Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Dehydrogenative coupling of 4-substituted pyridines mediated by a zirconium(II) synthon: reaction pathways and dead ends†
Lukas S.
Merz
a,
Hubert
Wadepohl
a,
Eric
Clot
*b and
Lutz H.
Gade
 *a
*a
aAnorganisch Chemisches Institut, Universität Heidelberg, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany. E-mail: lutz.gade@uni-heidelberg.de
bInstitut Charles Gerhardt Montpellier, UMR 5253 CNRS-UM-ENSCM, Université de Montpellier, Place Eugène Bataillon, Bât 15, cc1501, 34095 Montpellier Cedex 5, France. E-mail: eric.clot@umontpellier.fr
First published on 16th May 2018
Abstract
The mechanism of the reductive homocoupling of pyridine derivatives mediated by the ZrII synthon [(PNP)Zr(η6-toluene)Cl] (1) has been investigated. Selective transformation into three different types of product complexes has been observed, depending on the N-heterocyclic substrate employed: the bipyridyl complexes 3-R (R = Me, Et, tBu, Bn, Ph, CHCHPh), which are the homocoupling products, the η2-((4-dimethylamino)pyridyl) complex 4 as well as the bis(isoquinolinyl) complex 5. By deuterium labelling experiments the participation of the ligand backbone in the pyridine coupling reaction via potential cyclometallation steps was ruled out. Based on DFT modelling of the possible reaction sequences a reaction mechanism for the coupling sequence could be identified. The latter is initiated by a reductive syn C–C coupling rather than based on an initial C–H activation of the pyridine substrate.
Introduction
Early transition metal complexes are known to induce characteristic types of C–H activations1–15 which complement the analogous reactivity patterns established for their d-electron rich late transition metal counterparts.16–25 Cyclometallations may be viewed as particularly favourable variations of this type of reactivity26–34 and in their simplest form give rise to three-membered metallacycles. In this context group 4 metal compounds were found to activate pyridine to give pyridyl complexes incorporating η2-N,C-metallazirine moieties.35–45 Generally, the formation of these η2-pyridyl ligands may occur in two ways: either an alkyl substituent in MIV alkyl complexes abstracts a proton from a coordinated pyridine molecule to generate the metallacycle33,38–43 or an MII species, commonly generated in situ from the corresponding MIV complexes, activates the pyridine molecule through oxidative addition with concomitant formation of an additional hydrido ligand.35,36,46 The former activation pattern was most recently utilized by Mindiola, Baik and coworkers in a detailed study which demonstrated that the transitory generation of a titanaazirine species from the benzopyridines, quinoline, and isoquinoline could result in the C–C coupling (as well as ring opening) of two substrate molecules.47It is notable that despite its broad application as a ligand in coordination chemistry,48–51 the generation of 2,2′-bipyridine directly from pyridine is still in its infancy especially since transition metal catalysed cross-coupling reactions generally require 2-halopyridines and 2-metallated pyridines.52 Without prior functionalization of the pyridine cycle, the direct synthesis of 2,2′-bipyridines is hitherto almost entirely restricted to the application of heterogeneous catalysts such as RANEY® nickel and Pd/C.53–56 To our knowledge, only three homogeneous catalytic systems, using di- or trinuclear Ru- or Ru/Co-complexes,57–59 have been reported that are capable of catalysing the homocoupling of pyridines. Furthermore, even examples for the stoichiometric transformation of pyridine to 2,2′-bipyridine are rare and have only been described for a limited number of substrates (cf.Chart 1).60–63 The reaction pathways involved remain incompletely understood.
Recently, we reported the synthesis of the Cbz(PNP)ZrCl(η6-arene) complex 1, in which the η6-toluene ligand displays a puckered arrangement of the arene ring similar to other early TM arene complexes.64–68 This indicates a significant arene-1,4-diido character of the tolyl ring, and the zirconium atom in this complex is therefore to be assigned to the oxidation state +4.
However, complex 1 can be regarded as a ZrII synthon, since the displacement of a neutral toluene molecule would provide access to a transient ZrII species. This could be demonstrated inter alia by the reductive coupling of pyridine to form a dianionic bipyridyl ligand coordinated to the zirconium centre (Scheme 1).69 The latter raised the question about the scope of this transformation, on the one hand, and the reaction mechanism which leads to this dehydrogenative C–C coupling of two N-heterocycles. The latter was deemed to involve a complex sequence of bond scission and formation steps within the coordination sphere of the metal atom. In this work new light will be shed on this type of reaction highlighting the specific preconditions for such a transformation as well as the unproductive dead ends of the underlying network of reaction steps.
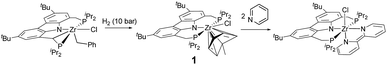 | ||
| Scheme 1 Hydrogenolytic formation of ZrII synthon 1 from a cyclometallated benzyl complex and its reaction with two molar equivalents of pyridine. | ||
Discussion
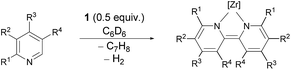
To obtain the first experimental mechanistic clues for the zirconium mediated reductive coupling of pyridine, we probed the substrate scope for this transformation using the isolated η6-arene complex 1.69In the first assay, monitored in situ by 31P NMR spectroscopy, four methyl substituted pyridines were tested (Table 1) and it was found that the previously observed reductive coupling reaction strongly depended on the substitution pattern. For pyridines methylated in the meta-position, unselective conversion into a variety of unidentifiable products was observed (Table 1, entries 1–2), 2-picoline underwent cyclometallation at the metal but no subsequent C–C coupling occurred (Table 1, entry 3 and Scheme 2, left) whereas 4-picoline was transformed to the expected dimethylated bipyridine (Table 1, entry 4 and Scheme 2, right).
| Entry | Substrate | Tc [°C] | Time [h] | Ratio A:Bde |
|---|---|---|---|---|
| a The reactions were carried out in NMR tubes fitted with J. Young valves with 20 mg (26 μmol, 1.0 equiv.) of 1 with 2.0 equivalents of the corresponding pyridine. b Unselective conversion to unidentified products was observed. c Temperature was gradually increased from RT to either 60, 80 or 100 °C in case no reaction occurred. d A = main product; B = unidentified by-product(s); judged by 31P spectroscopy. e Starting complex 1 was fully consumed in the stated reaction time; judged by 31P NMR spectroscopy. | ||||
| 1 | R2 = Me, R1,3,4 = H | 60 | 16 | —b |
| 2 | R2,4 = Me, R1,3 = H | 80 | 48 | —b |
| 3 | R1 = Me, R2–4 = H | 100 | 16 | 66:34 |
| 4 | R3 = Me, R1,2,4 = H | 60 | 16 | 80:20 |
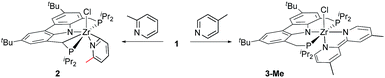 | ||
| Scheme 2 Reactivity of arene complex 1 with 2-picoline in contrast to the reactivity with 4-picoline (product 2 could not be purely isolated; observed via1H NMR and X-ray analysis). | ||
For the 2-picoline substrate the observation of two doublet resonances in the 31P{1H} NMR spectrum (δ(31P) [ppm] = 18.2 (d, JPP = 25.4 Hz), −1.2 (d, JPP = 25.4 Hz)) indicated the cyclometallation of one of the methylene bridges of the CbzPNP pincer ligand.70 The result of a single crystal X-ray structure analysis confirmed the identity of complex 2 as an η2-pyridyl species (see ESI†). The results obtained for 2-, 3- and 4-substituted methyl pyridines indicated that the reductive C–C coupling reaction mediated by η6-arene complex 1 proceeded exclusively with 4-substituted pyridines.
The formation of the dianionic bipyridine ligand in the reaction of 1 with 4-picoline was accompanied by a change of colour from brown to deep purple and conversion to 3-Me was completed after 16 h at 60 °C (cf.Scheme 2 and Table 1, entry 4). The reaction product was identified by 31P{1H} NMR spectroscopy (δ(31P{1H}) = 23.4 ppm (bs)) as well as a characteristic set of signals in the 1H NMR spectrum. In the case of 3-Me, this set consists of three different doublet and two singlet signals spread over a range of 5 ppm representing the six protons of the bipyridyl ligand [δ(1H) = 9.18 (d, JHH = 7.1 Hz, 1H); δ(1H) = 7.31 (bs, 1H); δ(1H) = 6.33 (s, 1H); 6.31 (s, 1H); δ(1H) = 4.87 (d, JHH = 7.0 Hz, 1H); 4.29 (d, JHH = 7.0 Hz, 1H) ppm].
To further investigate the scope of the coupling reaction, a series of 4-substituted pyridines was reacted with complex 1 (cf.Table 2). A general reactivity trend emerged in this study: pyridine substrates with reduced electron density in the aromatic ring did not undergo the reductive coupling reaction (cf.Table 2, entry 1 + 2) while the reaction with an electron-rich pyridine resulted in the formation of the coupled heterocycle along with significant amounts of side products, precluding the isolation of the respective bipyridyl complex (cf.Table 2, entry 10). However, substrates with similar electronic properties to the unsubstituted pyridine were readily transformed to the desired bipyridyl ligand. These included a variety of alkyl as well as sp2 substituted pyridines (Table 2, entries 3–9).
| Entry | Substrate | T [°C]c | Time [h] | Isolated yield [%] |
|---|---|---|---|---|
| a Unselective conversion to unidentified products was observed. | ||||
| 1 | R = CF3 | 80 | 24 | —a |
| 2 | R = CN | 50 | 24 | —a |
| 3 | Pyridine-d5 | 50 | 19 | 21 |
| 4 | R = Me | 60 | 16 | 18 |
| 5 | R = Et | 50 | 19 | 25 |
| 6 | R = tBu | 50 | 19 | 34 |
| 7 | R = CH2Ph | 50 | 19 | 42 |
| 8 | R = Ph | 60 | 19 | 59 |
| 9 | R = CHCHPh | 50 | 19 | 44 |
| 10 | R = OMe | 50 | 19 | —a |
| 11 | R = NMe2 | 25 | 20 | 42 |
As expected, all bipyridyl complexes possess very similar properties: they are deeply coloured in the solid state and in solution, highly sensitive towards oxygen and moisture, and all display a characteristic set of proton resonances for the newly formed bipyridine ligand between 9.2 and 4.5 ppm (in addition to the resonances of their respective substituents). The extreme solubility of all complexes in hydrocarbons, including n-pentane and n-hexane, led to significant discrepancies between the observed degree of conversion and the actual isolated yields in the purification based on washing or recrystallization.
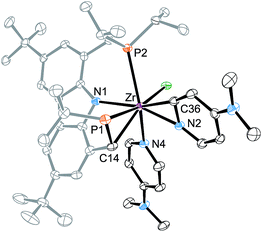
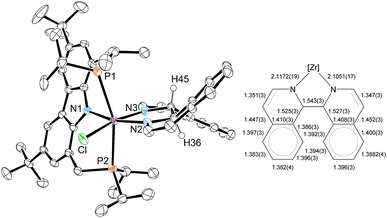
For 3-tBu, the crystal structure was determined from single crystals grown by cooling saturated n-pentane solutions to −40 °C (cf.Fig. 1). As expected, the molecular structure displays octahedral coordination geometry with the Cbz(PNP) ligand in a meridional coordination mode while the other three coordination sites are occupied by the chlorido and the bipyridyl ligand. The molecule is approximately Cs symmetric along the equatorial plane of the octahedron, which is in agreement with the symmetry derived from the NMR signal patterns in solution.
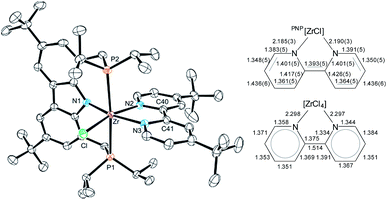 | ||
| Fig. 1 Left: molecular structure of 3-tBu (hydrogen atoms were omitted for clarity, ellipsoids set at 50% probability). Selected bond lengths [Å] and angles [°] for 3-tBu: Zr–Cl 2.4931(9), Zr–P1 2.7490(10), Zr–P2 2.7436(10), Zr–N1 2.243(3), Zr–N2 2.190(3), Zr–N3 2.185(3), Cl–Zr–P1 87.16(3), Cl–Zr–P2 91.52(3), P2–Zr–P1 175.39(3), N1–Zr–Cl 105.42(8), N1–Zr–N2 88.79(11), N2–Zr–N3 71.09(11), N3–Zr–Cl 94.48(8), N2–Zr–P1 87.99(8), N2–Zr–P2 94.38(8). Right: comparison between bond lengths: bipy2− of 3-tBu to bipy0.71 | ||
The dianionic character of the bipyridyl ligand becomes apparent upon comparison of its C–C and Zr–N bond lengths with those of a zirconium bipy0 complex reported in the literature (cf.Fig. 1).71 In the molecular structure of 3-tBu, contracted Zr–N bond lengths (Zr–N2 2.190(3); Zr–N3 2.185(3)) as well as alternatingly shortened C–C bond lengths within and between the two pyridine rings were found, suggesting the (partial) loss of aromatic character in the bipyridyl ligand and the localization of the double bonds. The presence of a bipy2− ligand is further corroborated by two broad absorption bands in the UV/Vis spectrum that can be attributed to the intraligand π–π* transitions of the bipy2− ligands.72–74
As mentioned above, an increase of electron density in the pyridine substrate tended to give rise to an inseparable product mixture (cf.Table 2, entry 10). However, further increase in the electron donating character of the substituent in the 4-position (in this case an N,N-dimethylamine substituent in 4-(dimethylamino)pyridine (DMAP)) led to the selective conversion into cyclometallated pyridyl complex 4 (cf.Table 2, entry 11 and Scheme 3). As for the analogous complex 2, the 1H, 13C and 31P NMR spectra of 4 indicated a C–H activated ligand backbone of the PNP pincer.
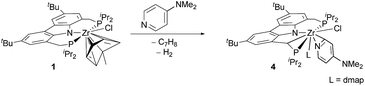 | ||
| Scheme 3 Conversion of η6-arene complex 1 with 4-(N,N-dimethylamino)pyridine to cyclometallated η2-pyridyl complex (L = DMAP). | ||
The 1H NMR spectrum of the isolated product, in particular, illustrated the activation of the ligand backbone. Due to the loss of one proton in the CH2-linkers, both linker units give rise to a characteristic signal pattern: three separate signals with the relative intensities of one proton each (δ(1H) [ppm] = 4.95 (dd, JHH = 16.0 Hz, JHP = 4.4 Hz), 3.60 (dd, JHH = 14.9 Hz, JHP = 14.9 Hz), 2.39 (m)).
To establish the structural details of 4, a single-crystal X-ray structure analysis was carried out (Fig. 2). It confirmed the presence of the cyclometallated ligand backbone of the Cbz(PNP) pincer ligand causing a deformation of the natural meridional coordination mode drawing the coordinating phosphines towards each other. As in compound 2, the pyridine substrate had undergone ortho C–H activation to form an η2-pyridyl unit; however, in the case of 4 an additional substrate molecule is coordinated to the metal atom. As opposed to the solid-state structure of 3-tBu (Fig. 1) the coordination mode for the CbzPNP ligand in 4 is better described as distorted facial than as meridional with the remaining three coordination sites being inhabited by the η2-pyridyl moiety, and the chlorido and the DMAP ligand.
Given the formation of the η2-pyridyl complexes 2 and 4, there appeared to be a pronounced tendency of the Cbz(PNP) ligand in combination with early TM to undergo cyclometallation reactions.70 It therefore was conceivable that the activation of the ligand backbone played a part in the reaction pathway resulting in the reductive coupling of pyridines. In this context, an initial C–H activation step forming an (η2-pyridyl)zirconium(IV) hydride species appeared to be possible.36 Such a potential intermediate in turn could activate the ligand backbone to provide A through elimination of H2 (cf.Chart 2). After the coordination of a second substrate molecule, C–C coupling could take place forming complex B while the Cbz(PNP) ligand would be restored in the last step through an H-atom transfer from the coupled bipyridyl ligand to generate complex C.
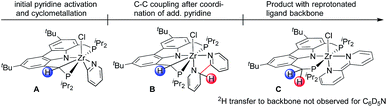 | ||
| Chart 2 Illustration of essential intermediates envisioned for the reductive coupling of pyridine involving a cyclometallated ligand backbone. | ||
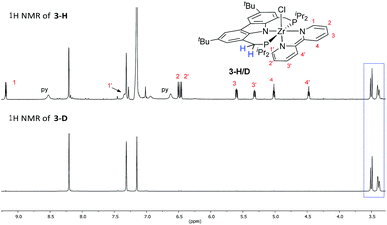
Such a reaction pathway would imply hydrogen atom exchange between the methylene hydrogen atoms in the PNP pincer and the pyridine substrate. Thus, in order to probe this specific consequence of such a mechanism the coupling reaction was repeated with pyridine-d5 in benzene-d6. This did not lead to deuterium incorporation into the ligand backbone (cf.Fig. 3) which allowed us to exclude such a cyclometallation step involving the PNP ligand backbone. It also implied that the isolated complexes 2 and 4 were the result of a competitive reaction pathway not leading to the formation of a bipy2− complex but rather representing a dead end for this type of transformation.
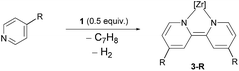
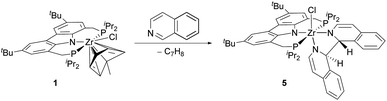
In the course of the substrate screening a remarkable new reactivity pattern was observed. When isoquinoline was reacted with ZrII synthon 1, the isoquinoline indeed underwent a C–C coupling reaction yielding bisisoquinoline complex 5. However, the NMR data of the product indicated that the coupling step occurred without the loss of the two hydrogen atoms at the bridge carbons75 as it was the case for several of the 4-substituted pyridine substrates (Scheme 4).
An X-ray diffraction study of 5 established the details of its molecular structure (Fig. 4). The molecular structure revealed a complex with distorted octahedral coordination geometry, in which a bisisoquinoline ligand occupies two coordination sites. The C1–C1′ bond length of the bisisoquinoline ligand is consistent with a single bond between the separate isoquinoline moieties. Moreover, the solid-state structure confirmed that no formal elimination of dihydrogen had occurred and that the remaining hydrogen atoms at the bridge carbons are arranged in the anti-disposition. In combination with the twisted isoquinoline moieties, these hydrogen atoms break the Cs symmetry generally found in complexes lacking an activated ligand backbone. As a result, the 1H and 31P NMR spectra show the signal patterns corresponding to a C1 symmetric complex.
DFT modelling of the reductive coupling of 4-substituted pyridines
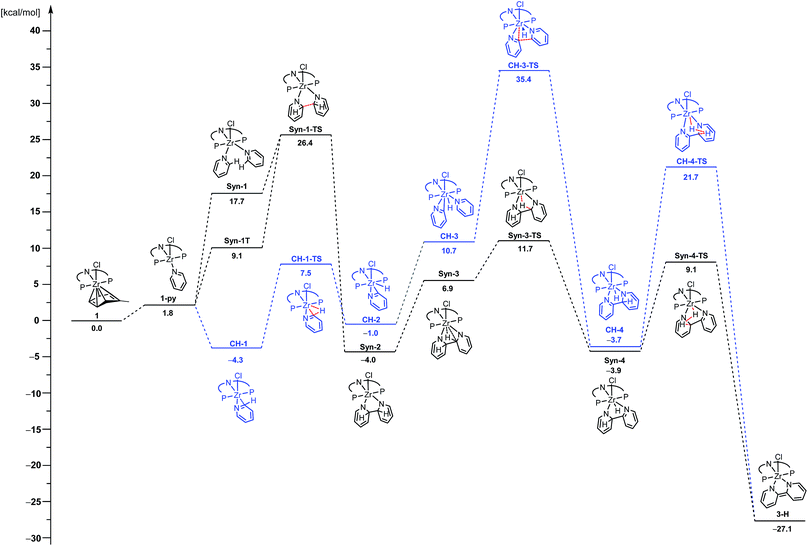
As group 4 complexes are known to promote the C–H activation of pyridines, the formation of an η2-pyridyl complex was initially assumed as the logical ZrIV intermediate in the mechanism to form complexes 3-R. However, there are reports of a high propensity of ZrII species, such as the one generated from 1 on elimination of toluene, to facilitate C–C coupling reactions. Exemplary work has been reported by Rosenthal and co-workers with utilization of zirconacenes.76–81 Due to this reactivity of low-valent zirconium species and the occurrence of complex 5, the possibility of the C–C coupling of the substrates prior to their C–H activation could not be neglected as a viable reaction mechanism. Hence, two general reaction sequences were envisaged: (i) the initial C–H activation of one pyridine molecule, as it has previously been proposed,61 and (ii) the C–C bond formation as the first reaction step. To distinguish between these sequences of events, DFT (PBE0) modelling for the two alternative reaction pathways was performed.82 The calculated reaction energy profiles for the reductive C–C coupling of pyridine are summarized in Fig. 5. Both pathways start with the extrusion of a neutral toluene molecule by pyridine to form the ZrII species 1-py in an almost thermoneutral transformation.In the case of the C–H activation pathway (Fig. 5, blue), isomerisation to a geometry, CH-1, with the C–H bond at the 2-position ready to be cleaved is exergonic (ΔG = −6.1 kcal mol−1). The actual C–H bond cleavage is effective through CH-1-TS with an activation barrier of ΔG‡ = 11.8 kcal mol−1, leading to the η2-N,C-pyridyl intermediate CH-2. After the endergonic coordination of an additional pyridine molecule to yield CH-3, the formation of the new C–C bond between the two heterocycles is effective through CH-3-TS with an activation barrier of ΔG‡ = 24.7 kcal mol−1 from CH-3. The resulting product of this C–C coupling is barely less stable than the initial complex CH-1 (ΔG = +0.6 kcal mol−1). The final product of the transformation, 3-H, is obtained when H2 is formed through CH-4-TS with an activation barrier of ΔG‡ = 25.4 kcal mol−1 from CH-4. Overall, the energy barrier to overcome is ΔG# = 39.7 kcal mol−1. This elevated activation barrier is consistent with a recent study by Mindiola, Baik and co-workers. They computed the cleavage of an η2-N,C-quinolyl unit [bonded as titanaaziridine moiety] with subsequent nucleophilic attack on a quinoline molecule at similar elevated energies of 27.9 to 40.1 kcal mol−1 (depending on the substrate and spin state).47 In light of our results and the congruence with previous findings, it was concluded that the reductive coupling reaction of pyridine unlikely proceeds along this reaction pathway.
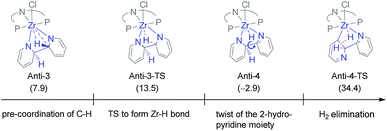
Hence, the focus was shifted towards an initial C–C coupling step of two pyridine molecules. Two reaction sequences are possible: the two pyridine moieties could undergo C–C coupling with the hydrogen atoms in the 2-positions pointing to (i) the same (syn) or (ii) to the opposite (anti) direction of the formed bipyridine plane. The latter was eliminated as H2 elimination from the anti-coupled intermediate, Anti-3, appeared unlikely (cf.Chart 3).83 Even though the first C–H activation to transfer one hydrogen atom onto Zr is easy (ΔG‡ = 5.6 and ΔG = −10.8 kcal mol−1), forming Anti-4, the subsequent H-transfer to form H2 is associated with a high lying TS (Anti-4-TS). In addition, the TS for rotation around the central C–C bond to reach, from Anti-4, a geometry more suited to C–H bond cleavage in Anti-4-TS could not be found. Therefore, the energy difference between Anti-4-TS and Anti-4, ΔG‡ = 37.3 kcal mol−1, is a lower limit of the actual activation barrier. This is too high a value to be representative of the situation observed experimentally.
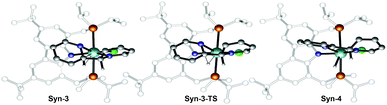
Therefore, attention was focused on the syn C–C coupling reaction sequence (Fig. 5, black). After the almost thermoneutral substitution of toluene by pyridine and the concomitant formation of a low-valent ZrII species, the coordination of a second pyridine molecule generates the precursor of the C–C coupling process, Syn-1, at ΔG = 17.7 kcal mol−1 with respect to the starting reactants. The exergonic formation of the C–C bond leading to Syn-2 (ΔG = −21.7 kcal mol−1 from Syn-1) is effective through Syn-1-TS with ΔG‡ = 8.7 kcal mol−1 (Fig. 6) and transforms the ZrII species, Syn-1, into the ZrIV species, Syn-2. Considering this, two spin states are possible: Syn-1 or Syn-1T and Syn-1-TS (singlet or triplet). A geometry for Syn-1 in the triplet state, Syn-1T, could be located on the potential energy surface at ΔG = 9.1 kcal mol−1 with respect to 1. Fig. 7 shows the spin density for Syn-1T and clearly highlights significant accumulation of spin density on both the ortho and para carbon atoms of the coordinated pyridine ligands. This electronic pattern is computed to be more stable than the singlet state Syn-1 and supports electron transfer from Zr to the coordinated pyridine before the actual C–C coupling step. However, attempts to locate a triplet transition state similar to Syn-1-TS failed as the same spin density on the ortho carbon atoms would prevent formation of the C–C bond, indicating the limitations of the computational strategy adopted in this paper. Subsequent isomerization to Syn-3 to position the C–H bond close to Zr is endergonic (ΔG = 10.9 kcal mol−1) but leads to an easy C–H bond cleavage through Syn-3-TS (ΔG‡ = 4.8 kcal mol−1, Fig. 8). The product of this C–H activation, Syn-4, lies at the same energy as the product of C–C syn coupling Syn-2.
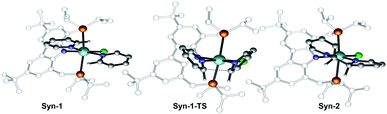 | ||
| Fig. 6 Optimized geometries of the extrema located along the reaction pathway for the C–C bond formation. | ||
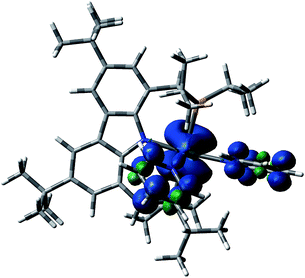 | ||
| Fig. 7 Visualization of the spin density in Syn-1T, showing the spin density in the ortho and para carbon atoms of the coordinated pyridines. | ||
 | ||
| Fig. 8 Optimized geometries of the extrema located along the reaction pathway for the hydrogen transfer from the C–C coupled 2H,2′H-[2,2′-bipyridine]-1,1′-diide ligand to the zirconium central atom. | ||
The second C–H bond cleavage is achieved through Syn-4-TS, in a σ-bond metathesis with ΔG‡ = 13 kcal mol−1 (Fig. 9). The last transformation is strongly exergonic and forms the product 3-H. Overall the energy barrier to overcome in order to form 3-H along the reaction pathway depicted in black in Fig. 5 is 26.4 kcal mol−1, associated with the TS for the C–C bond formation between two coordinated pyridine ligands.
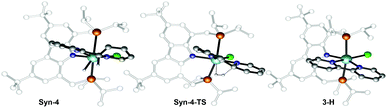 | ||
| Fig. 9 Optimized geometries of the extrema located along the reaction pathway for the hydrogen elimination from Syn-4 to form bipyridyl complex 3-H. | ||
Such a value is in very good agreement with the observed experimental conditions in terms of reaction time and temperature. To further probe the validity of this mechanism, the theoretical kinetic isotope effect (KIE) of the rate determining step for the Syn and the CH reaction sequences was modelled through normal coordinate analysis of the DFT reactant and the transition state structures (reactant (Syn-1/CH-3) → ts (Syn-1-TS/CH-3-TS)) by the Bigeleisen–Mayer approach.84–86 This resulted in the KIE(Syn-1-TS)theor = 1.32 for the transformation from Syn-1 to Syn-2, whereas a value of KIE(CH-3-TS)theor = 0.92 was found for the conversion of CH-3 to CH-4. The experimental KIE was evaluated through the reaction of Cbz(PNP)ZrCl(η6-arene) complex 1 with an excess of both, pyridine and pyridine-d5, and determined to be KIEexp = 1.29. The experimental value agrees well with KIE(Syn-1-TS)theor and further corroborates that the dehydrogenative coupling of 4-substituted pyridines proceeds along the suggested syn C–C coupling sequence (cf.Fig. 5 – black).
DFT modelling of the competitive reaction paths for 2-picoline, DMAP and isoquinoline
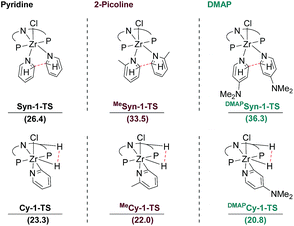
Based on deuteration experiments as well as DFT calculations, the reaction mechanisms involving a cyclometallated ligand backbone or an anti-arranged C–C coupling step were precluded as viable reaction pathways for the reductive coupling of pyridine and a variety of its 4-substituted analogues. Nevertheless, it was observed that selected substrates were converted into intermediates of the two aforementioned reaction pathways (cf.Schemes 3 and 4). To get more insight into these experimental observations, the computations for the crucial transition states found for the reductive coupling of pyridine were repeated with the respective substrate molecules, namely 2-picoline, DMAP and isoquinoline.For 2-picoline and DMAP, the initial syn C–C coupling transition states were modelled. The calculated activation barriers were significantly higher for 2-picoline (ΔG‡ = 15.3 kcal mol−1) and DMAP (ΔG‡ = 22.3 kcal mol−1) than the value obtained for pyridine (ΔG‡ = 8.7 kcal mol−1). As a consequence, the corresponding TS (MeSyn-1-TS and DMAPSyn-1-TS) lie at too high free energies with respect to the separated reactants to provide reactive pathways (Fig. 10).
In the case of pyridine, the reaction pathway involving a cyclometallated ligand was excluded based on the deuterium labelling experiments. Nevertheless, the transition state Cy-1-TS corresponding to the extrusion of H2 from CH-2 leading to the cyclometallated η2-pyridyl complex Cy-2 was computed to lie at ΔG = 23.3 kcal mol−1 with respect to the separated reactants (Fig. 10). The computed Gibbs free energies for Syn-1-TS (26.4 kcal mol−1) and Cy-1-TS (23.3 kcal mol−1) support preferred formation of Cy-2 with respect to Syn-2, both lying at the same energy (ΔG = −4.0 kcal mol−1). However, the values of the Gibbs free energies for the two competing transition states are rather to one another.
Interestingly, the difference in energy between these two crucial transition states deciding the final outcome of the reaction is strongly influenced by the nature of the substituent on the pyridine. With 2-picoline and DMAP, the transition states, MeCy-1-TS and DMAPCy-1-TS, leading to the cyclometallated η2-pyridyl complex, MeCy-2 and DMAPCy-2, lie at lower energy (22.0 and 20.8 kcal mol−1, respectively) compared to the unsubstituted case (Fig. 10). With the substituted pyridine rings there is thus a clear preference for the pathway leading to the cyclometallated η2-pyridyl complex in perfect agreement with the experimental observations. This situation is the result of both a more energy-demanding C–C coupling for the substituted heterocycles and an easier H2 extrusion upon cyclometallation.
With 2-picoline and DMAP, the cyclometallated η2-pyridyl complexes are dead-ends as further reaction with another equivalent of substituted pyridine is associated with high lying transition states. The activation barrier for C–C coupling from MeCy-2 and DMAPCy-2, through MeCy-3-TS and DMAPCy-3-TS, are ΔG# = 40.6 kcal mol−1 and ΔG# = 34.3 kcal mol−1, respectively. The reactivity of 2-picoline can be traced back to a steric repulsion of the methyl substituents with the isopropyl groups of the ligand destabilizing MeSyn-1-TS. In contrast, the reactivity of DMAP is electronically induced due to a more facile activation of C–H bonds of electron rich arenes.12 Furthermore, other 4-substituted pyridines with sterically more demanding substituents were readily transformed to 3-R, ruling out steric effects of the NMe2 substituent in DMAP.
Further attention was given to the competitive reaction path resulting in the anti C–C coupling of isoquinoline to form complex 5. For this purpose, the previously described syn- and anti-coupling steps were modelled with isoquinoline as the substrate and compared to the ones computed for pyridine. However, despite numerous attempts no transition state leading to complex 5, such as IqAnti-1-TS depicted in Fig. 11, could be located. A scan of the potential energy surface along the C–C distance between the C1 and C1′ isoquinoline carbon atoms only showed shallow maxima (cf. ESI†). In this context, a comparison of the energies of the transition states obtained for the competition between syn and anti-CC couplings for pyridine and the value obtained for syn-CC coupling for isoquinoline are instructive (Fig. 11). Notably, the anti-coupling step for pyridine (Anti-1-TS) is 13.3 kcal mol−1 lower in energy than the corresponding syn-transformation (Syn-1-TS). With isoquinoline as the substrate, the Gibbs free energy for the syn-coupling transition state (IqSyn-1-TS) was calculated to be 14 kcal mol−1 lower than that obtained for the pyridine transition state. Consequentially, if a similar energy lowering is applied to IqAnti-1-TS, then the anti-CC coupling for isoquinoline should be associated with a very low activation barrier if any. Moreover, the resulting anti-CC coupling step is associated with a very exergonic transformation with ΔG = −19.7 kcal mol−1. It can be concluded that, in the case of isoquinoline, an anti-coupling is favoured over a syn-coupling, thus explaining the reactivity observed experimentally.
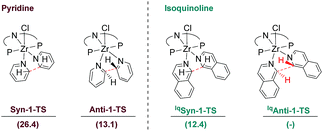 | ||
| Fig. 11 Gibbs free energies (kcal mol−1), relative to the separated reactants, for the syn- and anti-transition state for pyridine as well as isoquinoline. | ||
Conclusions
We investigated the reductive coupling of pyridine mediated by the ZrII synthon, Cbz(PNP)ZrCl(η6-toluene) 1. In this context, we were able to gain insights into the range of possible substrates and expanded the substrate scope for this reaction. Through experimental and extensive computational investigations, we were able to rule out several possible reaction paths and put forward a reaction mechanism which has been disregarded in previous publications concerning pyridine homocoupling reactions. To our knowledge, we report the first reductive coupling of pyridine proceeding via an initial syn C–C coupling step, instead of an initial C–H activation of the pyridine substrate. Further DFT calculations provided explanations for the observed deviation from the main reaction path in case of the substrates 2-picoline, DMAP and isoquinoline.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the University of Heidelberg and the Deutsche Forschungsgemeinschaft (SFB 1249) for funding this work and acknowledge support by the state of Baden-Württemberg through bwHPC.Notes and references
- I. P. Rothwell, Polyhedron, 1985, 4, 177–200 CrossRef.
- P. J. Chirik, Organometallics, 2010, 29, 1500–1517 CrossRef.
- H. Brintzinger and J. E. Bercaw, J. Am. Chem. Soc., 1971, 93, 2045–2046 CrossRef.
- W. A. Nugent, D. W. Ovenall and S. J. Holmes, Organometallics, 1983, 2, 161–162 CrossRef.
- H. Brunner, G. Gehart, W. Meier, J. Wachter, A. Riedel, S. Elkrami, Y. Mugnier and B. Nuber, Organometallics, 1994, 13, 134–140 CrossRef.
- A. Bertuleit, C. Fritze, G. Erker and R. Fröhlich, Organometallics, 1997, 16, 2891–2899 CrossRef.
- J. Pflug, A. Bertuleit, G. Kehr, R. Fröhlich and G. Erker, Organometallics, 1999, 18, 3818–3826 CrossRef.
- B. C. Bailey, H. Fan, J. C. Huffman, M.-H. Baik and D. J. Mindiola, J. Am. Chem. Soc., 2007, 129, 8781–8793 CrossRef PubMed.
- M. Manßen, N. Lauterbach, J. Dörfler, M. Schmidtmann, W. Saak, S. Doye and R. Beckhaus, Angew. Chem., Int. Ed., 2015, 54, 4383–4387 CrossRef PubMed.
- M. E. Thompson and J. E. Bercaw, Pure Appl. Chem., 1984, 56, 1–11 CrossRef.
- I. P. Rothwell, Acc. Chem. Res., 1988, 21, 153–159 CrossRef.
- M. E. Thompson, S. M. Baxter, A. R. Bulls, B. J. Burger, M. C. Nolan, B. D. Santarsiero, W. P. Schaefer and J. E. Bercaw, J. Am. Chem. Soc., 1987, 109, 203–219 CrossRef.
- G. Erker, J. Organomet. Chem., 1977, 134, 189–202 CrossRef.
- C. C. Cummins, S. M. Baxter and P. T. Wolczanski, J. Am. Chem. Soc., 1988, 110, 8731–8733 CrossRef.
- C. P. Schaller, C. C. Cummins and P. T. Wolczanski, J. Am. Chem. Soc., 1996, 118, 591–611 CrossRef.
- A. E. Shilov, Activation of Saturated Hydrocarbons by Transition Metal Complexes, D. Reidel, 1984 Search PubMed.
- S. Murai, Activation of Unreactive Bonds and Organic Synthesis, Springer-Verlag, 1999 Search PubMed.
- J. Halpern, Inorg. Chim. Acta, 1985, 100, 41–48 CrossRef.
- G. W. Parshall, Acc. Chem. Res., 1970, 3, 139–144 CrossRef.
- R. H. Crabtree, J. Organomet. Chem., 2004, 689, 4083–4091 CrossRef.
- A. E. Shilov and A. A. Shteinman, Coord. Chem. Rev., 1977, 24, 97–143 CrossRef.
- D. E. Webster, in Advances in Organometallic Chemistry, ed. R. W. F.G.A. Stone, Academic Press, 1977, vol. 15, pp. 147–188 Search PubMed.
- G. W. Parshall, Acc. Chem. Res., 1975, 8, 113–117 CrossRef.
- D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2010, 110, 624–655 CrossRef PubMed.
- D. Balcells, E. Clot and O. Eisenstein, Chem. Rev., 2010, 110, 749–823 CrossRef PubMed.
- M. I. Bruce, Angew. Chem., Int. Ed., 1977, 16, 73–86 CrossRef.
- I. Omae, Coord. Chem. Rev., 1979, 28, 97–115 CrossRef.
- I. Omae, Chem. Rev., 1979, 79, 287–321 CrossRef.
- R. H. Crabtree, Chem. Rev., 1985, 85, 245–269 CrossRef.
- M. Albrecht, Chem. Rev., 2010, 110, 576–623 CrossRef PubMed.
- I. Omae, Cyclometalation Reactions, Springer Japan, Tokyo, 2014 Search PubMed.
- J. Dehand and M. Pfeffer, Coord. Chem. Rev., 1976, 18, 327–352 CrossRef.
- E. C. Constable, Polyhedron, 1984, 3, 1037–1057 CrossRef.
- I. Omae, Coord. Chem. Rev., 1988, 83, 137–167 CrossRef.
- C. A. Bradley, E. Lobkovsky and P. J. Chirik, J. Am. Chem. Soc., 2003, 125, 8110–8111 CrossRef PubMed.
- D. P. Krut’ko, R. S. Kirsanov, S. A. Belov, M. V Borzov, A. V Churakov and J. A. K. Howard, Polyhedron, 2007, 26, 2864–2870 CrossRef.
- R. F. Jordan and D. F. Taylor, J. Am. Chem. Soc., 1989, 111, 778–779 CrossRef.
- R. F. Jordan, D. F. Taylor and N. C. Baenziger, Organometallics, 1990, 9, 1546–1557 CrossRef.
- R. F. Jordan and A. S. Guram, Organometallics, 1990, 9, 2116–2123 CrossRef.
- S. Kuppuswamy, I. Ghiviriga, K. A. Abboud and A. S. Veige, Organometallics, 2010, 29, 6711–6722 CrossRef.
- M. Oishi, T. Kato, M. Nakagawa and H. Suzuki, Organometallics, 2008, 27, 6046–6049 CrossRef.
- M. Oishi, M. Oshima and H. Suzuki, Inorg. Chem., 2014, 53, 6634–6654 CrossRef PubMed.
- T. Kurogi, M. E. Miehlich, D. Halter and D. J. Mindiola, Organometallics, 2018, 37, 165–167 CrossRef.
- S. Ren and Z. Xie, Organometallics, 2011, 30, 5953–5959 CrossRef.
- G. P. McGovern, F. Hung-Low, J. W. Tye and C. A. Bradley, Organometallics, 2012, 31, 3865–3879 CrossRef.
- I. M. Piglosiewicz, S. Kraft, R. Beckhaus, D. Haase and W. Saak, Eur. J. Inorg. Chem., 2005, 938–945 CrossRef.
- S. Baek, T. Kurogi, D. Kang, M. Kamitani, S. Kwon, D. P. Solowey, C.-H. Chen, M. Pink, P. J. Carroll, D. J. Mindiola and M.-H. Baik, J. Am. Chem. Soc., 2017, 139, 12804–12814 CrossRef PubMed.
- G. R. Newkome, A. K. Patri, E. Holder and U. S. Schubert, Eur. J. Org. Chem., 2004, 235–254 CrossRef.
- G. Chelucci and R. P. Thummel, Chem. Rev., 2002, 102, 3129–3170 CrossRef PubMed.
- L. A. Summers, in Advances in Heterocyclic Chemistry, ed. A. R. Katritzky, Academic Press, 1984, pp. 281–374 Search PubMed.
- F. Kröhnke, Synthesis, 1976, 1–24 CrossRef.
- R. Chinchilla, C. Nájera and M. Yus, Chem. Rev., 2004, 104, 2667–2722 CrossRef PubMed.
- G. M. Badger and W. H. F. Sasse, J. Chem. Soc., 1956, 616 RSC.
- W. H. F. Sasse and C. P. Whittle, J. Chem. Soc., 1961, 1347–1350 RSC.
- P. E. Rosevear and W. H. F. Sasse, J. Heterocycl. Chem., 1971, 8, 483–485 CrossRef.
- H. Hagelin, B. Hedman, I. Orabona, T. Åkermark, B. Åkermark and C. A. Klug, J. Mol. Catal. A: Chem., 2000, 164, 137–146 CrossRef.
- T. Kawashima, T. Takao and H. Suzuki, J. Am. Chem. Soc., 2007, 129, 11006–11007 CrossRef PubMed.
- T. Takao, T. Kawashima, H. Kanda, R. Okamura and H. Suzuki, Organometallics, 2012, 31, 4817–4831 CrossRef.
- M. Nagaoka, T. Kawashima, H. Suzuki and T. Takao, Organometallics, 2016, 35, 2348–2360 CrossRef.
- B. R. Cockerton and A. J. Deeming, J. Organomet. Chem., 1992, 426, 92–95 CrossRef.
- H. Sen Soo, P. L. Diaconescu and C. C. Cummins, Organometallics, 2004, 23, 498–503 CrossRef.
- M. E. Viguri, J. Pérez and L. Riera, Chem.–Eur. J., 2014, 20, 5732–5740 CrossRef PubMed.
- Y. Shibata, H. Nagae, S. Sumiya, R. Rochat, H. Tsurugi and K. Mashima, Chem. Sci., 2015, 6, 5394–5399 RSC.
- R. J. P. Corriu, C. Guerin, B. Henner and Q. Wang, Organometallics, 1991, 10, 2297–2303 CrossRef.
- J. R. Hagadorn and J. Arnold, Angew. Chem., 1998, 110, 1813–1815 CrossRef.
- O. V. Ozerov, B. O. Patrick and F. T. Ladipo, J. Am. Chem. Soc., 2000, 122, 6423–6431 CrossRef.
- D. Kissounko, A. Epshteyn, J. C. Fettinger and L. R. Sita, Organometallics, 2006, 25, 531–535 CrossRef.
- W. A. Chomitz, A. D. Sutton, J. L. Krinsky and J. Arnold, Organometallics, 2009, 28, 3338–3349 CrossRef.
- G. T. Plundrich, H. Wadepohl, E. Clot and L. H. Gade, Chem.–Eur. J., 2016, 22, 9283–9292 CrossRef PubMed.
- G. T. Plundrich, H. Wadepohl and L. H. Gade, Inorg. Chem., 2016, 55, 353–365 CrossRef PubMed.
- V. B. R. S. I. Troyanov and G. N. Mazo, Zh. Neorg. Khim., 1988, 33, 2798–2801 Search PubMed.
- E. König and S. Kremer, Chem. Phys. Lett., 1970, 5, 87–90 CrossRef.
- A. C. Bowman, J. England, S. Sproules, T. Weyhermüller and K. Wieghardt, Inorg. Chem., 2013, 52, 2242–2256 CrossRef PubMed.
- A. K. Hickey, M. G. Crestani, A. R. Fout, X. Gao, C.-H. Chen and D. J. Mindiola, Dalton Trans., 2014, 43, 9834–9837 RSC.
- D. Chen, G. Xu, Q. Zhou, L. W. Chung and W. Tang, J. Am. Chem. Soc., 2017, 139, 9767–9770 CrossRef PubMed.
- J. E. Bercaw, P. T. Wolczanski and D. L. Davies, Organometallics, 1986, 5, 443–450 CrossRef.
- L. Becker, P. Arndt, H. Jiao, A. Spannenberg and U. Rosenthal, Angew. Chem., Int. Ed., 2013, 52, 11396–11400 CrossRef PubMed.
- L. Becker, V. V. Burlakov, P. Arndt, A. Spannenberg, W. Baumann, H. Jiao and U. Rosenthal, Chem.–Eur. J., 2013, 19, 4230–4237 CrossRef PubMed.
- L. Becker, F. Strehler, M. Korb, P. Arndt, A. Spannenberg, W. Baumann, H. Lang and U. Rosenthal, Chem.–Eur. J., 2014, 20, 3061–3068 CrossRef PubMed.
- L. Becker, P. Arndt, A. Spannenberg, H. Jiao and U. Rosenthal, Angew. Chem., Int. Ed., 2015, 54, 5523–5526 CrossRef PubMed.
- K. Altenburger, P. Arndt, L. Becker, F. Reiß, V. V. Burlakov, A. Spannenberg, W. Baumann and U. Rosenthal, Chem.–Eur. J., 2016, 22, 9169–9180 CrossRef PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox, Gaussian, Inc., Gaussian 09, Revision D.01, Wallingford CT, 2013 Search PubMed.
- The bis-coordinated pyridine complex prior to anti-CC coupling, Anti-1, is computed at ΔG = 6.0 kcal mol-1. The C-C coupling through Anti-1-TS has an activation barrier of ΔG# = 7.1 kcal mol-1 and the reaction is exoergic with Anti-2 lying at ΔG = −8.8 kcal mol-1 with respect to the reactants.
- J. Bigeleisen, J. Chem. Phys., 1949, 17, 675–678 CrossRef.
- M. Wolfsberg, Acc. Chem. Res., 1972, 5, 225–233 CrossRef.
- The program diagonalizes the mass-weighted Hessian matricies to obtain harmonic vibrational frequencies and Bigeleisen-Mayer Reduced Isotopic Partition Function Ratios.
Footnote |
| † Electronic supplementary information (ESI) available: Includes experimental details and characterization data of all new compounds, synthetic protocols, spectral data, X-ray crystallographic information (CIF), and xyz-files of the DFT-optimized geometries. CCDC 1826625–1826628. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8sc01025k |
| This journal is © The Royal Society of Chemistry 2018 |