatives using aldehydes, malononitriles and different 1,3-dicarbonyl compounds (4-hydroxy coumarin, 5,5-dimethyl-1,3cyclohexanedione/1,3-cyclohexanedione and 1,3-cyclohexanedione, respectively) in the presence of [Bmim]Sac (5 mol%) in an aqueous medium under reflux conditions. The results have been summarized in Tables 2 and 3. Indeed, there is no difference in reactivities among 5,5-dimethyl-1,3cyclohexanedione/1,3-cyclohexanedione, 1,3-cyclohexanedione and 4-hydroxycoumarin. The effect of electron-withdrawing substituents, electron-releasing substituents and halogens of the aromatic ring of aldehydes on the reaction results was investigated. The reaction time of aromatic aldehydes having electron-withdrawing substituents and halogens produced higher yield of products and faster reactions than that observed for their electron-rich counterparts (Table 2, entry 2).
| S. no. | Ra | Product | Time (min) | Yieldb | Melting point | Literature melting point18 |
|---|---|---|---|---|---|---|
| a Reaction conditions: aldehyde (1 mmol), malononitrile (1 mmol), 4-hydroxy coumarin/4-hydroxy-6-methyl-2-pyrone (1 mmol), water (2 ml), [Bmim]Sac (5 mol%).b Isolated yield. | ||||||
| 1 | Ph | 7a | 10 | 95 | 262–263 | 260–261 |
| 2 | 4-MeOC6H4 | 7b | 50 | 85 | 252–253 | 250–251 |
| 3 | 4-NO2C6H4 | 7c | 35 | 90 | 264–265 | 261–263 |
| 4 | 4-OHC6H4 | 7d | 75 | 81 | 266–267 | 267–269 |
| 5 | 4-ClC6H4 | 7e | 45 | 85 | 265–267 | 266–268 |
| 6 | 4-FC6H4 | 7f | 45 | 84 | 257–258 | 258–259 (ref. 10d) |
| 7 | 3-NO2C6H4 | 7g | 30 | 93 | 255–256 | 250–251 |
| 8 | 4-BrC6H4 | 7h | 40 | 89 | 257–258 | 255–258 |
| 9 | 2-C5H4OS | 7i | 70 | 70 | 226–230 | 228–230 (ref. 19) |
| 10 | CH3(CH2)2 | 7j | 50 | 85 | 195–200 | 193–195 (ref. 17) |
| 11 | 4-BrC6H4 | 11a | 45 | 87 | 239–242 | 240–242 (ref. 11d) |
| 12 | CH3(CH2)2 | 11b | 40 | 90 | 218–220 | 220–222 (ref. 10i) |
| 13 | Ph | 11c | 15 | 93 | 236–238 | |
| 14 | 4-MeOC6H4 | 11d | 45 | 88 | 222–224 | 223–225 (ref. 10m) |
| S. no. | Ra | Product | Time (min) | Yieldb | Melting point | Literature melting point18 |
|---|---|---|---|---|---|---|
| a Reaction conditions: aldehyde (1 mmol), malononitrile (1 mmol), 5,5-dimethyl-1,3cyclohexanedione/1,3-cyclohexanedione (1 mmol), water (2 ml) [Bmim]Sac (5 mol%).b Isolated yield. | ||||||
| 15 | Ph | 9a | 10 | 96 | 238–240 | 227–228 |
| 16 | 4-MeOC6H4 | 9b | 25 | 82 | 201–203 | 194–196 |
| 17 | 4-NO2C6H4 | 9c | 20 | 91 | 179–181 | 178–180 |
| 18 | 4-OHC6H4 | 9d | 75 | 80 | 269–270 | 265–266 |
| 19 | 4-ClC6H4 | 9e | 45 | 88 | 212–213 | 207–209 |
| 20 | 4-FC6H4 | 9f | 25 | 92 | 195–197 | 191–193 |
| 21 | 3-NO2C6H4 | 9g | 15 | 94 | 209–211 | 208–211 |
| 22 | 4-BrC6H4 | 9h | 35 | 90 | 200–201 | 196–198 |
| 23 | 2-C5H4OS | 9i | 60 | 88 | 226–228 | 230–231 (ref. 19) |
| 24 | CH3(CH2)2 | 9j | 45 | 89 | 193–194 | 192–193 (ref. 11h) |
| 25 | C6H5CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH CH |
9k | 60 | 80 | 200–202 | 205–207 (ref. 11h) |
| 26 | 4-ClC6H4 | 12a | 40 | 90 | 223–225 | 224–226 (ref. 11g) |
| 27 | Ph | 12b | 15 | 92 | 219–221 | 220–222 (ref. 10k) |
| 28 | 4-BrC6H4 | 12c | 40 | 89 | 196–200 | — |
| 29 | 4-MeOC6H4 | 12d | 30 | 86 | 189–191 | 186–189 (ref. 10k) |
| 30 | 4-MeC6H4 | 12e | 30 | 90 | 228–230 | — |
| 31 | CH3(CH2)2 | 12f | 25 | 92 | 200–205 | — |
| 32 | 4-FC6H4 | 12g | 30 | 93 | 198–201 | — |
The attempt to synthesise 3,4-dihydropyrano[c]chromene and tetrahydrobenzo[b]pyran derivatives using aliphatic aldehyde (n-butyraldehyde) was successful, and the results are summarized in Table 2 (entry 10) and Table 3 (entry 24 and 25). To expand the scope of the present catalytic system, we used substrate 4-hydroxy-6-methyl-2-pyrone as the cyclic 1,3-dicarbonyl compound for the synthesis of 4,5-dihydropyrano[4,3-b]pyran derivatives. As expected, the reaction proceeded smoothly, giving the corresponding products in good to excellent yields with aliphatic as well as aromatic aldehydes (Table 2 , entry 11–14).
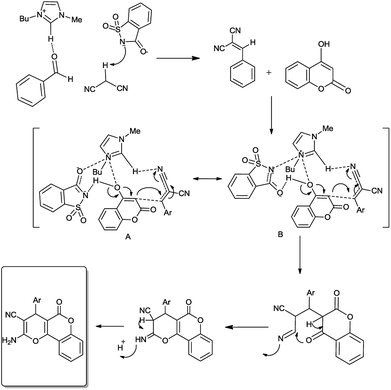
A mechanism for the probable sequence of events is given in Fig. 3. The reaction proceeds via three steps: Knoevenagel condensation, Michael addition, and then intramolecular cyclization, as presented in Fig. 3. The Knoevenagel adduct formed from the ionic liquid-catalyzed condensation of aldehydes and malononitrile subsequently undergoes Michael reaction with carbonyl compounds possessing a reactive methylene group (4-hydroxycoumarin, 4-hydroxy-6-methyl-2-pyrone, 5,5-dimethyl-1,3cyclohexanedione and 1,3-cyclohexanedione); after cyclization, it affords pyran annulated heterocyclic systems.
Bmim cations of ionic liquids activate electrophiles by the proton in the 2-position of the imidazolium ring through hydrogen-bond interaction with the carbonyl and nitrile groups of aldehyde and malononitrile. Simultaneously, anions of ionic liquids activate nucleophiles by accepting the hydrogen bond. The dual activation of nucleophiles and electrophiles by the cations and anions of ionic liquids is crucial to promote the reaction in high yields. As can be seen in Fig. 3, saccharinate anions also play an important role in the dual activation of 1,3-dicarbonyl intermediate nucleophile. It is proposed that an “electrophile nucleophile dual activation” phenomenon of [Bmim]Sac through “dual hydrogen bond formation by saccharinate anions and charge–charge interactions” occurs (Fig. 3).16
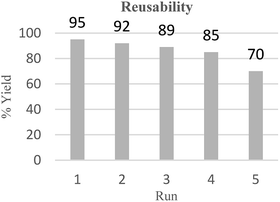
The reusability of ionic liquid [Bmim]Sac was also investigated using the reaction between benzaldehyde, malononitrile and 4-hydroxycoumarin as a model system. Ionic liquid can be recovered from the reaction system and it is interesting to note that the recovered IL was reused for five successive batches of reactions to afford pure products after crystallization (Fig. 4). Therefore, it can be concluded that this catalytic system has great potential in industrial applications (Fig. 4).
Conclusions
We have introduced a green domino Knoevenagel–Michael multicomponent reaction procedure for novel and highly efficient synthesis of 3,4-dihydropyrano[c]chromene and tetrahydrobenzo[b]pyran derivatives in the presence of [Bmim]Sac as a non-toxic and green ionic liquid in aqueous media. This procedure also offers other significant advantages including simple operation, excellent yield, short reaction time, atom economy, scaling up to multigram quantities, and ease of separation. Also, the catalyst can be easily recovered and reused for five consecutive reaction cycles without significant loss of activity.Conflicts of interest
There are no conflicts to declare.Acknowledgements
H. S. and S. S. are thankful to DST for the financial assistance. SAIF-CDRI and IIT Mandi were acknowledged for providing the spectral analytical data.Notes and references
- (a) S. Mallakpour and M. Dinari, in Ionic Liquids as Green Solvents: Progress and Prospects, Green Solvents II, ed. A. Mohammad and D. Inamuddin, Springer, Dordrecht, 2012, pp. 1–32 Search PubMed; (b) M. J. Earle and K. R. Seddon, Pure Appl. Chem., 2000, 72(7), 1391–1398 CAS; (c) M. Petkovic, K. R. Seddon, L. P. N. Rebelo and C. S. Pereira, Chem. Soc. Rev., 2011, 40, 1383–1403 RSC.
- (a) V. I. Pârvulescu, and C. Hardacre, Chem. Rev., 2007, 107, 6, 2615–2665 Search PubMed; (b) H. Olivier-Bourbigou, L. Magna and D. Morvan, Appl. Catal., A, 2010, 373(1–2), 1–56 CrossRef CAS; (c) H. Li, P. S. Bhadury, B. Song and S. Yang, RSC Adv., 2012, 2, 12525–12551 RSC; (d) T. Welton, Coord. Chem. Rev., 2004, 248(21–24), 2459–2477 CrossRef CAS.
- (a) S. G. Lee, Chem. Commun., 2006, 1049–1063 RSC; (b) J. H. Davis, Jr, Chem. Lett., 2004, 33, 1072–1077 CrossRef; (c) A. D. Sawant, D. G. Raut, N. B. Dervatkar and M. M. Salunkhe, Green Chem. Lett. Rev., 2011, 4, 41–54 CrossRef CAS.
- A. C. Cole, J. L. Jensen, I. Ntai, K. L. T. Tran, K. J. Weaver, D. C. Forbes and J. H. Davis, Jr, J. Am. Chem. Soc., 2002, 124, 5962–5963 CrossRef CAS PubMed.
- G. Y. Zhao, T. Jiang, H. X. Gao, B. X. Han, J. Huang and D. H. Sun, Green Chem., 2004, 6, 75–77 RSC.
- J. M. Xu, B. K. Liu, W. B. Wu, C. Qian, Q. Wu and X. F. Lin, J. Org. Chem., 2006, 71, 3991–3993 CrossRef CAS PubMed.
- (a) B. C. Ranu and S. Banerjee, Org. Lett., 2005, 7, 3049–3052 CrossRef CAS PubMed; (b) L. Yang, L. W. Xu, W. Zhou, L. Li and C. G. Xia, Tetrahedron Lett., 2006, 47, 7723–7726 CrossRef CAS.
- S. Ren, Y. Hou, S. Tian, X. Chen and W. Wu, J. Phys. Chem. B, 2013, 117(8), 2482–2486 CrossRef CAS PubMed.
- (a) W. O. Foye, Prinicipi di Chemica Farmaceutica, Piccin, Padova, Italy, 1991, p. 416 Search PubMed; (b) G. R. Green, J. M. Evans and A. K. Vong, in Comprehensive Heterocyclic Chemistry II, ed. A. R. Katritzky, C. W. Ress and E. F. V. Scriven, Pergamon Press, Oxford, 1995, vol. 5, p. 469 Search PubMed; (c) Y. L. Zhang, B. Z. Chen, K. Q. Zheng, M. L. Xu and X. H. Lei, Chin. Acta Pharm. Sin., 1982, 17, 17–22 (Chem. Abstr., 1982, 96, 135383e) CAS; (d) L. Bonsignore, G. Loy, D. Secci and A. Calignano, Eur. J. Med. Chem., 1993, 28, 517–520 CrossRef CAS; (e) L. L. Andreani and E. Lapi, Boll. Chim. Farm., 1960, 99, 583–586 Search PubMed; (f) E. C. Witte, P. Neubert and A. Roesch, Ger. Offen D.E. 3427985, 1986Chem. Abstr. 104 (1986) 224915f; (g) R. M. Shaker, Pharmazie, 1996, 51(3), 148–151 CAS.
- (a) S. Nagaraju, B. Paplal, K. Sathish, S. Giri and D. Kashinath, Tetrahedron Lett., 2017, 58(44), 4200–4204 CrossRef CAS; (b) R. Ghorbani-Vaghei, J. Mahmoodi, Y. Maghbooli and A. Shahriari, Curr. Org. Synth., 2017, 14(6), 904–911 CrossRef CAS; (c) R. Tayebee, A. Pejhan, H. Ramshini, B. Maleki, N. Erfaninia, Z. Tabatabaie and E. Esmaeili, Appl. Organomet. Chem., 2018, 32(1), 3924 CrossRef; (d) D. S. Patel, J. R. Avalani and D. K. Raval, J. Saudi Chem. Soc., 2016, 20, S401–S405 CrossRef CAS; (e) S. Kanakaraju, B. Prasanna, S. Basavoju and G. V. P. Chandramouli, Arabian J. Chem., 2017, 10, S2705–S2713 CrossRef CAS; (f) J. Tiwari, M. Saquib, S. Singh, F. Tufail, M. Singh, J. Singh and J. Singh, Green Chem., 2016, 18, 3221–3231 RSC; (g) S. S. Mansoor, K. Logaiya, K. Aswin and P. N. Sudhan, J. Taibah Univ. Sci., 2015, 9, 213–226 CrossRef; (h) K. Niknam and A. Piran, Green Sustainable Chem., 2013, 3, 1–8 CrossRef; (i) M. Abaszadeh and M. Seif, Res. Chem. Intermed., 2015, 41(10), 7715–7723 CrossRef CAS; (j) H. Mehrabi and H. Abusaidi, J. Iran. Chem. Soc., 2010, 7, 890–894 CrossRef CAS; (k) J. Zheng and Y. Li, Mendeleev Commun., 2011, 21, 280–281 CrossRef CAS; (l) N. Hazeri, M. T. Maghsoodlou, F. Mir, M. Kangani, H. Saravani and E. Molashahi, Chin. J. Catal., 2014, 35, 391–395 CrossRef CAS; (m) J. M. Khurana, B. Nand and P. Saluja, Tetrahedron, 2010, 66, 5637–5641 CrossRef CAS.
- (a) C.-W. Lu, J.-J. Wang, F. Li, S.-J. Yu and Y. An, Res. Chem. Intermed., 2018, 44(2), 1035–1043 CrossRef CAS; (b) D. Azarifar and Y. Abbasi, Synth. Commun., 2016, 745–758 CrossRef CAS; (c) B. D. Rupnar, S. S. Bhagat, A. J. Sirsat and R. P. Pawar, International Journal of Scientific Research in Science, Engineering and Technology, 2018, 4(3), 30–34 Search PubMed; (d) M. Esmaeilpour, J. Javidi, F. Dehghania and F. N. Dodejib, RSC Adv., 2015, 5, 26625–26633 RSC; (e) M. Khoobi, L. Ma'mani, F. Rezazadeh, Z. Zareie, A. Foroumadi, A. Ramazani and A. Shafiee, J. Mol. Catal. A: Chem., 2012, 359, 74–80 CrossRef CAS; (f) H. R. S. Moshtaghin and F. M. Zonoz, Mater. Chem. Phys., 2017, 199, 159–165 CrossRef; (g) M. Seifi and H. Sheibani, Catal. Lett., 2008, 126, 275–279 CrossRef CAS; (h) H. Hu, F. Qiu, A. Ying, J. Yang and H. Meng, Int. J. Mol. Sci., 2014, 15, 6897–6909 CrossRef CAS PubMed.
- (a) A. Kumar, S. Maurya, Kemant and S. Srivastava, Chem. Commun., 2016, 52, 2795–2798 RSC; (b) A. Kumar, G. Gupta and S. Srivastava, Org. Lett., 2011, 13, 6366–6369 CrossRef CAS PubMed.
- (a) A. Kumar, S. Srivastava and G. Gupta, Green Chem., 2012, 14, 3269–3272 RSC; (b) A. Kumar, G. Gupta and S. Srivastava, J. Comb. Chem., 2010, 12, 458–462 CrossRef CAS PubMed; (c) A. Kumar, P. kumar, V. D. Tripathi and S. Srivastava, RSC Adv., 2012, 2, 11641–11644 RSC; (d) A. Kumar, S. Srivastava, G. Gupta, V. Chaturvedi, S. Sinha and R. Srivastava, ACS Comb. Sci., 2011, 13, 65–71 CrossRef CAS PubMed; (e) A. Kumar, G. Gupta and S. Srivastava, Green Chem., 2011, 13, 2459–2463 RSC; (f) A. Kumar, S. Srivastava and G. Gupta, Tetrahedron Lett., 2010, 51, 517–520 CrossRef CAS.
- E. B. Carter, S. L. Culver, P. A. Fox, R. D. Goode, I. Ntai, M. D. Tickell, R. K. Traylor, N. W. Hoffman and J. H. Davis, Jr., Chem. Commun., 2004, 0, 630–631 RSC.
- A. Kumar, S. Srivastava, G. Gupta, P. Kumar and J. Sarkar, RSC Adv., 2013, 3, 3548–3552 RSC.
- P. Nockemann, B. Thijs, K. Driesen, C. R. Janssen, K. V. Hecke, L. V. Meervelt, S. Kossmann, B. Kirchner and K. J. Binnemans, J. Phys. Chem. B, 2007, 111, 5254–5263 CrossRef CAS PubMed.
- P. Das, A. Dutta, A. Bhaumik and C. Mukhopadhyay, Green Chem., 2014, 16, 1426–1435 RSC.
- E. Mollashahi and M. Nikraftar, J. Saudi Chem. Soc., 2018, 22(1), 42–48 CrossRef CAS.
- A. Mobinikhaledi, H. Moghanian and A. Zohari, Rev. Roum. Chim., 2016, 61(1), 35–39 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ra06889e |
| This journal is © The Royal Society of Chemistry 2018 |