Open Access Article
Open Access ArticleThe performance and associated mechanisms of carbon transformation (PHAs, polyhydroxyalkanoates) and nitrogen removal for landfill leachate treatment in a sequencing batch biofilm reactor (SBBR)
Wenjun Yin*a,
Kai Wanga,
Jingtao Xua,
Daoji Wu *a and
Congcong Zhaob
*a and
Congcong Zhaob
aSchool of Municipal and Environmental Engineering, Shandong Jianzhu University, Jinan, 250101, China. E-mail: yinwenjun1991@163.com; 1353007350@qq.com; Fax: +86 13708922504; Tel: +86 13256741911
bCollege of Geography and Environment, Collaborative Innovation Center of Human-Nature and Green Development in Universities of Shandong, Shandong Normal University, Jinan 250014, P. R. China
First published on 19th December 2018
Abstract
A modified sequencing batch biofilm reactor (SBBR, adding a pre-anoxic phase before the aeration phase) was used to treat landfill leachate. The overall SBBR operation period was divided into a load-increasing period I (69 days) and a steady operation period II (41 days). In period I, the influent total nitrogen (TN) and chemical oxygen demand (COD) concentrations increased from approximately 60 and 400 mg L−1 to 1000 and 6500 mg L−1, respectively, and these were kept for period II. In period II, the COD and TN removal rates were 83–88% and 95–98%, with effluent COD and TN concentrations of less than 500–600 and 10–20 mg L−1, respectively. The end of pre-anoxic phase PHA (polyhydroxyalkanoate) content increased from 0.11 Cmol (start of period I) to 0.22 Cmol (end of period II). The contributions from simultaneous nitrification and denitrification (SND) and endogenous denitrification to the TN removal rate were approximately 60% and 40%, respectively. The mechanisms of carbon transformation and nitrogen removal were: (1) the synthesis of PHAs in the pre-anoxic phase; (2) short-range nitrification; (3) simultaneous nitrification and denitrification (SND); and (4) endogenous denitrification. Microbial diversity analysis revealed that Proteobacteria and Bacteroidetes accounted for 89.66% of the total bacteria. Ammonia-oxidizing bacteria (AOB, Nitrosomonas) and denitrifying bacteria with the ability to transform organic matter into PHAs (Paracoccus and Thauera) are the dominant bacterial communities.
1. Introduction
The main treatment method for municipal solid waste world-wide involves the use of sanitary landfills. However, sanitary landfills generate landfill leachate, containing high concentrations of organic matter, nitrogen, inorganic salts, and heavy metals,1–3 resulting in serious pollution of the external aquatic environment.4 Therefore, treatment technologies for landfill leachate with improved efficiency and low cost are important for sewage treatment strategies.Currently, the main treatment methods for landfill leachate are physical–chemical and biological methods. Physical–chemical methods include ammonia stripping technology, advanced oxidation, and adsorption;5–7 these methods are typically used in pre-treatment or post-treatment steps due to their secondary pollution and high cost. Biochemical methods, including sequencing batch reactors (SBRs), up-flow anaerobic sludge beds (UASBs), anaerobic sequencing batch reactors (ASBRs), membrane bio-reactors (MBRs) and related methods, could treat landfill leachate effectively and environmentally.8,9 However, anaerobic methods are unable to remove NH4+–N;10 traditional aerobic methods need the addition of extra carbon sources to remove nitrogen.11
PHAs (polyhydroxyalkanoates) composed of polyhydroxybutyrate (PHB) and polyhydroxyvalerate (PHV) have a significant impact on carbon substrate conversion in activated sludge processes.12 Some microbes can convert organic matter into intracellular PHAs when there is enough organic matter, then use PHAs as a carbon source for denitrification to remove nitrogen.13 Zhu et al. used PHAs as a carbon source for endogenous denitrification to remove nitrogen in SBR systems, therefore the strategy of using PHAs to remove nitrogen is feasible.14,15
PHAs can be used as a carbon source for simultaneous nitrification and denitrification (SND) to remove nitrogen.16,17 The realization of SND depends on the anaerobic reaction zone of the system, so there is no SND in the aeration phase of a SBR.14,15 A SBR can achieve SND through controlling the dissolved oxygen (DO) concentration over a range of 0.5 to 1 mg L−1,18,19 but a lower DO concentration can limit the nitrification reaction rate. Anaerobic reaction zones exist in the biofilm inner layer of a SBBR system because of the dissolved oxygen gradient; therefore, a SBBR could achieve SND when the DO concentration is controlled over the range of 2 to 3 mg L−1. If an anoxic phase is added before the aeration phase to modify a SBBR, the biofilm can store PHAs during the pre-anoxic phase and the modified SBBR could achieve SND in the anaerobic reaction zone of the aeration phase. SND facilitates the removal of nitrogen through these properties: (1) the alkali produced by SND makes up for alkali consumed by the nitrification reaction, which maintains a stable pH range; (2) there is reduced inhibition from free ammonia and free NO2−–N of the nitrification reaction, with enhanced nitrification reaction rates; and (3) SND can use PHAs as a carbon source. A survey of the literature shows that no work has been done so far regarding landfill leachate treatment by SND in a modified SBBR, but it is very meaningful to realize SND in a SBBR system.
The objectives of this study were: (1) to determine the operating parameters and investigate the variation in biofilm indicators, such as biomass, density and extracellular polymeric substance (EPS) concentration; (2) to monitor the variation in COD, TN, NO2−–N, NO3−–N, COD and TN removal rates, PHA content, pH, DO, oxidation-reduction potential (ORP), etc. over the whole operation period and typical cycles of period II; (3) to investigate mechanisms of carbon transformation and nitrogen removal; and (4) to investigate the structures and characteristics of dominant bacterial communities.
2. Materials and methods
2.1 Experimental setup
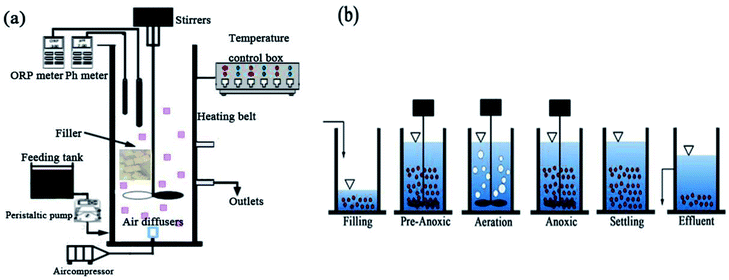
A polymethyl methacrylate SBBR reactor was used for the experiments, as shown in Fig. 1a. A laboratory scale cylindrical SBBR reactor with a height of 50 cm, diameter of 20 cm, and working volume of 10 L was used. The SBBR reactor included a Multi 3620 analyzer for the detection of pH, DO and ORP, mechanical stirrers, an air diffuser, an air compressor, a heating belt, and a temperature control box. The air diffuser is connected to the air compressor for the aeration of the SBBR. The heating belt and temperature control box constitute a temperature control device to regulate the temperature. The biofilm filler consists of polyurethane and other polymer materials, referred to as PPC, and the PPC characteristics are shown in Table 1.| Item | Edge length (mm) | Density (kg m−3) | Surface area (m2 m−1) | Porosity (%) | Hanging film time (days) | Attachments (mg) |
|---|---|---|---|---|---|---|
| Parameter | 10 ± 1 | 12.5 ± 0.7 | >4000 | 98 | 3–7 | 0.02 |
2.2 Influent media, sludge and filler
Raw landfill leachate for this study was taken from the Ji Yang MSW Sanitation Landfill Site, Jinan, Shandong Province, China. The characteristics of the landfill leachate are shown in Table 2. The seed sludge was obtained from return sludge from the Everbright Sewage Treatment Plant, Jinan, Shandong Province, China. The concentrations of mixed liquid suspended solids (MLSSs), mixed liquor volatile suspended solids (MLVSSs) and the sludge volume index (SVI) were approximately 9230 mg L−1, 7523 mg L−1 and 123 mL g−1, respectively.2.3 Operational procedure
The operation mode of the SBBR system includes: (a) a filling phase (5 min); (b) a pre-anoxic phase (60 min); (c) an aeration phase (aeration time); (d) an anoxic phase (anoxic time); (e) a settling phase (30 min); (f) an effluent phase (5 min); and (g) an idle phase (idle time), as shown in Fig. 1b. The aeration time and anoxic time were determined from the ammonium valley (pH variation) and nitrate knee (ORP variation) real-time control methods.20The volumetric exchange rate was 30%. The temperature was controlled at 25 ± 1 °C. The DO concentration was maintained over a range from 2.0 to 3.0 mg L−1 during the aeration phase.
2.4 Method determination and calculations
The pH, DO, ORP, and temperature were monitored using a Multi 3620 analyzer (WTW Company, Germany). NH4+–N, NO2−–N, NO3−–N, COD, MLSSs, MLVSSs, and the SVI were measured using standard methods.21 The total nitrogen (TN) was analyzed using a TN analyzer (Multi N/C3000, Germany). The extraction and determination of EPS were performed using the method of Zhang et al.22 The extraction of PHAs was performed using the method described in Albuquerque et al.23 The PHA content was determined via gas chromatography, calculated using eqn (1) and (2). The determination method for the biofilm indices (biomass and density) comes from the literature;24 biomass and density were calculated using eqn (3) and (4). The contribution of SND to the TN removal rate was calculated using eqn (5). The nitrite accumulation rate was calculated using eqn (6). Eqn (7) shows the reduction process of NO2−–N. DNA extraction and Illumina high-throughput sequencing analysis of the SBBR and seed-sludge were performed by Novogene Co., Ltd (Beijing, China).
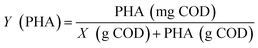 | (1) |
| X = DW (g COD) − PHA (g COD) | (2) |
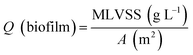 | (3) |
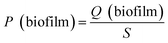 | (4) |
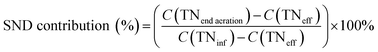 | (5) |
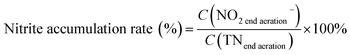 | (6) |
| NO2− + 0.67CH3OH + 0.53H2CO3 → 0.04C5H7O2N + 0.48N2↑ + 1.23H2O + HCO3− | (7) |
2.5 Statistical analysis
All statistical analyses were performed using the statistical program SPSS 19.0 (SPSS, Chicago, USA). Three-sample t-tests were used to evaluate the significance difference for each group. The data are all averages of two or more replicates. The results were analyzed to be statistically significant when p < 0.05 (significance above 95%).3. Results and discussion
3.1 Variation of biofilm indices
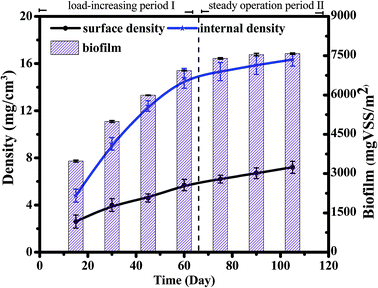
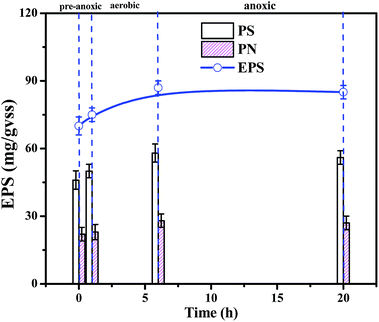
The biomass, density and EPS concentration are important biofilm indices, and the stability of the biofilm determines the stability of the SBBR system. The overall SBBR operation period was divided into the load-increasing period I (69 days) and the steady operation period II (41 days). As shown in Fig. 2, the biomass and density increased rapidly in period I and then gradually stabilized in period II as the influent load increased. The density of the internal and surface biofilm layers increased from 5 ± 0.5 to 16 ± 1 mg cm−3 and from 3 ± 0.5 to 7 ± 1 mg cm−3, respectively, which demonstrates that the density increases significantly with depth. The biomass increased with the increase in density; biomass increased from 3026 ± 20 to 7504 ± 30 VSS/m2.25EPS consists of polysaccharides (PS), proteins (PN), and nucleic acids.26 As shown in Fig. 3, the EPS content is basically maintained at 70–80 mg/VSS during typical cycles of the stability period II, indicating that the biofilm system is stable and that EPS forms and stabilizes the biofilm structure together with filler, sludge, and biofilm.27 And polysaccharides (PSs) were the main EPS component (60%) in the SBBR system. The stability of the biomass, density and EPS concentration during period II indicated that this operational set-up of the SBBR system is stable.
3.2 Treatment performance
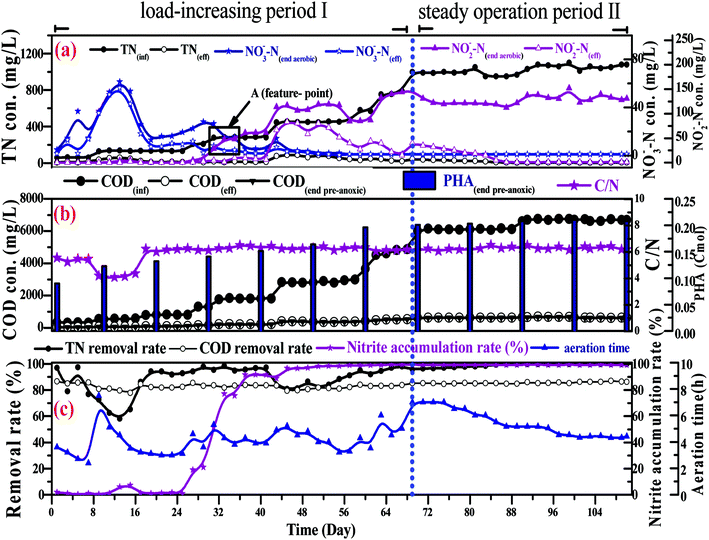
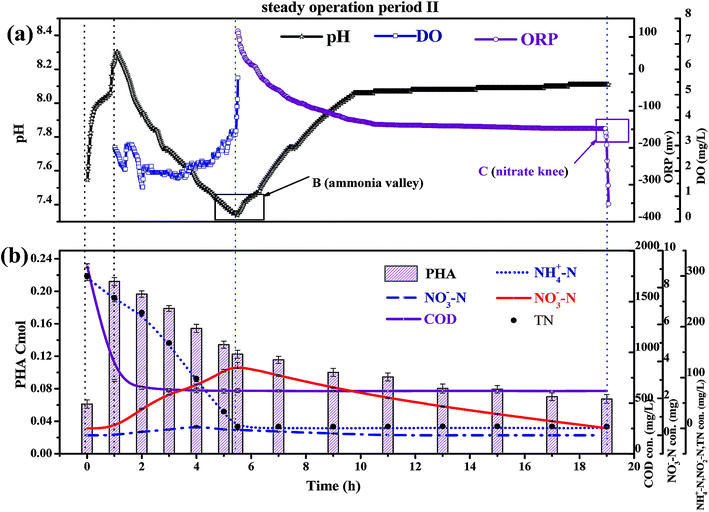
In a conventional SBBR, the removal of organic matter depends on the oxidation of aerobic heterotrophic bacteria in the aerobic phase; aerobic heterotrophic bacteria compete for oxygen (O2) more than nitrifying bacteria, and the nitrification reaction begins after the organic matter is removed.28 As shown in Fig. 5b, the little variation between the CODeff and the end of pre-anoxic phase COD concentrations (CODend pre-anoxic) explained that the removal of organic matter mainly occurred in the pre-anoxic phases. There is no biodegradable organic matter present in the aeration phase; aerobic heterotrophic bacteria are gradually eliminated and nitrifying bacteria become the dominant bacteria. Therefore, the addition of pre-anoxic phases could not only remove organic matter, but could also promote the rapid occurrence of the nitrification reaction.
As shown in Fig. 5b, the PHA content increased with decreasing CODinf in the pre-anoxic phase, which indicated that biofilm converted organic matter into PHAs. The PHA content (start of the pre-anoxic phase) increased from 0.065 ± 0.001 Cmol to a maximum content (end of the pre-anoxic phase) of 0.223 ± 0.005 Cmol, then decreased sharply to 0.125 ± 0.005 Cmol (end of the aeration phase), and slowly reduced to 0.065 ± 0.005 Cmol (end of the anoxic phase). The NO2−–N concentration decreased as the PHA content decreased synchronously in the aeration and anoxic phases, which indicates that the removal of NO2−–N requires the consumption of PHAs. The NH4+–N concentration was not obviously increased during the aeration and anoxic phases, which explains that the biofilm used PHAs for the removal of NO2−–N, rather than carbon sources from cell lyses. The synthesis of PHAs occurs in the pre-anoxic phase, and the consumption of PHAs occurs in the aeration and anoxic phases, indicating that biofilm converts organic matter into intracellular PHAs in the pre-anoxic phase and consumes PHAs for the removal of NO2−–N in the aeration phase and the anoxic phase.
Fig. 4b shows that the end of pre-anoxic phase PHA content (PHA end pre-anoxic) increased from 0.1 Cmol to 0.22 Cmol, as the improvement in CODinf demonstrated that the PHA content threshold in the microbial cells can be increased. A theoretical COD conversion value of 1 Cmol PHA is approximately 36 g COD, so the theoretical COD conversion value of PHA end pre-anoxic (0.22 Cmol) is 5.38 g COD; the reduction value of COD was 6.0 ± 0.3 g COD, so the theoretical COD conversion value was less than the reduction value of COD, which suggests that part of the organic matter was converted to PHAs and part of it was used for metabolism or other aspects.29,30
Fig. 5b shows that the NO2−–N and NO3−–N concentrations quickly increase as the NH4+–N concentration decreases during the whole aeration phase. The nitrification reaction becomes the dominant aerobic reaction in the aeration phase and aerobic heterotrophic bacteria were gradually eliminated due to a lack of organic matter; this result is consistent with the conclusions when discussing carbon transformation. With the increase in the influent load, the end of aeration phase NO2−–N concentration (NO2−–Naeration) started to exceed the end of aeration phase NO3−–N concentration (NO3−–Naeration) at point A (Fig. 4a), then the NO3−–Naeration concentration gradually decreased below 1 mg L−1 and the nitrite accumulation rate increased to over 98%. It is indicated that the SBBR system realizes the short-range nitrification reaction in the aeration phase. The achievement of the short-range nitrification reaction depends on the influent load; a high influent load has high free ammonia (FA) and leachate toxicity concentrations. High FA and leachate toxicity concentrations can inhibit the activity of ammonia-oxidizing bacteria (AOB) and nitrite-oxidizing bacteria (NOB), but the inhibition thresholds of AOB (10–150 mg L−1) were smaller than those of NOB (0.1–1.0 mg L−1), and AOB shows stronger adaptability and resistance than NOB.31 In this study, NOB was gradually eliminated and AOB eventually became the dominant nitrobacteria, resulting in the short-cut nitrification reaction becoming the dominant nitrification reaction, with the product after the end of the aeration phase being basically all NO2−–N. The short-cut nitrification reaction can increase the TN removal rate through the following properties: (1) economizing carbon source and oxygen consumption; (2) reducing the aeration time; (3) reducing alkali consumption; and (4) increasing the PHA content of the initial anoxic phase.
Biofilm has a large amount of PHAs at the beginning of the aeration phase; the PHAs could be used as a carbon source for simultaneous nitrification and denitrification (SND) to remove NO2−–N. As shown in Fig. 5b, the theoretical NO2−–N value produced by nitrification is 3 ± 0.3 g N, but the actual end of aeration phase NO2−–N value (in the range of 1.3 to 1.5 g N) is less than the theoretical value; the PHA content is reduced from the aeration stage, indicating that some NO2−–N is reduced through SND. SND only exist in an anaerobic area: a traditional activated sludge process achieves SND through controlling the DO concentration in the range of 0.5 to 1 mg L−1. However, this SBBR system could achieve SND when the DO concentration was controlled in the range of 2–3 mg L−1, which was due to the presence of anaerobic zones in the biofilm inner layer. SND could increase the TN removal rate through the following properties: (1) alkalis produced by SND made up for alkalis consumed by short-cut nitrification, which maintained a stable pH range; (2) the reduced inhibition of short-range nitrification by free nitrite; and (3) enhanced nitrification reaction rates.
The concentration of NO2−–N decreased as the PHA content decreased during the anoxic phase, which indicated that endogenous denitrification used PHAs as a carbon source for nitrogen removal. Van Aalst et al. showed that cells preferentially used PHAs as a metabolic carbon source rather than a denitrification carbon source when the PHA content was at a critical level,32 suggesting that the PHA content determined the contribution of endogenous denitrification to the TN removal rate. Given that: (1) the NO2−–N reduction value was 3 ± 0.3 g N; (2) each reduction of 1 g of NO2−–N could consume 1.71 g COD, theoretically (eqn (7)); (3) the theoretical COD value (COD-1) for the reduction of NO2−–N was 5.13 g COD; (4) the PHApre-anoxic content was above 0.2 Cmol; (5) the theoretical COD value corresponding to the PHA content was approximately 7.2 g COD (COD-2); and (6) COD-2 is more than COD-1, the PHA content was sufficient to achieve the reduction of NO2−–N. In other words, the biofilm has sufficient PHA content to act as a carbon source for SND and endogenous denitrification to remove TN. Endogenous denitrification could use PHAs as a carbon source for nitrogen removal to guarantee a lower effluent TN concentration.
The mechanisms for carbon transformation and nitrogen removal during landfill leachate treatment are: (1) biofilm converts influent organic matter into intracellular PHAs during the pre-anoxic phase; (2) the realization of short-range nitrification saves carbon sources and aeration time during the aeration phase; (3) the synergistic effects of SND and short-range nitrification promote the nitrification reaction rate and maintain a constant pH; and (4) SND and endogenous denitrification use PHAs as a carbon source to remove nitrogen.
3.3 diversity of microbial communities
Statistics showing the alpha diversity of the SBBR and seed-sludge at a 97% consistency threshold are shown in Table 3. The coverage index indicates the good coverage of the sequencing. The community composition, species richness, and diversity of the seed-sludge were higher than those of the SBBR. At the phylum level for the SBBR, as shown in Fig. 6a, Proteobacteria (59.38%) and Bacteroidetes (30.28%) were the dominant communities, accounting for 89.66% of the total bacteria. The abundance of Bacteroides in the SBBR was higher than that in the seed sludge, and this may be related to the quality of the landfill leachate.33 At the genera level for the SBBR, as shown in Fig. 6b, the top four genera (with relative abundances higher than 1%) were Paracoccus (28.60%), Thauera (10.34%), Nitrosomonas (5.89%) and Truepera (3.42%), accounting for 48.25% of the total bacteria.| Group | Observed species | Shannon | Simpson | Chao | Coverage |
|---|---|---|---|---|---|
| SBBR | 572 | 4.80 | 0.889 | 655.59 | 0.998 |
| Seed-sludge | 1424 | 8.09 | 0.995 | 1506.28 | 0.997 |
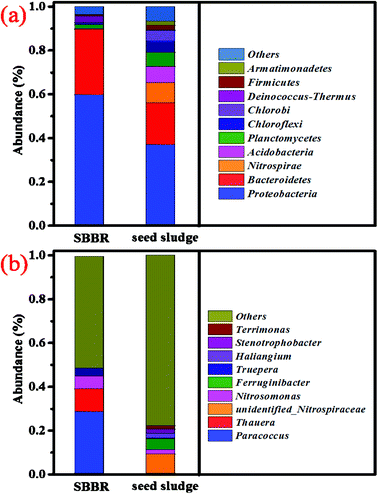 | ||
| Fig. 6 The microbial diversity of the top 10 examples at the phylum level (a); and the microbial diversity of the top 9 examples at the genus level (b). | ||
In the nitrobacteria community, unidentified-Nitrospiraceae (NOB) presented in the seed sludge finally disappears; Nitrosomonas (AOB) becomes the dominant nitrobacteria and Nitrosomonas europaea (AOB, 5.57%) becomes the dominant species with the most abundance. This explains that the short-cut nitrification reaction is the dominant nitrification reaction, and this result is consistent with the conclusions made when discussing nitrogen removal. Paracoccus, Thauera, and Truepera belong to the denitrifying bacteria community, and account for 42.36% of the total bacteria. Paracoccus34 and Thauera35 have the ability to transform organic matter into PHAs, accounting for 38.94% of the total bacteria. Thauera and Truepera can degrade bio-refractory organic matter and resist harsh environments.36 This shows that ammonia-oxidizing bacteria and denitrifying bacteria with the ability to transform organic matter into PHAs become the dominant bacterial communities.
4. Conclusions
A modified SBBR, with the addition of a pre-anoxic phase before the aeration phase in a conventional SBBR, was used to treat landfill leachate. The system achieves stable operation with 110 days of operation, and COD and TN removal rates were 83–88% and 95–98%. In the pre-anoxic phase, biofilm converts organic matter into intracellular PHAs. In the aeration phase, short-range nitrification removes NH4+–N to produce NO2−–N, and SND uses PHAs as a carbon source to remove NO2−–N. In the anoxic phase, endogenous denitrification uses PHAs as a carbon source to remove residual NO2−–N. Ammonia-oxidizing bacteria and denitrifying bacteria with the ability to transform organic matter into PHAs become the dominant bacterial communities.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Shandong Provincial Natural Science Foundation, China (ZR2017BEE067), the Science and Technology Planning of the Ministry of Housing and Urban-Rural Development of the People’s Republic of China (UDC2017031712), the National Key Research and Development Program of China (2017YFF0209903), the Shandong Provincial Natural Science Foundation, China (ZR2018BEE036), the National Natural Science Foundation of China (21307078), and the Natural Science Foundation of Shandong Province (ZR2016DB13).References
- G. Insel, M. Dagdar, S. Dogruel, N. Dizge, C. E. Ubay and B. Keskinler, Biodegradation characteristics and size fractionation of landfill leachate for integrated membrane treatment, J. Hazard. Mater., 2013, 260, 825–832 CrossRef CAS PubMed.
- W. G. Moravia, M. C. S. Amaral and L. C. Lange, Evaluation of landfill leachate treatment by advanced oxidative process by fenton's reagent combined with membrane separation system, Waste Manag., 2013, 331, 89–101 CrossRef PubMed.
- C. Amor, T. E. De, J. A. Peres, M. I. Maldonado, I. Oller and S. Malato, Mature landfill leachate treatment by coagulation/flocculation combined with fenton and solar photo-fenton processes, J. Hazard. Mater., 2015, 286, 261–268 CrossRef CAS PubMed.
- R. Zhao, A. Gupta, J. T. Novak, C. D. Goldsmith and N. Driskill, Characterization and treatment of organic constituents in landfill leachates that influence the UV disinfection in the publicly owned treatment works (POTWs), J. Hazard. Mater., 2013, 258, 1–9 Search PubMed.
- H. Liang, Z. Guangming, C. Guiqiu, D. Haoran and L. Yutang, Treatment of landfill leachate using immobilized Phanerochaete chrysosporium loaded with nitrogen-doped TiO2 nanoparticles, J. Hazard. Mater., 2016, 301, 106–118 CrossRef PubMed.
- P. Asaithambi, B. Sajjadi, A. R. A. Aziz and M. A. B. W. D. Wan, Ozone (O3) and sono (US) based advanced oxidation processes for the removal of color, COD and determination of electrical energy from landfill leachate, Sep. Purif. Technol., 2017, 172, 442–449 CrossRef CAS.
- W. Yin, C. Zhao, J. Xu, J. Zhang, Z. Guo and Y. Shao, Removal of Cd(II) and Ni(II) from aqueous solutions using activated carbon developed from powder-hydrolyzed-feathers and Trapa natans husks, Colloids Surf., A, 2019, 560, 426–433 CrossRef CAS.
- J. Liu, et al., Influence of reflux ratio on two-stage anoxic/oxic with MBR for leachate treatment: Performance and microbial community structure, Bioresour. Technol., 2018, 256, 69–76 CrossRef CAS PubMed.
- J. Liu, et al., Denitrification of landfill leachate under different hydraulic retention time in a two-stage anoxic/oxic combined membrane bioreactor process: performances and bacterial community, Bioresour. Technol., 2018, 250, 110–116 CrossRef CAS PubMed.
- O. N. Agdag and D. T. Sponza, Anaerobic/aerobic treatment of municipal landfill leachate in sequential two-stage up-flow anaerobic sludge blanket reactor (UASB)/completely stirred tank reactor (CSTR) systems, Process Biochem., 2005, 40, 895–902 CrossRef CAS.
- R. Zaloum and M. Abbott, Anaerobic pretreatment improves single sequencing batch reactor treatment of landfill leachates, Water Sci. Technol., 1997, 35, 207–214 CrossRef CAS.
- C. Krishna and M. C. M. V. Loosdrecht, Effect of temperature on storage polymers and settleability of activated sludge, Water Res., 1999, 33, 2374–2382 CrossRef CAS.
- H. Takabatake, H. Satoh, T. Mino and T. Matsuo, Recovery of biodegradable plastics from activated sludge process, Water Sci. Technol., 2000, 42(3–4), 351–356 CrossRef CAS.
- J. J. Beun, F. Paletta, M. C. Van Loosdrecht and J. J. Heijnen, Stoichiometry and kinetics of poly-beta-hydroxybutyrate metabolism in aerobic, slow growing, activated sludge cultures, Biotechnol. Bioeng., 2000, 67, 379 CrossRef CAS PubMed.
- R. Zhu, S. Wang, J. Li, K. Wang, L. Miao and B. Ma, Biological nitrogen removal from landfill leachate using anaerobic-aerobic process: denitritation via organic matter in raw leachate and intracellular storage polymers of microorganisms, Bioresour. Technol., 2013, 128, 401 CrossRef CAS PubMed.
- Y. C. Chiu, L. L. Lee, C. N. Chang and A. C. Chao, Control of carbon and ammonium ratio for simultaneous nitrification and denitrification in a sequencing batch bioreactor, Int. Biodeterior. Biodegrad., 2007, 59, 1–7 CrossRef CAS.
- J. Yin, et al., Simultaneous biological nitrogen and phosphorus removal with a sequencing batch reactor-biofilm system, Int. Biodeterior. Biodegrad., 2015, 103, 221–226 CrossRef CAS.
- K. A. Third, N. Burnett and R. Cord-Ruwisch, Simultaneous nitrification and denitrification using stored substrate (PHB) as the electron donor in an SBR, Biotechnol. Bioeng., 2003, 83, 706 CrossRef CAS PubMed.
- J. Li, Y. Peng and G. Gu, Factors Affecting Simultaneous Nitrification and Denitrification in an SBBR Treating Domestic Wastewater, Front. Environ. Sci. Eng. China, 2007, 1(2), 246–250 CrossRef.
- C. Wu, Z. Chen, X. Liu and Y. Peng, Nitrification-denitrification via nitrite in sbr using real-time control strategy when treating domestic wastewater, Biochem. Eng. J., 2007, 36, 87–92 CrossRef CAS.
- APHA, Standard Methods for the Examination of Water and Wastewater, American Public Health Association, New York, 1995 Search PubMed.
- X. Zhang and P. L. Bishop, Spatial distribution of extracellular polymeric substances in biofilms, J. Environ. Eng., 2001, 127, 850–856 CrossRef CAS.
- M. G. Albuquerque, M. Eiroa, C. Torres, B. R. Nunes and M. A. Reis, Strategies for the development of a side stream process for polyhydroxyalkanoate (PHA) production from sugar cane molasses, J. Biotechnol., 2007, 130, 411–421 CrossRef CAS PubMed.
- A. Shrestha, R. Riffat and C. Bott, Denitrification Stoichiometry and Kinetics of Moving Bed Biofilm Reactor, Proceedings of the Water Environment Federation, 2009, 4, 153–165 CrossRef.
- A. Eldyasti, G. Nakhla and J. Zhu, Influence of particles properties on biofilm structure and energy consumption in denitrifying fluidized bed bioreactors (DFBBRs), Bioresour. Technol., 2012, 126, 162 CrossRef CAS PubMed.
- R. Aryal, J. Lebegue, S. Vigneswaran, J. Kandasamy and A. Grasmick, Identification and characterisation of biofilm formed on membrane bio-reactor, Sep. Purif. Technol., 2009, 67, 86–94 CrossRef CAS.
- V. Lazarova and J. Manem, Biofilm characterization and activity analysis in water and wastewater treatment, Water Res., 1995, 29, 2227–2245 CrossRef CAS.
- R. Nogueira, L. F. Melo, U. Purkhold, S. Wuertz and M. Wagner, Nitrifying and heterotrophic population dynamics in biofilm reactors: effects of hydraulic retention time and the presence of organic carbon, Water Res., 2002, 36, 469–481 CrossRef CAS PubMed.
- Q. Wen, Z. Chen, C. Wang and N. Ren, Bulking sludge for pha production:energy saving and comparative storage capacity with well-settled sludge, J. Environ. Manage., 2012, 24, 1744–1752 CAS.
- J. J. Beun, K. Dircks, M. C. M. V. Loosdrecht and J. J. Heijnen, Poly-β-hydroxybutyrate metabolism in dynamically fed mixed microbial cultures, Water Res., 2002, 36, 1167–1180 CrossRef CAS PubMed.
- C. Mota, M. A. Head, J. A. Ridenoure, J. J. Cheng and F. L. D. L. Reyes, Effects of aeration cycles on nitrifying bacterial populations and nitrogen removal in intermittently aerated reactors, Appl. Environ. Microbiol., 2005, 71, 8565–8572 CrossRef CAS PubMed.
- M. A. Van Aalst-van Leeuwen, M. A. Pot, M. C. M. van Loosdrecht and J. J. Heijnen, Kinetic modeling of poly (β-hydroxybutyrate) production and consumption by paracoccus pantotrophus under dynamic substrate supply, Biotechnol. Bioeng., 1997, 55, 773–782 CrossRef CAS PubMed.
- T. Zhang, M. F. Shao and L. Ye, 454 Pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants, ISME J., 2012, 6, 1137–1147 CrossRef CAS PubMed.
- S. S. Sawant, B. K. Salunke and B. S. Kim, Degradation of corn stover by fungal cellulase cocktail for production of polyhydroxyalkanoates by moderate halophile Paracoccus sp. LL1, Bioresour. Technol., 2015, 194, 247–255 CrossRef CAS PubMed.
- P. C. Lemos, C. Levantesi, L. S. Serafim, S. Rossetti, M. A. Reis and V. Tandoi, Microbial characterisation of polyhydroxyalkanoates storing populations selected under different operating conditions using a cell-sorting RT-PCR approach, Appl. Microbiol. Biotechnol., 2008, 78, 351 CrossRef CAS PubMed.
- B. Liang, L. Y. Wang, S. M. Mbadinga, J. F. Liu, S. Z. Yang and J. D. Gu, et al., Anaerolineaceae, and Methanosaeta, turned to be the dominant microorganisms in alkanes-dependent methanogenic culture after long-term of incubation, AMB Express, 2015, 5, 37 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |