Open Access Article
Open Access ArticlePhytotoxicity and anti-phytopathogenic activities of marine-derived fungi and their secondary metabolites†
Rui-Huan Huang‡
a,
Jian-Yu Gou‡b,
Dong-Lin Zhao *a,
Dan Wanga,
Jing Liub,
Guo-Yong Mab,
Yi-Qiang Lia and
Cheng-Sheng Zhang*a
*a,
Dan Wanga,
Jing Liub,
Guo-Yong Mab,
Yi-Qiang Lia and
Cheng-Sheng Zhang*a
aMarine Agriculture Research Center, Tobacco Research Institute of Chinese Academy of Agricultural Sciences, Qingdao 266101, China. E-mail: zhaodonglin@caas.cn; zhchengsheng@126.com; Tel: +86-532-8870-2115
bZunyi Branch, Guizhou Tobacco Company, Zunyi 563000, China
First published on 8th November 2018
Abstract
To find new pesticides for agricultural use, 133 fungal strains were isolated from coastal marine habitats, from which 37 independent isolates were identified, belonging to 20 genera in nine orders, and the diversity of the isolated fungi were discussed. The phytotoxicity and anti-phytopathogenic fungal and bacterial activities of these 37 extracts, and two previously isolated fungal extracts were evaluated, displaying different levels of bioactivity. Based on the bioactive and chemical screening, an Alternaria sp. (P8) strain, which showed prominent bioactivity and contained abundant secondary metabolites was selected for further chemical investigation; one new compound, a benzopyranone (1), and seven known compounds (2–8) were obtained. Their structures were determined by analysing extensive NMR spectroscopic data and ECD comparisons. Compounds 1, 2, and 6–8 showed obvious phytotoxicity, especially against amaranth, and compound 1 also showed potent antifungal activity toward Alternaria brassicicola. To the best of our knowledge, this is the first report of the phytotoxicity of marine-derived fungi and their secondary metabolites. These studies should provide the foundation for future research into the use of such fungal extracts to combat weeds and diseases in agriculture.
1 Introduction
It has been estimated that at least 10% of global food production is lost to plant diseases; among these, weeds, plant pathogenic fungi, and bacteria are three major pests causing drastic loss in yield.1 Conventional chemicals have brought many benefits to mankind in the agricultural area, but their toxicity to both humans and animals has always been a concern.2 Therefore, new pesticides with high efficacy and safety need to be discovered and developed to replace the conventional ones.3Natural products (NPs) play an important role in the search for new pesticides. According to the literature, NPs accounted for the majority (35.7%) of new active ingredient registrations with the Environmental Protection Agency (EPA) from 1997 to 2010.4 As a major component of NPs, microbial-produced biopesticides have been very successful in the past, with such products as bialaphos, spinosyns, blasticidin, destruxins, bassianolide, and isarolides.5 However, after more than half a century of combinatorial chemistry research of terrestrial microorganisms, a declining number of new compounds have been found, which has prompted scientists to find new biopesticides from marine microorganisms. During the last two decades, marine-derived fungi have gained much attention among all marine organisms, as they can produce large numbers of novel biological compounds, including polyketides, meroterpenoids, terpenoids, peptides, alkaloids, and steroids, with antimicrobial, anticancer, antiviral, anti-inflammatory, antioxidant, and insecticidal activities.6 However, most of the bioactive compounds were utilized for medical, rather than agricultural applications. Hence, there is tremendous potential to find biopesticides from marine-derived fungi.
During our previous study of the agricultural applications of marine-derived fungi, 24 out of 31 fungal strains displayed obvious anti-phytopathogenic activities, and four bioactive compounds were isolated from two fungal strains.7 Due to the potential applications of marine-derived fungi for plant protection which few groups focused on, it is necessary to continue studying marine-derived fungi and their secondary metabolites that have agricultural bioactivities. In the present study, the isolation, identification, and bioactivity screening (phytotoxic and anti-phytopathogenic activities) of marine-derived fungi are reported, and the structure elucidation, as well as biological activities of isolated compounds from one selected strain are presented and discussed.
2 Results and discussion
2.1 Diversity of the isolated marine-derived fungi
Marine fungal strains have been obtained from nearly every possible marine habitat, including marine plants (most notably algae and mangrove plants), marine invertebrates (e.g., sponges, corals, ascidians, holothurians, bivalves, and crustaceans), vertebrates (mainly fish), and inorganic matter (sediments and sea water).8 In this study, a total of 133 fungal strains were isolated from marine plants (algae and mangroves), animals (fish, crab and starfish), sediments, and seawater, which were collected from two coastal marine habitats in Qingdao and Haikou, China, in May, 2017. Among these, 88 fungal strains were from Haikou and 45 were from Qingdao. The mangrove-derived fungi were the dominant group that we obtained (59 strains), accounting for 44% in this study, followed by 37 animal-derived (28%), 20 alga-derived (15%), 15 sediment-derived (11%), and two seawater-derived (2%) strains (Fig. S1†).It was reported that there were 530 species in 321 genera of filamentous marine fungi, among which 424 species were within Ascomycota (in 251 genera), 94 species were anamorphic fungi (in 61 genera), and 12 species were within Basidiomycota (in nine genera). These data apply to those marine taxa that can be isolated or cultivated by classical microbial techniques, and that have been studied in detail and taxonomically described by marine mycologists; the overall figures are expected to be much higher.8 Among our isolated marine-derived fungi, according to the morphotypes, strains of Penicillium sp. and Aspergillus sp. accounted for a large proportion of the 45 isolates from the Qingdao intertidal zone, while Trichoderma sp. strains seemed to be the predominant fungi of the 88 isolates from the Hainan coastal habitat. Redundant isolates were excluded by analysis of their morphological characteristics and 37 independent strains were selected for sequencing and identification according to ITS sequences. Based on the sequences deposited into the National Center for Biotechnology Information, 36 strains belonged to the phylum Ascomycota, including eight taxonomic orders: Botryosphaeriales, Diaporthales, Eurotiales, Glomerellales, Hypocreales, Mucorales, Pleosporales, and Xylariales (Fig. 1 and Table S1†). Particularly, one representative strain was grouped in the genus Poitrasia, of the order Mucorales and phylum Mucoromycota, which was the first reported Poitrasia sp. strain from marine environments. Isolates of Eurotiales (38%), Hypocreales (27%), and Pleosporales (16%) were dominant. According to the phylogenetic analysis, all identified fungi belonged to 20 genera, among which Penicillium sp. (five strains), Trichoderma sp. (four strains), Talaromyces sp. (four strains), Aspergillus sp. (three strains), and Fusarium sp. (three strains) were the dominant groups, accounting for 51.4% in this study; the remaining genera occurred as singletons or doubletons.
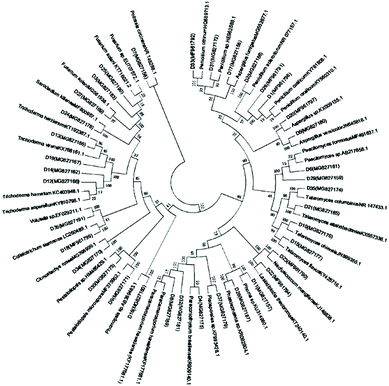 | ||
| Fig. 1 Neighbor-joining phylogenetic tree of 37 representative marine-derived fungi based on 18S rRNA gene sequences. | ||
2.2 Exploring for the bioactivities of marine-derived fungal extracts
In the present study, 37 newly isolated marine fungi and two fungal strains that we had previously obtained and showed to have potent agricultural biological activity, were tested for their phytotoxicity toward seedling growth and leaf health. The effects of the marine fungal extracts toward seedling growth of amaranth (Amaranthus retroflexus L.) and lettuce (Lactuca sativa) are shown in Table 1. Two fungal extracts (D2 and D3) significantly inhibited the seed germination of amaranth at 1.0 mg mL−1, while no significant difference was found between the control and most of the fungal extracts for the germination of lettuce seeds. However, despite germination not being inhibited noticeably, the marine fungal isolates showed excellent inhibition of the root and hypocotyl growth of the two tested plants. Fifteen fungal extracts displayed obvious phytotoxicity toward seedling growth, among which D2, D3, D12, D14, D22, and P8 were the most effective since they showed the strongest inhibition of the root or hypocotyl growth of at least one of the two tested plants. It was obvious that the effect of the marine fungal isolates on seedling growth was greater with amaranth than with lettuce, and the phytotoxicity was stronger toward root growth than toward hypocotyl growth. The genera Trichoderma was the most effective fungi as all three strains (D12, D13, and D14) showed potent phytotoxicity toward radicle and embryonal elongations. It is well-known that fungi such as Alternaria sp., Fusarium sp., and Colletotrichum sp. can produce phytotoxins that have considerable potential as models to develop herbicides with new modes of action unrelated to those in current use. Trichoderma spp. have been studied as biopesticides and biofertilizers due to their abilities to protect crops from plant pathogens and promote vegetative growth. However, products for weed control from Trichoderma spp. are commercially limited and the few known studies in this area are restricted to T. virens.11 Therefore, our report provides a new area for the application of Trichoderma sp. in agriculture.
| Strains | Root length (mm) | Hypocotyl length (mm) | ||
|---|---|---|---|---|
| Amaranth | Lettuce | Amaranth | Lettuce | |
| a “gp” was glyphosate. The length < 2.0 mm was regarded as no germination. “—” means no obvious effect on seedling growth. | ||||
| D1 | 6.47 ± 0.86 | 5.73 ± 0.70 | 4.70 ± 1.07 | — |
| D2 | 0.00 ± 0.00 | — | 0.00 ± 0.00 | — |
| D3 | 0.00 ± 0.00 | — | 0.00 ± 0.00 | — |
| D5 | 7.55 ± 1.24 | — | 4.15 ± 0.56 | — |
| D12 | 0.00 ± 0.00 | — | 5.70 ± 0.79 | — |
| D13 | 5.15 ± 0.34 | 6.65 ± 1.42 | — | — |
| D14 | 2.30 ± 0.27 | 2.40 ± 0.07 | 4.55 ± 1.28 | — |
| D20 | 5.45 ± 0.59 | — | — | — |
| D21 | 9.10 ± 1.00 | — | — | — |
| D22 | 3.20 ± 1.92 | — | 2.15 ± 1.46 | — |
| D25 | 4.40 ± 0.55 | — | 4.50 ± 1.42 | — |
| D35 | 9.25 ± 1.06 | — | — | — |
| D36 | 8.55 ± 1.86 | — | — | — |
| P8 | 7.55 ± 0.81 | 0.00 ± 0.00 | 0.00 ± 0.00 | — |
| P18 | 10.00 ± 1.17 | — | 5.20 ± 0.47 | — |
| gp | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.10 ± 0.10 | 4.40 ± 0.73 |
| ck | 22.05 ± 1.75 | 19.35 ± 1.12 | 9.45 ± 0.40 | 11.11 ± 2.39 |
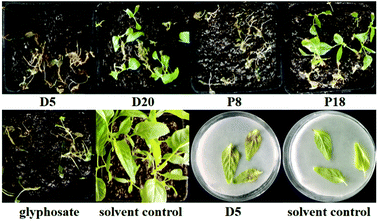
The leaf spray experiments were performed on amaranth and Chinese pennisetum (Pennisetum alopecuroides (L.) Spreng.) in pot assays. The development of necrotic lesions after 3–7 days of treatment with 39 marine fungal extracts indicated that 10 strains showed obvious phytotoxicity toward amaranth, while six ones were effective on Chinese pennisetum (Table 2). It is notable that D5 and P8 showed phytotoxicity toward amaranth leaves that was as strong as the positive control glyphosate, and D5 still showed phytotoxicity when its concentration was decreased to 1.0 mg mL−1 (Fig. 2). Of importance, the phytotoxicity manifested within three days, which was faster than that of glyphosate, indicating that these fungi could produce secondary metabolites with potent phytotoxicity. Amaranth was much more sensitive to the marine fungal extracts than was Chinese pennisetum, and the newer leaves were more sensitive than the older ones were. Further investigation on the phytotoxicity toward amaranth and Chinese pennisetum leaves was carried out by leaf puncture assays at a low concentration of 1.0 mg mL−1. Only one fungal isolate (D5) induced necrotic lesions on amaranth at 20 μg per droplet 3 days after its application, demonstrating great potential of being developed as a bioherbicide (Fig. 2).
| Strains | Amaranth | Chinese pennisetum |
|---|---|---|
| a 0 means no effect (control), 1 was inhibition rate <50%, 2 was 50% < inhibition rate < 70%, 3 was 70% < inhibition rate < 90%, and 4 was inhibition rate >90%. | ||
| D1 | 2 | 0 |
| D3 | 1 | 0 |
| D5 | 4 | 0 |
| D6 | 1 | 2 |
| D7 | 1 | 1 |
| D10 | 2 | 1 |
| D13 | 1 | 0 |
| D17 | 0 | 1 |
| D20 | 3 | 3 |
| P8 | 4 | 3 |
| P18 | 3 | 0 |
| gp | 4 | 3 |
From the results above, it was obvious that seven fungal strains (D1, D3, D5, D13, D20, P8, and P18) showed phytotoxicity against not only seedling growth, but also leaf health. To the best of our knowledge, we are exploring the phytotoxicity of marine-derived fungi and the genera Poitrasia, Talaromyces, and Volutella for the first time.
2.3 Investigation of the bioactive compounds from Alternaria sp. P8
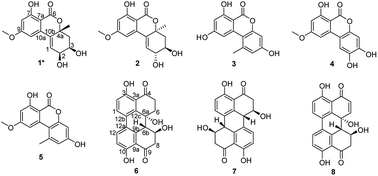
We previously reported the antibacterial activity of a marine-derived fungus Alternaria sp. P8 and its two bioactive secondary metabolites.7 In the present study, the potent phytotoxicity and antifungal activity of this fungus were also discovered. This suggests a broad agricultural bioactivity spectrum of P8, which warranted further investigation. Fifty Erlenmeyer flasks of fungi were cultivated in solid medium. Chemical investigations of the P8 extracts led to the isolation of one new benzopyranone, (+)-(2S,3R,4aR)-altenuene (1), and seven known compounds, (+)-isoaltenuene (2),14 alternariol (3),15 altenuisol (4),16 alternariol 9-methyl ether (5),15 altertoxin I (6),17 stemphyperylenol (7),7 and alterperylenol (8) (Fig. 5).7Compound 1 was isolated as a white, amorphous powder and assigned the molecular formula C15H16O6 by HRESIMS, indicating seven degrees of unsaturation. The 1H NMR spectrum displayed signals for three aromatic or olefinic protons at δH 6.75 (d, J = 2.0 Hz), 6.50 (d, J = 2.0 Hz), and 6.30 (d, J = 3.5 Hz); two oxymethines at δH 3.95 (t, J = 3.5 Hz) and 3.70 (dt, J = 7.5, 3.5 Hz); one methoxy group at δH 3.86 (s); one set of nonequivalent methylene protons at δH 2.26 (dd, J = 14.0, 3.5 Hz) and 1.95 (dd, J = 14.0, 7.5 Hz); and one methyl group at δH 1.47 (s). The 13C NMR and distortion less enhancement by polarization transfer (DEPT) spectra showed resonances for one ester carbonyl (δC 168.2), eight aromatic or olefinic carbon atoms (δC 165.8, 163.0, 139.2, 131.8, 131.0, 102.3, 100.9, and 100.0), one sp3 oxygenated quaternary carbon (δC 81.2), two oxymethines (δC 69.5 and 68.8), one methoxyl group (δC 55.8), one methylene (δC 38.5) and one methyl group (δC 27.4). These spectroscopic features, in addition to the 2D NMR data, suggested that 1 belonged to the benzopyranone family and is very similar to altenuene, which was isolated from an endolichenic fungal strain, Nigrospora sphaerica (No. 83-1-1-2), cultured from tissues of the lichen, Parmelinella wallichiana (Taylor) Elix & Hale collected from the Zixi Mountains of the Yunnan Province of China.14
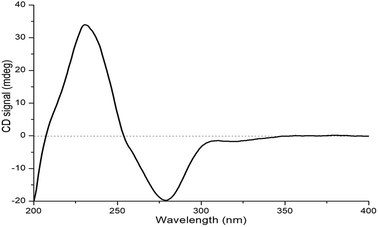
The relative configuration of 1 was deduced by J-based configurational analysis and 1D nuclear Overhauser effect (NOE) correlations. The 1H–1H coupling constants of H-2 (δH 3.95, t, J = 3.5 Hz) and H-3 (δH 3.69, dt, J = 7.5, 3.5 Hz) indicated a cis-relationship of these two protons. The signal for Ha-4 (δH 1.95) showed one vicinal coupling constant (J = 7.5 Hz) characteristic of axial-axial-type coupling, leading to the assignment of an axial orientation for Ha-4 and H-3. In the NOE difference spectrum, irradiation of 4a-CH3 enhanced the resonance of Ha-4, indicating a cis relationship between them. This evidence suggested that 4a-CH3 and H-2/H-3 were on the opposite face of the molecule. The absolute configuration of 1 was determined by ECD analysis. The negative first Cotton effect at 279 nm (Δε −41.95) and the positive second one at 232 nm (Δε +71.88) (Fig. 6) in the ECD spectrum indicated a 4aR-configuration.14 Thus, the absolute configuration of 1 was determined to be 2S,3R,4aR.
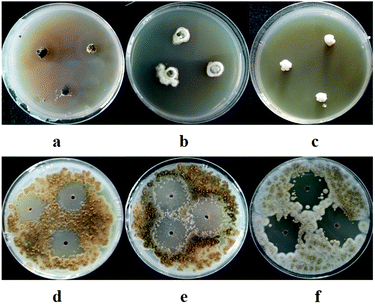
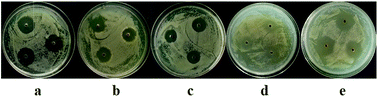
In our previous study, we found that compound 8 exhibited potent antibacterial activity against Clavibacter michiganensis and 7 displayed obvious antifungal activity against A. brassicicola and P. theae. In the present study, the phytotoxicity of 1–8, and the antifungal and antibacterial activities of 1–6 were determined. Compounds 1, 2, and 6–8 showed obvious phytotoxicity against the seedling growth of amaranth and lettuce at 200 ppm (Table 3). The perylenequinones (6–8) were more bioactive than the benzopyranones (1 and 2) were, since they could inhibit seed germination at 200 ppm and still display strong phytotoxicity when their concentration decreased to 50 ppm (Table 4). It seemed that the isolated compounds inhibited the seedling growth of lettuce less than that of amaranth, and their effects on root elongation were much more obvious than their effects on hypocotyl elongation. Because no phytotoxicity of 3–5 was observed, preliminary analysis of the structure–activity relationships of the benzopyranones (1–5) indicated that the replacement of benzene by cyclohexene could positively influence phytotoxicity. Compound 1 also showed potent antifungal activity toward A. brassicicola, with a minimum inhibitory concentration (MIC) of 125 μg mL−1, which was equivalent to that of the positive control carbendazim. Compound 6 displayed moderate antifungal activity against D. medusaea, with an MIC of 62.5 μg mL−1, compared to 31.3 μg mL−1 for carbendazim. Compounds 1–6 exhibited no obvious antibacterial activity.
| Strains | Root length (mm) | Hypocotyl length (mm) | ||
|---|---|---|---|---|
| Amaranth | Lettuce | Amaranth | Lettuce | |
| a Values means ± SD. The length < 2.0 mm was regarded as no germination. | ||||
| 1 | 4.65 ± 0.53 | 19.30 ± 3.06 | 4.96 ± 0.45 | 6.65 ± 1.14 |
| 2 | 4.30 ± 0.10 | 14.01 ± 1.77 | 7.42 ± 0.27 | 7.78 ± 0.87 |
| 6 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 7 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| 8 | 0.00 ± 0.00 | 4.45 ± 0.43 | 0.00 ± 0.00 | 4.60 ± 0.34 |
| gp | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| H2O | 12.08 ± 0.43 | 21.00 ± 1.07 | 7.34 ± 0.26 | 9.00 ± 0.54 |
| Strains | Root length (mm) | Hypocotyl length (mm) | ||
|---|---|---|---|---|
| Amaranth | Lettuce | Amaranth | Lettuce | |
| a Values means ± SD. The length < 2.0 mm was regarded as no germination. | ||||
| 6 | 0.00 ± 0.00 | 0.00 ± 0.00 | 4.15 ± 0.19 | 0.00 ± 0.00 |
| 7 | 0.00 ± 0.00 | 2.25 ± 0.26 | 4.90 ± 0.45 | 3.45 ± 0.26 |
| 8 | 0.00 ± 0.00 | 7.90 ± 0.41 | 6.00 ± 0.39 | 5.07 ± 0.21 |
| gp | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| H2O | 12.08 ± 0.43 | 21.00 ± 1.07 | 7.34 ± 0.26 | 9.00 ± 0.54 |
3 Experimental section
3.1 Fungal materials, isolation, identification, and fermentation
The marine-derived fungi in this study were isolated from seawater, sediments, animals, and algae collected from coastal marine habitats of the Yellow Sea, Qingdao, and South China Sea, Haikou, China, during May, 2017. These samples were immediately processed for isolation and purification of fungi, using potato dextrose agar (PDA) culture media with a salinity of 3%, according to the method we previously described.7 The isolated fungi were transferred into cryogenic vials containing potato dextrose water (PDW) culture media and glycerol (v/v = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), and were deposited at the Marine Agriculture Research Center, Tobacco Research Institute of Chinese Academy of Agricultural Sciences, Qingdao, China. The obtained fungi were identified according to morphological characteristics and a molecular protocol by amplification and DNA sequencing of the internal transcribed spacer (ITS) region of the rRNA gene, as well as phylogenetic analysis.
1), and were deposited at the Marine Agriculture Research Center, Tobacco Research Institute of Chinese Academy of Agricultural Sciences, Qingdao, China. The obtained fungi were identified according to morphological characteristics and a molecular protocol by amplification and DNA sequencing of the internal transcribed spacer (ITS) region of the rRNA gene, as well as phylogenetic analysis.
The separated marine-derived fungi were cultivated in PDW culture media with 3% salinity in three, 1 L Erlenmeyer flasks (each containing 400 mL of culture broth) at 28 °C without shaking for 40 days. The pooled cultures (1.2 L) were filtered to separate the broth from the mycelia. The mycelia were extracted three times with CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (1
MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), and the solution was concentrated under reduced pressure to afford a residue, which was extracted with EtOAc three times. Then, the broth was extracted three times with an equal volume of EtOAc, and the mycelia extracts were combined and concentrated under vacuum to afford a total extract.
1, v/v), and the solution was concentrated under reduced pressure to afford a residue, which was extracted with EtOAc three times. Then, the broth was extracted three times with an equal volume of EtOAc, and the mycelia extracts were combined and concentrated under vacuum to afford a total extract.
3.2 Bioassays of the marine-derived fungal extracts
In the seedling growth assays, seeds of two dicotyledons, amaranth and lettuce were used. The procedure was conducted according to a published protocol with slight modifications.18 The plant seeds were soaked in sterile water for 5 h and in 0.3% NaClO for 15 min, then were rinsed three times with sterile water. The extracts were dissolved in methanol to a final concentration of 1.0 mg mL−1, and 200 μL per well was added to 24-well plats containing filter paper at the bottom of the wells (Whatman no. 1). Glyphosate and methanol were used as a positive control and solvent control, respectively. After the methanol evaporated, five seeds were placed in each well of the 24-well plates, and 200 μL of sterile water per well was added. The plates were then incubated at 28 °C for 96 h with a cycle of 12 h light and 12 h dark. The lengths of the roots and hypocotyls were recorded.
The leaf spray assays were performed using the leaves of amaranth and Chinese pennisetum, according to a previously described method, with modifications.19 Plastic pots (10 cm diameter) were filled with nutrient soil bought from Shouguang Wode Nursery Substrate Co. Ltd., Weifang, China. Twenty seeds were planted in each pot, and after seeding, the pots were placed in trays without drainage holes and watered from the bottom. The plants were grown in an illuminating incubator at 28 °C with 12 h illumination and 12 h darkness. Fourteen-day-old amaranth (3–4 true leaf stage) and 7 day-old Chinese pennisetum (10–12 cm high) were used for experiments. The fungal extracts were dissolved in 4% MeOH with 0.25% surfactant (pesticide emulsifier 1602) to final concentrations of 10.0, 5.0, and 1.0 mg mL−1. Glyphosate and 4% MeOH were used as a positive control and solvent control, respectively. The solutions (5.0 mL) were sprayed on the plants and a qualitative estimation of phytotoxicity was obtained by using a visual rating scale from 0 to 4, where 0 was no effect (solvent control), 1 was an inhibition <50%, 2 was 50% < inhibition < 70%, 3 was 70% < inhibition < 90%, and 4 was inhibition >90%.
The leaf puncture assays were carried out using the leaves of amaranth and Chinese pennisetum by a previously described method.20 The fungal extracts were dissolved in 4% MeOH to a final concentration of 1.0 mg mL−1. A droplet (20 μL) of solution was applied to detached leaves previously punctured with a needle. Glyphosate and 4% MeOH were used as a positive control and solvent control, respectively. The leaves were kept in a moistened chamber under continuous fluorescent lighting. Symptoms were estimated visually 3–7 days after droplet application.
| Inhibition of growth (%) = [1 − (D − 4)/(Dck − 4)] × 100 |
The antifungal activity of the EtOAc extracts against the plant pathogenic fungi, A. alternata (Fries) Keissler, A. brassicicola, A. niger van. Tiegh, and P. theae, was also tested using an agar diffusion method, with modifications.22 Briefly, the spores of the fungi were adjusted to 1 × 105 CFU mL−1, and 100 μL suspensions were then spread on Petri dishes (diameter = 9 cm) containing 20 mL PDA media. Three wells (diameter = 6 mm each) were created in this pathogen-embedded agar with sterile glass tubes. The lyophilized EtOAc extracts were dissolved in DMSO to a final concentration of 10.0 mg mL−1 and 10 μL of the solutions was added to each well on the plates. Prochloraz and DMSO were employed as a positive control and solvent control, respectively. The Petri dishes were incubated at 28 °C for 72 h in darkness and the diameter of each zone of inhibition was measured.
3.3 Isolation and structure elucidation of compounds from Alternaria sp. P8
Optical rotations were measured on a Jasco P-1020 digital polarimeter (Jasco, Inc., Easton, MD, USA), and UV spectra were recorded on a Techcomp UV2310II spectrophotometer (Techcomp, Ltd., Shanghai, China). Electronic circular dichroism (ECD) spectra were obtained on a Jasco J-815-150S circular dichroism spectrometer (Jasco, Inc., Tokyo, Japan). NMR spectra were recorded on an Agilent DD2 500 MHz NMR spectrometer (500 MHz for 1H and 125 MHz for 13C; Agilent Technologies, Santa Clara, CA, USA) using tetramethylsilane as an internal standard. Electrospray ionization mass spectrometry (ESIMS) and high-resolution electrospray ionization mass spectrometry (HRESIMS) spectra were obtained with a Micromass Q-TOF spectrometer and Thermo Scientific LTQ Orbitrap XL spectrometer. Semi-preparative HPLC was performed on a Waters C18 (5 μm, 10 × 250 mm) column using a Waters 2695 separation module equipped with a Waters 2996 photodiode array (PDA) detector (Waters, MA, USA). Silica gel (200–300 mesh; Qing Dao Hai Yang Chemical Group Co., Qingdao, China), octadecylsilyl-silica gel (45–60 μm; Merck, Darmstadt, Germany), and Sephadex LH-20 (GE Healthcare, Uppsala, Sweden) were used for column chromatography. Compounds were monitored by thin layer chromatography (TLC) (G60, F-254; Yan Tai Zi Fu Chemical Group Co., Yantai, China), and spots were visualized by heating the silica gel plates after spraying with 12% H2SO4 in H2O containing saturated vanillins.The fungal strain Alternaria sp. P8 was fermented on rice media in 50 Erlenmeyer flasks (each containing 80 g rice and 120 mL H2O) at 28 °C for 40 days. The culture medium was extracted three times with CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (1
MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), and the pooled solutions were concentrated under reduced pressure to afford a residue, which was extracted with EtOAc three times. The pooled EtOAc extracts were concentrated under reduced pressure and subjected to vacuum liquid chromatography (VLC) on silica gel. An eluent gradient of EtOAc in petroleum ether (0/100 to 100/0, v/v) followed by MeOH in EtOAc (0–100%) was used to give five fractions (Fr. 1−Fr. 5). Fr. 3 was first subjected to silica gel column chromatography (mobile phase, 30% EtOAc in petroleum ether), and then separated by octadecyl-silica column, eluting with 30–60% MeOH in H2O, to obtain Fr. 3-1–Fr. 3-3. Fr. 3-2 was applied to Sephadex LH-20 column chromatography (mobile phase, CH2Cl2
1, v/v), and the pooled solutions were concentrated under reduced pressure to afford a residue, which was extracted with EtOAc three times. The pooled EtOAc extracts were concentrated under reduced pressure and subjected to vacuum liquid chromatography (VLC) on silica gel. An eluent gradient of EtOAc in petroleum ether (0/100 to 100/0, v/v) followed by MeOH in EtOAc (0–100%) was used to give five fractions (Fr. 1−Fr. 5). Fr. 3 was first subjected to silica gel column chromatography (mobile phase, 30% EtOAc in petroleum ether), and then separated by octadecyl-silica column, eluting with 30–60% MeOH in H2O, to obtain Fr. 3-1–Fr. 3-3. Fr. 3-2 was applied to Sephadex LH-20 column chromatography (mobile phase, CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 1
MeOH, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), followed by purification on HPLC with 60% MeOH to afford 5 (8.9 mg). Fr. 4 was fractionated on silica gel column chromatography using a gradient elution with petroleum ether
1, v/v), followed by purification on HPLC with 60% MeOH to afford 5 (8.9 mg). Fr. 4 was fractionated on silica gel column chromatography using a gradient elution with petroleum ether![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EtOAc to afford three subfractions (Fr. 4-1–Fr. 4-4). Fr. 4-3 was applied to an octadecyl-silica column, eluting with 50% MeOH and then was purified by Sephadex LH-20 column chromatography (mobile phase, CH2Cl2
EtOAc to afford three subfractions (Fr. 4-1–Fr. 4-4). Fr. 4-3 was applied to an octadecyl-silica column, eluting with 50% MeOH and then was purified by Sephadex LH-20 column chromatography (mobile phase, CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH, 1
MeOH, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to obtain three subfractions (Fr. 4-3-1–4-3-3). Fr. 4-3-2 was purified on HPLC with 25% MeCN in H2O to give 6 (15.3 mg), 7 (8.5 mg), and 8 (11.2 mg), while Fr. 4-3-3 was purified by semipreparative HPLC, eluting with 50% MeOH to give 3 (14.6 mg) and 4 (5.1 mg). Fr. 4-4 was purified on HPLC with 35% MeCN to give 1 (17.2 mg) and 2 (18.9 mg).
1, v/v) to obtain three subfractions (Fr. 4-3-1–4-3-3). Fr. 4-3-2 was purified on HPLC with 25% MeCN in H2O to give 6 (15.3 mg), 7 (8.5 mg), and 8 (11.2 mg), while Fr. 4-3-3 was purified by semipreparative HPLC, eluting with 50% MeOH to give 3 (14.6 mg) and 4 (5.1 mg). Fr. 4-4 was purified on HPLC with 35% MeCN to give 1 (17.2 mg) and 2 (18.9 mg).
(+)-(2S,3R,4aR)-Altenuene (1): white, amorphous powder; [α]20D −6.4° (c 0.10, MeOH); UV (MeOH) λmax (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ε) 240 (4.80), 278 (4.33), 319 (4.10) nm; ECD (0.14 mM, MeOH) λmax (Δε) 232 (+71.88), 279 (−41.95) nm; 1H NMR (DMSO-d6, 500 MHz) δ 11.30 (s, 7-OH), 6.75 (1H, d, J = 2.0 Hz, H-10), 6.54 (1H, d, J = 2.0 Hz, H-8), 6.30 (1H, d, J = 3.5 Hz, H-1), 3.95 (1H, t, J = 3.5 Hz, H-2), 3.86 (3H, s, 9-OCH3), 3.69 (1H, dt, J = 7.5, 3.5 Hz, H-3), 2.26 (1H, dd, J = 14.0, 3.5 Hz, He-4), 3.69 (1H, dt, J = 7.5, 3.5 Hz, H-3), 1.95 (1H, dd, J = 14.0, 7.5 Hz, Ha-4), 1.47 (1H, s, J = 14.0, 7.5 Hz, 4a-CH3); 13C NMR (DMSO-d6, 125 MHz) δ 168.2 (C, C-6), 165.8 (C, C-9), 163.0 (C, C-7), 139.2 (C, C-10a), 131.8 (C, C-10b), 131.0 (CH, C-1), 102.3 (CH, C-10), 100.9 (CH, C-8), 100.0 (C, C-7a), 81.2 (C, C-4a), 69.5 (CH, C-2), 68.8 (CH, C-3), 55.9 (CH3, 9-OCH3), 38.5 (CH2, C-4), 27.4 (CH3, 4a-CH3); HRESIMS m/z 291.0878 [M − H]− (calcd for C15H15O6, 291.0874).
ε) 240 (4.80), 278 (4.33), 319 (4.10) nm; ECD (0.14 mM, MeOH) λmax (Δε) 232 (+71.88), 279 (−41.95) nm; 1H NMR (DMSO-d6, 500 MHz) δ 11.30 (s, 7-OH), 6.75 (1H, d, J = 2.0 Hz, H-10), 6.54 (1H, d, J = 2.0 Hz, H-8), 6.30 (1H, d, J = 3.5 Hz, H-1), 3.95 (1H, t, J = 3.5 Hz, H-2), 3.86 (3H, s, 9-OCH3), 3.69 (1H, dt, J = 7.5, 3.5 Hz, H-3), 2.26 (1H, dd, J = 14.0, 3.5 Hz, He-4), 3.69 (1H, dt, J = 7.5, 3.5 Hz, H-3), 1.95 (1H, dd, J = 14.0, 7.5 Hz, Ha-4), 1.47 (1H, s, J = 14.0, 7.5 Hz, 4a-CH3); 13C NMR (DMSO-d6, 125 MHz) δ 168.2 (C, C-6), 165.8 (C, C-9), 163.0 (C, C-7), 139.2 (C, C-10a), 131.8 (C, C-10b), 131.0 (CH, C-1), 102.3 (CH, C-10), 100.9 (CH, C-8), 100.0 (C, C-7a), 81.2 (C, C-4a), 69.5 (CH, C-2), 68.8 (CH, C-3), 55.9 (CH3, 9-OCH3), 38.5 (CH2, C-4), 27.4 (CH3, 4a-CH3); HRESIMS m/z 291.0878 [M − H]− (calcd for C15H15O6, 291.0874).
3.4 Bioassays of compounds 1–8
The phytotoxicity of 1–8 against seed germination and seedling growth of amaranth and lettuce (200 and 50 ppm) was conducted as mentioned above. The antibacterial and antifungal activities were evaluated by a conventional broth-dilution assay according to the National Center for Clinical Laboratory Standards recommendations, with modifications as we previously reported.74 Conclusions
In summary, 37 marine-derived fungi were identified from 133 strains that were isolated from several marine biotopes. The diversity of the obtained fungi was investigated, leading to the discovery of the first marine-derived Poitrasia sp. strain. The phytotoxicity, as well as the anti-phytopathogenic fungal and bacterial activities of the fungal extracts were tested, and one strain, Alternaria sp. P8, was selected for chemical investigation according to its bioactivity. This resulted in the isolation of eight compounds, including one new compound, which exhibited potent phytotoxicity, antifungal activity, and antibacterial activity. The phytotoxicity of marine-derived fungi in this study is very interesting, since this is the first report, to the best of our knowledge, on the discovery and evaluation of the phytotoxic efficacy of marine-derived fungi. As few studies focus on agricultural bioactivities, our results suggest that exploring the application of marine-derived fungi in agriculture represents a promising strategy for discovering new biopesticides.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (41806194), the Fundamental Research Funds for Central Non-profit Scientific Institution (1610232017013), and the Science Foundation for Young Scholars of Tobacco Research Institute of Chinese Academy of Agricultural Sciences (2017B08).Notes and references
- C. Buttimer, O. Mcauliffe, R. P. Ross, C. Hill, J. O'Mahony and A. Coffey, Front. Microbiol., 2017, 8, 34 Search PubMed.
- S. Mostafalou and M. Abdollahi, Arch. Toxicol., 2016, 91, 1–51 Search PubMed.
- J. N. Seiber, J. Coats, S. O. Duke and A. D. Gross, J. Agric. Food Chem., 2014, 62, 11613–11619 CrossRef CAS PubMed.
- C. L. Cantrell, F. E. Dayan and S. O. Duke, J. Nat. Prod., 2012, 75, 1231–1242 CrossRef CAS PubMed.
- S. Saxena, Allelopathy J., 2014, 33, 1–24 Search PubMed.
- J. W. Blunt, A. R. Carroll, B. R. Copp, R. A. Davis, R. A. Keyzers and M. R. Prinsep, Nat. Prod. Rep., 2018, 35, 8–53 RSC.
- D. L. Zhao, D. Wang, X. Y. Tian, F. Cao, Y. Q. Li and C. S. Zhang, Mar. Drugs, 2018, 16, 36 CrossRef PubMed.
- M. E. Rateb and R. Ebel, Nat. Prod. Rep., 2011, 28, 290–344 RSC.
- M. Vurro, A. Boari, A. Evidente, A. Andolfi and N. Zermane, Pest Manage. Sci., 2010, 65, 566–571 CrossRef PubMed.
- J. F. Imhoff, Mar. Drugs, 2016, 14, 19 CrossRef PubMed.
- A. Javaid and S. Ali, Nat. Prod. Res., 2011, 25, 730–740 CrossRef CAS PubMed.
- S. Johanna, K. Annemarie, L. Antje and T. Deniz, Mar. Drugs, 2016, 14, 137 CrossRef PubMed.
- L. Xu, W. Meng, C. Cao, J. Wang, W. Shan and Q. Wang, Mar. Drugs, 2015, 13, 3479–3513 CrossRef CAS PubMed.
- J. W. He, G. D. Chen, H. Gao, F. Yang, X. X. Li, T. Peng, L. D. Guo and X. S. Yao, Fitoterapia, 2012, 83, 1087–1091 CrossRef CAS PubMed.
- W. Gu, World J. Microbiol. Biotechnol., 2009, 25, 1677–1683 CrossRef CAS.
- N. Kim, M. J. Sohn, H. Koshino, E. H. Kim and W. G. Kim, Bioorg. Med. Chem. Lett., 2014, 24, 83–86 CrossRef CAS PubMed.
- T. Okuno, I. Natsume, K. Sawai, K. Sawamura, A. Furusaki and T. Matsumoto, Tetrahedron Lett., 1983, 24, 5653–5656 CrossRef CAS.
- Q. Zhang, S. Q. Wang, H. Y. Tang, X. J. Li, L. Zhang, J. Xiao, Y. Q. Gao, A. L. Zhang and J. M. Gao, J. Agric. Food Chem., 2013, 61, 11447–11452 CrossRef CAS PubMed.
- M. G. Corral, J. Leroux, S. Tresch, T. Newton, K. A. Stubbs and J. S. Mylne, Pest Manage. Sci., 2018, 74, 1558–1563 CrossRef CAS PubMed.
- A. Cimmino, A. Andolfi, M. C. Zonno, C. Troise, A. Santini, A. Tuzi, M. Vurro, G. Ash and A. Evidente, J. Nat. Prod., 2012, 75, 1130–1137 CrossRef CAS PubMed.
- Q. Li, L. Qiu, W. Tan, G. Gu and Z. Guo, RSC Adv., 2017, 7, 42225–42232 RSC.
- C. H. Bock, D. I. Shapiro-Ilan, D. E. Wedge and C. L. Cantrell, J. Pest Sci., 2014, 87, 155–162 CrossRef.
- A. Zhu, M. Y. Yang, Y. H. Zhang, C. L. Shao, C. Y. Wang, L. D. Hu, F. Cao and H. J. Zhu, Sci. Rep., 2018, 8, 10621 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Table S1: Identification and phylogenetic affiliations of the isolated marine-derived fungal strains, Table S2: antifungal activity against spore germination of the marine-derived fungal extracts (10.0 mg mL−1), Table S3: antifungal activity against mycelial growth (10.0 mg mL−1) of plant pathogenic fungi, Table S4. Antibacterial activity of the marine-derived fungal extracts, Fig. S1: the sources of the isolate marine-derived fungal strains, Fig. S2–S8: 1H NMR, 13C NMR, COSY, HMQC, HMBC, 1D NOE, and HRESIMS spectra of compound 1. See DOI: 10.1039/c8ra08047j |
| ‡ Co-first authors. |
| This journal is © The Royal Society of Chemistry 2018 |