Open Access Article
Open Access ArticleRoles of a new drug-delivery healing abutment in the prevention and treatment of peri-implant infections: a preliminary study
Shuang Zhang†
a,
Min Wang†ac,
Tao Jiang ab,
Yi Zhou*ab and
Yining Wang
ab,
Yi Zhou*ab and
Yining Wang *ab
*ab
aThe State Key Laboratory Breeding Base of Basic Science of Stomatology (Hubei-MOST), Key Laboratory of Oral Biomedicine Ministry of Education, School and Hospital of Stomatology, Wuhan University, 237 Luoyu Road, Wuhan 430079, China. E-mail: dryizhou@163.com; wang.yn@whu.edu.cn; Fax: +86 27 87873260; Tel: +86 27 87686318
bDepartment of Prosthodontics, Hospital of Stomatology, Wuhan University, Wuhan 430079, China
cDepartment of Oral Implantology, Hospital of Stomatology, Wuhan University, Wuhan 430079, China
First published on 19th November 2018
Abstract
In this study we modified the common healing abutment into a specifically designed drug-delivery abutment (DDA), which is a hollow columnar system with drug-distribution holes in peripheral wall. The objective of this study was to investigate the possibility of the prevention and treatment of peri-implant diseases with this drug-delivery system. Minocycline hydrochloride was added to DDAs with different hole diameters, and then subjected to bacteria-inhibition tests in vitro with three oral bacterial strains, namely, Streptococcus mutans, Streptococcus sanguinis, and Porphyromonas gingivalis. The implants were placed into the mandible of beagle dogs. DDAs with or without minocycline and normal healing abutments were installed. One week after surgery, the plaques on all the abutments were analyzed by plaque stain. Following this, both abutments and adjacent teeth received oral hygiene to maintain a healing environment. Eleven weeks later, the ligature-induced experimental peri-implantitis model was set up for eight weeks. Periapical radiographs and clinical measurements were performed during the process. We found that inhibition zones were observed surrounding all the tested drug-delivery abutments in all three bacterial strains. One week after implant installation, oral plaque formed on the DDAs with minocycline was significantly less than that on normal abutments and DDAs without drugs. DDA with the minocycline group showed a relatively slower rate of deterioration of the mucosal inflammation and probing depth in the experimental peri-implant lesions. We suggest that this drug-delivery abutment could effectively deliver medications into peri-implant tissues to minimize plaque formation and relieve peri-implant inflammation in the experimental peri-implantitis model.
1. Introduction
On the basis of the osseointegration theory put forward by Branemark et al.,1–3 dental implantation has become a common and popular treatment for the loss of tooth. Despite the high survival rate that was reported to be greater than 89% at 10–15 years of follow-up,4 peri-implant disease, introduced as a collective term for inflammatory reactions in the tissues surrounding the implants, has been the major challenge to the long-term maintenance of the implant and fixtures.5 Peri-implant diseases are of two types: peri-implant mucositis and peri-implantitis. Peri-implant mucositis describes an inflammatory lesion that resides in the mucosa, while peri-implantitis also affects the supporting bone.6 Peri-implant mucositis is believed to be the precursor of peri-implantitis, and it is reversible when effectively treated. According to a recent meta-analyses result, the weighted mean prevalence of peri-implant mucositis and peri-implantitis were respectively 43% (CI: 32–54%) and 22% (CI: 14–30%).7Peri-implantitis occurs primarily as a result of an overwhelming bacterial insult and subsequent host immune response. Therefore, elimination of the established microbial plaque from the implant surface is the main objective in the prevention and treatment of peri-implant mucositis and peri-implantitis. Different to periodontal therapy, it is very hard to achieve successful plaque control due to the complex threaded structure and roughness of the implant surface.8,9 Drug therapy, including antibiotic and antimicrobial agent application, provides an antimicrobial concentration adequate enough to achieve a reduction of peri-implant pathogens. Thus, drug therapy has been used as an important adjunct to both mechanical debridement and surgery. Antibiotics can be delivered locally or systemically. With the great concern of bacterial resistance brought about by the systematic use of antibiotics, more studies have demonstrated clinical and microbiological improvements with local use.10–13 Therefore, a control-released drug delivery system is always required in the antibiotic treatment of peri-implant infections.8 Antibiotic gels, microspheres, chips, and fibers have been tested and have shown improved clinical results.14–17 However, the existence and circulation flow of oral salivary and gingival crevicular fluid decide that the effect of local drug treatment relies on a long-lasting necessary concentration of medications. The appliances of antibiotic gels, microspheres, chips, and fibers have been restricted to the existence of peri-implant sites pockets and well demarcated lesions, which occur in the late phase of peri-implantitis. Therefore, more efforts are needed to explore a new way of delivery of anti-bacteria agents.
Here, we present a new device for the local delivery of medications by the modification of healing abutments used in most implant cases, namely a drug-delivery abutment (DDA). Potentially, a DDA with anti-bacteria agents might be used in compromised implant sites for the prevention of a high risk of infection, or to peri-implantitis treatment as the adjunct to mechanical debridement and surgery. The aim of this study was to investigate the in vitro drug distribution ability of this device and the in vivo possibility for the prevention and treatment of peri-implant diseases with this drug-delivery system.
2. Experimental
2.1 Preparation of abutments and implants
The abutments and implants in this study were fabricated from commercial pure titanium (BaoJiXinNuo Ltd., Shanxi, China, TA1 grade according to ISO 5832-2:1993). The drug-delivery abutments consisted of two parts: a cylinder-shaped drug chamber (4 mm in diameter and 4 mm in height) with evenly distributed holes in the peripheral wall and the closure to seal the drug chamber (Fig. 1). Normal healing abutments were just cylinder-shaped (4 mm in diameter and 4 mm in height) with neither holes nor closure.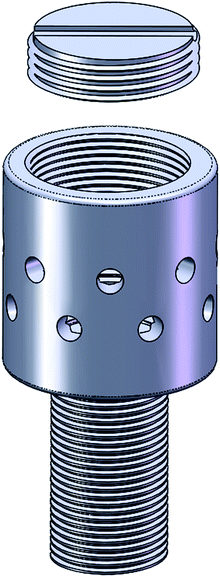 | ||
| Fig. 1 Design of the experimental drug-delivery abutment. 3D Schematic of DDA used in our experiments. | ||
The implants were 3.5 mm in diameter and 6 mm in height. The upper part of the implant was cylinder-shaped with screw threads around the surface and the bottom was hemisphere-shaped. Implants were sandblasted and etched as previously described in our publication as follows: all implants were coarse grit blasted with 0.25–0.50 mm corundum grit at 5 bars for 1 min, and then etched in an equal volume of mixed hydrochloric acid (12.4 mol l−1) and sulfuric acid (18.4 mol l−1) at 60 °C for 15 min.
All the abutments and treated implants were cleaned in a series of deionized water, ethanol, acetone, ethanol, deionized water, under ultrasonic conditions, and were sterilized in an autoclaving machine (SANYO, Japan) at 121 °C for 20 min, according to our previous publication.18
2.2 In vitro bacteria-inhibition tests
Bacteria were grown anaerobically in Brain Heart Infusion broth (BHI) (BD, USA) overnight at 37 °C after propagation from stocks frozen at −70 °C in the presence of 50% glycerol (SCR, China). The bacterial suspensions were prepared for further use, and a 0.5 McFarland standard was used as a reference for the turbidity (approximately 1.5 × 108 CFU ml−1).
After the application of the abutments, the plates were cultured in an anaerobic jar at 37 °C for 18 h. The diameter of the inhibition zone (including the diameter of the abutment) was then measured four times. The tests of each group of abutments were repeated three times and the results were expressed as the mean ± SD.
2.3 In vivo experiments
Three 18 months-old Beagle dogs (females; weight 14–16 kg) were involved in this study. During all the surgical procedures, general anaesthesia was induced with an intravenous injection of pentobarbital sodium (30 mg ml−1, 1 ml kg−1; Merck, German).
The 18 abutments were connected to the implants and the mucoperiosteal flaps were adjusted and sutured. A soft diet was applied to the dogs and no plaque-control program was initiated.
Seven days after surgery, all the abutments were carefully removed without touching the surface and replaced by new normal abutments. An oral hygiene program, including cleaning of the remaining natural teeth and the implant-supported abutments three times a week, was initiated and the soft diet was replaced by hard food.
The plaque formation on the three groups of abutments was observed by Basic Fuchsin staining. The abutments tested were stained with 2% Basic Fuchsin for 1 min and then washed with sterile water three times for 1 min. The stained plaque on the abutment was collected by a curette and the collection was dissolved in 200 μl ethanol. The absorbance of Fuchsin basic was measured at a wavelength of 543 nm and the OD values of all the abutments were recorded and analyzed.
Three months after implant installation (baseline, week 0), oral examination and standardized peri-apical intraoral radiograph were performed and the status of the peri-implant tissues was evaluated by clinical and radiographic measurements. The detailed measurements are as follows:
Clinical measurements:
Peri-implant sites bleeding on probing index (BOP);
Peri-implant probing depth (PPD);
Volume of peri-implant crevicular fluid (PICF);
Activity of the aspartate aminotransferase (AST) and alkaline phosphatase (ALP) in PICF;
Level of Tumor Necrosis Factor alpha (TNF-α) and Interleukine-1 beta (IL-1β) in PICF.
Radiographic measurements:
The distance between the implant shoulder (IC) and the marginal bone level (MBL) next to the implant;
BOP, PPD measurements were performed using a manual 15 mm periodontal probe with a 0.25 N force. All clinical parameters were recorded at six sites per implant (mesiobuccal, buccal, distobuccal, mesiolingual, lingual, distolingual). PICF samples were obtained from the mesial, distal, buccal, and lingual aspects of each implant with standard 2 mm × 8 mm Whatman 3# sterile paper strips (Whatman International Ltd., Maidstone, England). Any supragingival deposits were carefully removed and the PICF collection sites were separated by cotton rolls and air-dried. The paper strip was inserted into the sulcus until a slight resistance was encountered and left in situ for 30 s. Any strips with contamination of blood and salivary were discarded. Samples were taken from all implants in each animal and the strip was transferred into a 1.5 ml Eppendorf tube then immediately stored at −70 °C. Each single paper strip was weighed before the sampling procedure and immediately repeated after sampling. Considering the PICF density as 1, the volume of PICF could be calculated.
PICF were extracted from the strips when 100 μl PBS (pH = 7.4) were added to each tube. The tubes were shaken at room temperature for 1 h and then centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 4 °C for 10 min. The supernatant was taken to be measured. AST and ALP activity levels in PICF were measured with an automatic biochemical analyzer (Olympus AU5400, Olympus, Tokyo, Japan), and the results were expressed as units per ml in PICF. The levels of TNF-α and IL-1β in PICF were determined using a commercially available canine TNF-α and IL-1β ELISA kit (R&D Systems; MN, USA). The assay was performed according to the manufacturer's protocol.
000 rpm, 4 °C for 10 min. The supernatant was taken to be measured. AST and ALP activity levels in PICF were measured with an automatic biochemical analyzer (Olympus AU5400, Olympus, Tokyo, Japan), and the results were expressed as units per ml in PICF. The levels of TNF-α and IL-1β in PICF were determined using a commercially available canine TNF-α and IL-1β ELISA kit (R&D Systems; MN, USA). The assay was performed according to the manufacturer's protocol.
Radiographic measurements were determined both at the mesial and distal aspect of each implant and expressed in millimeters. Dimensional distortions and enlargements on the radiographs were adjusted.
After the clinical and radiographic examination at week 0, establishment of the experimental peri-implant infection model was initiated and oral hygiene was terminated. 4-0 Silk ligatures were placed in a sub-marginal position apical of the perimucosal margin of all the implants. These ligatures were exchanged every two weeks in the pocket of a receded mucosal margin. Clinical measurements were performed at weeks 0, 2, 4, and 8 and radiographic examinations were at weeks 0 and 8.
An outline of the in vivo experiment is shown in Fig. 2. The examiners were blinded with regard to the group identity of the abutments for all the measurements. All the operations were performed by the same operator.
2.4 Statistical analysis
Data were statistically analyzed by means of SPSS 16.0 for Windows (SPSS, Chicago, IL). Independent-sample t tests were applied for comparison of Ra between the two kinds of abutments. One-way analysis of variance and Student–Newman–Keuls (SNK) tests were used to determine level of significance for other parameters. A p-value < 0.05 was required for statistical significance.3. Results
3.1 In vitro inhibition of bacteria growth
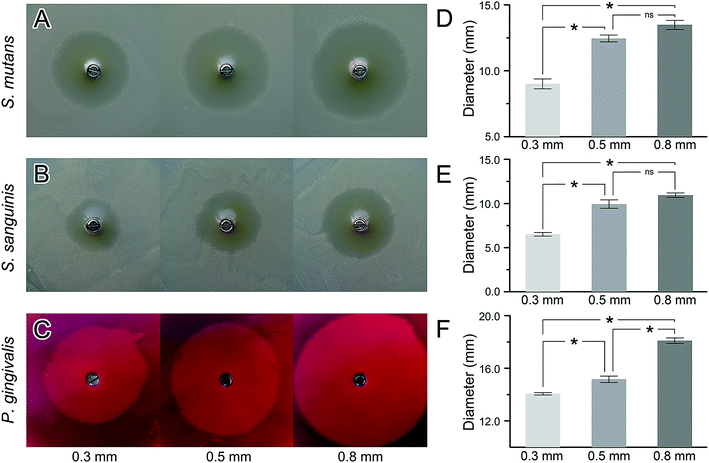
The bacterial-inhibition tests were performed to evaluate the drug distribution and bacteriostatic effects of DDA loaded with minocycline. Inhibition zones were found surrounding all the tested drug-delivery abutments in all three bacterial strains (Fig. 3A–C). As the pore size of the abutments increased, the diameters of the inhibition zones increased. The diameters of inhibition zones formed by DDA with 0.5 mm and 0.8 mm pores were significantly larger than that with 0.3 mm in all the strains. While the diameters of the inhibition zone formed by DDA with 0.8 mm pores on plates of Streptococcus mutans and Streptococcus sanguinis showed no significant differences with that of 0.5 mm pores (Fig. 3D–F).3.2 In vivo plaque on the abutment at an early stage of osseointegration
Seven days after the placement of implants and abutments, intact plaque spontaneously colonized on the abutment was stained with Fuchsin plaque indicator. Photographs of the front and back of the abutments are shown in Fig. 4A. More stained areas were vividly observed on the abutment surface of the control group and DDA group.The result of quantitative measurement of Basic Fuchsin in stained plaque was in accordance with the apparel observation (Fig. 4B). The OD value at 543 nm of DDA + minocycline group was 0.030 ± 0.010, whereas the OD value of DDA group and control group were respectively 0.454 ± 0.083 and 0.426 ± 0.105, which is significantly higher than DDA + minocycline group. No significant difference was found between control group and DDA group.
3.3 In vivo evaluation of inflammation development in the experimental peri-implant infection model
Visual inspection showed that peri-implant tissues of the DDA + minocycline group were in a relatively healthier condition compared with the control group in the perspective of plaque formation with signs of inflammation at the end of observation (week 8), though the inclination to bleed, tissue swelling, and redness were observed in all implant sites throughout the study period (Fig. 5A, B, D and E).
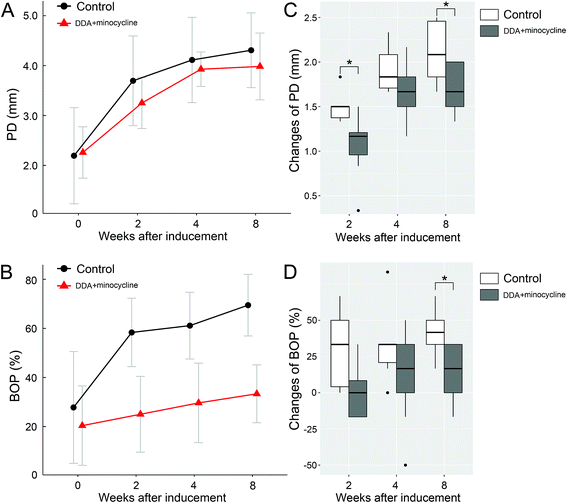
Baseline PPDs (2.26 mm and 2.19 mm for DDA + minocycline group and control group respectively) showed a continuous increase during the experimental peri-implant infections (Fig. 6A). The mean probing depths at week 8 were 3.98 mm and 4.30 mm for the DDA + minocycline group and control group, respectively, which were significantly different from the baseline. PPD increases (ΔPPD) from the baseline to different observation times are shown in Fig. 6C. The control group exhibited a greater increase than the DDA + minocycline group at weeks 2, 4, and 8, and the differences were significant at week 2 and week 8.
Both groups showed significant increases in BOP (Fig. 6B). The percentile of BOP grew from 20.37% (baseline) to 33.33% (week 8) in the DDA + minocycline group, 27.79% to 69.44% in the control group, and the increase in the control group was significantly greater than that in the DDA + minocycline group at week 8 (Fig. 6D).
As regard to the parameters of PICF, Table 1 shows the changes of PICF indexes from the baseline to final examination. Changes of PICF volume, IL-1β and AST levels were significantly different between the two groups. Changes of TNF-α and ALP in the control group were greater than those in the DDA + minocycline group, though the differences were not statistically significant.
| Group | Variable | |||||
|---|---|---|---|---|---|---|
| Abutments | ΔVolume (±SD) (μl) | ΔTNF-α (±SD) (pg ml−1) | ΔIL-1β (±SD) (ng ml−1) | ΔAST (±SD) (U ml−1) | ΔALP (±SD) (U ml−1) | |
| a Group A: DDA + minocycline group; Group B: control group. | ||||||
| Group A | 9 | 0.511 ± 0.341 | 15.269 ± 8.481 | 0.474 ± 0.105 | 0.733 ± 0.394 | 0.178 ± 0.259 |
| Group B | 6 | 1.517 ± 0.857 | 21.261 ± 10.461 | 0.906 ± 0.303 | 3.050 ± 1.498 | 0.233 ± 0.197 |
| P-value | — | 0.017 | 0.318 | 0.036 | 0.017 | 0.653 |
| Group A | Group B | P-value | |
|---|---|---|---|
| a Group A: DDA + minocycline group; Group B: control group. | |||
| Baseline | 0.560 ± 0.515 | 0.6390.739 | — |
| Week 8 | 1.715 ± 0.352 | 1.896 ± 0.374 | — |
| Difference | 1.209 ± 0.790 | 1.256 ± 0.691 | 0.713 |
4. Discussion
This research aimed to develop a drug-delivery device to assist in the non-surgical treatment of peri-implantitis in different phases of the inflammation. The cause and effect relationship was established for plaque accumulation at dental implants triggering the inflammatory host response resulting in peri-implant mucositis/peri-implantitis.19 Therefore, plaque control is one of the main aims of the prevention and of mucositis and peri-implantitis. However, due to the rough surface of the implant, mechanical means of decontaminating implants has a limited effect owing to technical difficulties and can certainly not remove all the bacteria. Also, it is very difficult. It seems that limited clinical improvements have been found following mechanical therapy alone using specially designed carbon-fiber curettes, ultrasonic devices, and titanium instruments.10,20 Therefore, mechanical therapy has been supplemented with local antimicrobials.The local administration of antimicrobial agents as adjuncts is usually implemented with controlled delivery devices, such as fibers, gels, chips, and microspheres. Such devices are expected to release a sustained high dose of antimicrobial agents precisely into the infective areas over several days. Although the results available so far have suggested that these devices have a positive effect on the controlled delivery of antimicrobial agents, clinical experience also point to the limitations of these methods.10 For example, in most of the cases studied in previous research, the existing peri-implant pocket and a well-demarcated saucer were seen around the peri-implant lesions. Also, the placement and retention of these carriers thus became possible. However, in some cases without the deep pocket and well-demarcated lesion or at the early stage of peri-implant inflammation, the usage of controlled delivery carries may meet obstacles.
In this experiment, we modified the common healing abutment into a specifically designed drug-delivery abutment (DDA), which is a hollow columnar system with drug distribution holes in the peripheral wall. It is supposed that drugs inside the drug-delivery abutment could effectively spread out through the holes, which was proved by the in vitro bacteria-inhibition test. To prevent the possible ectopic tissue growth into the abutment and maintain a decent mechanical strength, we chose the abutment with a Φ 0.5 mm pore size in the following experiments. We speculate that this device may be effective in the plaque control during the healing phase of the implant surgery. Therefore, the plaque formation on the abutment after implant placement was analyzed in a canine model. As a result, compared to the common healing abutment, a significant decrease of plaque formation was found in DDA with the minocycline group, which suggested that DDA might inhibit the peri-implant plaque formation. This effect might be related to the resolution of the minocycline by sulcus fluid and saliva, delivered to the zone around the abutment. It has been recommended that the anti-bacteria treatment could be performed in the event compromised local and systematic conditions increase the infection possibility of the implantation sites.21–23 We expect that this modified abutment might be used immediately after implant placement as a prophylaxis treatment, in patients with complex bone augmentation, at susceptible sites of an immediately placed implant, suffering from previous periodontitis or periapical lesion, or compromised systematic conditions (such as diabetes). Thus, the special healing abutment might play a role in the prevention of peri-implantitis and early infection.
Then, we built an experimental peri-implant infection model to observe the development of peri-implant infection in the DDA + minocycline group and control group. Progress of inflammation was evaluated by both clinical and radiographic measurements. Bleeding on probing index (BOP) indicates the inflammation of soft tissue and may be used as a predictor for loss of tissue support.24 An increase in peri-implant probing depth (PPD) over time is associated with the loss of attachment and supporting bone.25,26 Analysis of PICF, including its volume and constituents, can be used to evaluate the healing and inflammation process in peri-implant tissues. Several studies supported that the increase of PICF volume,27 ALP and AST activity,28,29 and TNF-α and IL-1β levels30–32 could be associated with the inflammation around dental implants. The alternation of distance between the implant shoulder and the marginal bone level indicates the loss of supporting bone. It was observed in this model that the DDA and minocycline combination could inhibit the peri-implant inflammations. Results showed that drug delivery abutment could prevent the plaque formation and retard the peri-implantitis progress in the animal model. Drugs can be released into the surrounding tissues through the circulation holes around the abutment, which can avoid the side effects of systemic administration and possible drug resistance.33 At the same time, it can effectively avoid the difficulties in the placement and retention of drug carriers in traditional local administration. This system might be used for the treatment of peri-implant inflammation accompanied by different types of defects and less limited by the periodontal pocket and the shape of defect.
Another potential application of this modified abutment can be an experimental platform for new antimicrobial drugs and controlled released system. This abutment can deliver not only antimicrobial drugs for the prevention and treatment of peri-implant diseases, but other agents aimed at inflammation control, tissue repair, bone healing promotion, and so on. Agents being studied can be released directly to the tissue around the implant through this device. It is easy to control the initial volume and concentration of the drugs.
5. Conclusions
Based on our in vitro and in vivo finding in this study, we could conclude that the drug-delivery abutment used in our experiment could effectively deliver drugs into peri-implant tissues and could possibly be applied in the prevention and treatment of peri-implant infections.This research is a preliminary study of the drug-delivery abutment. The more precise geometrical parameters of the abutment are still in need of optimization. In addition, the exact concentration variation of the drug around the abutment remains unclear. Therefore, more investigations are necessary in the future.
Conflicts of interest
There are no conflicts of interests to declare.Acknowledgements
This study was financially supported by National Natural Science Foundation of China (No. 81200812 and No. 81500866), and Science and Technology Project of Wuhan, China(No. 2016060101010043).Notes and references
- P. I. Branemark, R. Adell, U. Breine, B. O. Hansson, J. Lindstrom and A. Ohlsson, Scand. J. Plast. Reconstr. Surg., 1969, 3, 81–100 CrossRef CAS PubMed.
- R. Adell, B. O. Hansson, P. I. Branemark and U. Breine, Scand. J. Plast. Reconstr. Surg., 1970, 4, 19–34 CrossRef CAS PubMed.
- P. I. Branemark, J. Prosthet. Dent., 1983, 50, 399–410 CrossRef CAS PubMed.
- P. A. Norowski Jr and J. D. Bumgardner, J. Biomed. Mater. Res., Part B, 2009, 88, 530–543 CrossRef PubMed.
- C. Riben-Grundstrom, O. Norderyd, U. Andre and S. Renvert, J. Clin. Periodontol., 2015, 42, 462–469 CrossRef CAS PubMed.
- J. Lindhe, J. Meyle and D. o. E. W. o. P. Group, J. Clin. Periodontol., 2008, 35, 282–285 CrossRef PubMed.
- J. Derks and C. Tomasi, J. Clin. Periodontol., 2015, 42(suppl. 16), S158–S171 CrossRef PubMed.
- A. Mombelli, Periodontol. 2000, 2002, 28, 177–189 CrossRef PubMed.
- C. M. Murray, E. T. Knight, A. A. Russell, A. Tawse-Smith and J. W. Leichter, N. Z. Dent. J., 2013, 109, 55–62 Search PubMed.
- S. Renvert and I. N. Polyzois, Periodontol. 2000, 2015, 68, 369–404 CrossRef PubMed.
- S. Renvert, A. M. Roos-Jansaker and N. Claffey, J. Clin. Periodontol., 2008, 35, 305–315 CrossRef PubMed.
- A. J. van Winkelhoff, Eur. J. Oral Implantol., 2012,(suppl. 5), S43–S50 Search PubMed.
- M. De Araujo Nobre, C. Capelas, A. Alves, T. Almeida, R. Carvalho, E. Antunes, D. Oliveira, A. Cardador and P. Malo, Int. J. Dent. Hyg., 2006, 4, 84–90 CrossRef CAS PubMed.
- M. Bassetti, D. Schar, B. Wicki, S. Eick, C. A. Ramseier, N. B. Arweiler, A. Sculean and G. E. Salvi, Clin. Oral Implants Res., 2014, 25, 279–287 CrossRef PubMed.
- S. Renvert, J. Lessem, G. Dahlen, C. Lindahl and M. Svensson, J. Clin. Periodontol., 2006, 33, 362–369 CrossRef CAS PubMed.
- E. E. Machtei, S. Frankenthal, G. Levi, R. Elimelech, E. Shoshani, O. Rosenfeld, N. Tagger-Green and B. Shlomi, J. Clin. Periodontol., 2012, 39, 1198–1205 CrossRef CAS PubMed.
- A. Mombelli, A. Feloutzis, U. Bragger and N. P. Lang, Clin. Oral Implants Res., 2001, 12, 287–294 CrossRef CAS PubMed.
- Y. Zhou, T. Jiang, M. Qian, X. Zhang, J. Wang, B. Shi, H. Xia, X. Cheng and Y. Wang, Biomaterials, 2008, 29, 461–474 CrossRef PubMed.
- B. Klinge, T. Flemming, J. Cosyn, H. De Bruyn, B. M. Eisner, M. Hultin, F. Isidor, N. P. Lang, B. Lund, J. Meyle, A. Mombelli, J. M. Navarro, B. Pjetursson, S. Renvert and H. Schliephake, Clin. Oral Implants Res., 2015, 26(suppl. 11), 64–67 CrossRef PubMed.
- S. Renvert, E. Samuelsson, C. Lindahl and G. R. Persson, J. Clin. Periodontol., 2009, 36, 604–609 CrossRef PubMed.
- J. R. Keenan and A. Veitz-Keenan, Evid. Based Dent., 2015, 16, 52–53 CrossRef PubMed.
- S. Corbella, S. Taschieri, I. Tsesis and M. Del Fabbro, J. Oral Implantol., 2013, 39, 399–405 CrossRef PubMed.
- G. Bryce, D. I. Bomfim and G. S. Bassi, Br. Dent. J., 2014, 217, 171–176 CrossRef CAS PubMed.
- J. Ainamo and I. Bay, Int. Dent. J., 1975, 25, 229–235 CAS.
- U. Lekholm, I. Ericsson, R. Adell and J. Slots, J. Clin. Periodontol., 1986, 13, 558–562 CrossRef CAS PubMed.
- M. Quirynen, D. van Steenberghe, R. Jacobs, A. Schotte and P. Darius, Clin. Oral Implants Res., 1991, 2, 186–192 CrossRef CAS PubMed.
- A. Niimi and M. Ueda, Int. J. Oral Maxillofac. Implants, 1995, 10, 434–436 CAS.
- R. C. Page, J. Periodontol., 1992, 63, 356–366 CrossRef CAS PubMed.
- M. Paolantonio, G. Di Placido, V. Tumini, M. Di Stilio, A. Contento and G. Spoto, J. Periodontol., 2000, 71, 1151–1157 CrossRef CAS PubMed.
- H. Ataoglu, N. O. Alptekin, S. Haliloglu, M. Gursel, T. Ataoglu, B. Serpek and E. Durmus, Clin. Oral Implants Res., 2002, 13, 470–476 CrossRef PubMed.
- D. T. Graves and D. Cochran, J. Periodontol., 2003, 74, 391–401 CrossRef CAS PubMed.
- G. Schierano, G. Pejrone, P. Brusco, A. Trombetta, G. Martinasso, G. Preti and R. A. Canuto, J. Clin. Periodontol., 2008, 35, 532–538 CrossRef PubMed.
- F. Schwarz and J. Becker, Peri-implant Infection: Etiology, Diagnosis and Treatment, Quintessence Publishing Co. Ltd., London, 2009 Search PubMed.
Footnote |
| † Both authors contributed equally to this study. |
| This journal is © The Royal Society of Chemistry 2018 |