Regenerating infected bone defects with osteocompatible microspheres possessing antibacterial activity†
Peng-Fei
Wei
a,
Zuo-Ying
Yuan
a,
Wei
Jing
a,
Bin-Bin
Guan
b,
Zi-Hao
Liu
c,
Xu
Zhang
c,
Jian-Ping
Mao
d,
Da-Fu
Chen
e,
Qing
Cai
 *a and
Xiao-Ping
Yang
*a
*a and
Xiao-Ping
Yang
*a
aState Key Laboratory of Organic-Inorganic Composites; Beijing Laboratory of Biomedical Materials; Beijing University of Chemical Technology, Beijing 100029, P.R. China. E-mail: caiqing@mail.buct.edu.cn; yangxp@mail.buct.edu.cn
bDepartment of Stomatology, Tianjin Medical University General Hospital, Tianjin 300052, P.R. China
cDepartment of Endodontics, School and Hospital of Stomatology, Tianjin Medical University, Tianjin 300070, P.R. China
dDepartment of Spine Surgery, Beijing Jishuitan Hospital, Beijing 100035, P.R. China
eLaboratory of Bone Tissue Engineering, Beijing Research institute of Traumatology and Orthopaedics, Beijing Jishuitan Hospital, Beijing 100035, P.R. China
First published on 9th November 2018
Abstract
Treatment of infected bone defects still remains a formidable clinical challenge, and the design of bone implants with both anti-bacterial activity and -osteogenesis effects is nowadays regarded as a powerful strategy for infection control and bone healing. In the present study, bioresorbable porous-structured microspheres were fabricated from an amphiphilic block copolymer composed of poly(L-lactide) and poly(ethyl glycol) blocks. After being surface coated with mussel-inspired polydopamine, the microspheres were loaded with nanosilver via the reduction of silver nitrate and apatite via biomineralization in sequence. At optimized loading amounts, the nanosilver-loaded microspheres showed no unfavorable effects on the proliferation and differentiation of bone marrow mesenchymal stem cells despite preserving strong antibacterial activity in in vitro evaluations. For the critical-sized defects (ϕ = 8 mm) in the rat cranium that was pre-infected with Staphylococcus aureus, the filling of the dual-purpose microspheres demonstrated an effective way to kill bacteria in vivo, and in the meantime, it promoted new bone formation efficiently alongside the degradation of microspheres. Thus, the results suggested that bioresorbable microspheres with both osteoconductive and antibacterial activities were a good choice for treating infected bone defects.
Introduction
Bone tissue engineering is a promising method in regenerating and reconstructing bone defects, because it demonstrates significant advantages over auto-, allo- or artificial bone grafts in avoiding shortcomings such as limited donors and potential immune rejection.1,2 Essential requirements for bone tissue engineering scaffolds involve biocompatibility, biodegradability and bioactivity.3–6 Considering the possible infection associated with seriously injured bone tissues, which can be a challenging situation in the clinic, antibacterial activity is also expected from the scaffold, because infection often causes limited blood supply in defect sites to impede the restoration of bone structure and function.7–9 To achieve satisfactory bone regeneration, thereby, researches have begun to endow scaffolds with both osteogenic and bacteriostatic activities.10–12Biodegradable aliphatic polyesters based on lactones such as lactide and glycolide are widely used as scaffolding materials for tissue regeneration owing to their adjustable degradation rates, good biocompatibility and processibility.13,14 In recent years, injectable microspheres made from these materials have been highlighted because they have free form to allow for perfect filling into irregular shaped defects, and at the same time, improving patient comfort by minimally invasive manipulation.15–17 In addition, microspheres are good carriers to encapsulate bioactive compounds such as growth factors and/or antibacterial drugs such as vancomycin, which makes them promising substrates to induce regeneration.18–20 As for bone regeneration, to our knowledge, most studies have focused on incorporating osteoactive components into microspheres,21–23 while sparse reports can be found that focus on developing dual-purpose microspheres with both osteogenic and bacteriostatic activities to regenerate infected bone defects.24 To meet this goal, silver nanoparticles (AgNPs) are proposed to be proper antibacterial species to be incorporated into scaffolding materials, due to their broad-spectrum antibacterial properties.25,26 AgNPs are readily prepared by reducing Ag+ ions from aqueous solution with reductive agents such as formaldehyde,27 and the reduction is found to easily proceed even when Ag+ ions encounter reductive groups like the amino group in gelatin28 or the catechol group in polydopamine.29,30 Surface modification using oxidative polymerization of mussel-inspired dopamine is a popular method used in recent years to improve the cell affinity of biomaterials;31 therefore, the loading of AgNPs onto biodegradable microspheres is feasible by applying the polydopamine coating step. Different from those cases of antibacterial compounds being microencapsulated via the emulsion technique,32,33 the loading of AgNPs and the release of Ag+ ions are more controllable.
Simulated body fluid (SBF) soaking is a widely used technique to deposit biomimetric apatite onto scaffolds to thereby promote bone regeneration.34–37 Polylactide-based substrates, however, are not good at inducing mineral nucleation and growth due to the absence of functional groups.38 Pre-treatments using alkaline or plasma are usually effective at creating nucleation sites on the scaffold surface for mineral deposition.39,40 If polydopamine modification is conducted, however, the mineralization will become easy with the functional groups in polydopamine acting as nucleation sites for apatite deposition.41,42
In this study, therefore, a kind of injectable dual-purpose microsphere was designed and fabricated targeting the regeneration of infected bone defects. An amphipathic tri-block copolymer consisting of poly(L-lactide) (PLLA) and poly(ethylene glycol) (PEG) was chosen to prepare microspheres via the (water-in-oil)-in-water (W1/O/W2) double emulsion method, because the produced microspheres were porous with a rough surface, which favored cell attachment and proliferation.40 Polydopamine coating, AgNP loading and apatite deposition were then carried out in sequence. After in vitro evaluations of biocompatibility and antibacterial activity, the microspheres were injected into critical-sized rat cranial defects, which were pre-infected with Staphylococcus aureus (S. aureus). Then, the in vivo regeneration of infected bone defects was systematically assessed using characterization studies such as micro-CT, histological and immunohistological staining. The positive hypothesis of this study is to show that biodegradable microspheres with both AgNPs and apatite loadings are effectively dual-functional in repairing bone defects of critical size with associated infections.
Experimental section
Materials
L-Lactide was purchased from Shandong Pharmaceutical Sciences Pilot Plant (China). Polyvinyl alcohol (PVA, MW = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), Tween 60, Span 80, Tris, and salts for SBF preparation were all purchased from Aladdin (China). Dopamine and polyethylene glycol (PEG, MW = 6000) were purchased from Sigma-Aldrich (USA). Stannous octoate (Sn(Oct)2) and silver nitrate (AgNO3) were from Alfa Aesar (USA). All other reagents and solvents used were of analytical grade and supplied by Beijing Chemical Reagent Co., Ltd (China). Referring to our previous work,43 PLLA-PEG-PLLA tri-block copolymer was synthesized in the lab via ring-opening polymerization of L-lactide initiated by PEG6000 with two end hydroxyl groups in the presence of Sn(Oct)2 as a catalyst. The molar ratio of L-lactide to PEG 6000 was set at 9
000), Tween 60, Span 80, Tris, and salts for SBF preparation were all purchased from Aladdin (China). Dopamine and polyethylene glycol (PEG, MW = 6000) were purchased from Sigma-Aldrich (USA). Stannous octoate (Sn(Oct)2) and silver nitrate (AgNO3) were from Alfa Aesar (USA). All other reagents and solvents used were of analytical grade and supplied by Beijing Chemical Reagent Co., Ltd (China). Referring to our previous work,43 PLLA-PEG-PLLA tri-block copolymer was synthesized in the lab via ring-opening polymerization of L-lactide initiated by PEG6000 with two end hydroxyl groups in the presence of Sn(Oct)2 as a catalyst. The molar ratio of L-lactide to PEG 6000 was set at 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and the resulting copolymer demonstrated a molecular weight of ∼60
1, and the resulting copolymer demonstrated a molecular weight of ∼60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and a polydispersity of ∼1.3, which were determined by gel permeation chromatography (GPC, Waters 1515) using polystyrene as a standard and THF as an eluent.
000 and a polydispersity of ∼1.3, which were determined by gel permeation chromatography (GPC, Waters 1515) using polystyrene as a standard and THF as an eluent.
Microsphere preparation and polydopamine coating
Microspheres were prepared via the W1/O/W2 double emulsion method. Briefly, the PLLA-PEG-PLLA copolymer (1.0 g) was dissolved in 20 mL dichloromethane and 0.1% Span 80 was added. Under ultrasonication, 2 mL deionized water was added dropwise into the polymeric solution to form W1/O emulation. Then, the W1/O emulation was transferred into 200 mL aqueous solution of PVA (1%, containing 0.1% Tween 60) under continuous stirring (300 rpm). After 4 h of solvent evaporation at R.T., the formed microspheres (termed M) were collected by centrifugation and washed three times with deionized water, followed by freeze-drying. To perform polydopamine coating, the microspheres were re-suspended in an aqueous solution of dopamine (2 mg mL−1) buffered at pH 8.5 with Tris-HCl. The oxidative polymerization of dopamine was continued for 24 h; after that, polydopamine coated microspheres (termed M + D) were washed, collected and freeze-dried.Loading of AgNPs and apatite
To 100 mL deionized water, AgNO3 was added at different concentrations (5, 10, or 20 mg). Then, the M + D microspheres (100 mg) were suspended in the solutions, followed by 4 h of gentle stirring at R.T. The resulting AgNP-loaded microspheres (termed M + D + Ag) were washed with deionized water and collected by centrifugation. M + D + Ag microspheres prepared from 5, 10, 20 mg of AgNO3 were termed M + D + Ag(5), M + D + Ag(10) and M + D + Ag(20), respectively, for simplification. Subsequently, the retrieved M + D + Ag microspheres were re-suspended in a 5 times concentrated SBF (5SBF), which was prepared by dissolving NaCl, NaHCO3, KCl, K2HPO4·3H2O, MgCl2·6H2O, Na2SO4 and CaCl2 in 1000 mL deionized water, using Tris-HCl to adjust the solution pH ∼ 6.5.44 The system was incubated at 37 °C for 12 h to induce mineral deposition on microspheres. Finally, the biomineralized microspheres (termed M + D + Ag + B) were washed, collected and freeze-dried for further use. For comparison, biomineralization on M + D microspheres was conducted similarly by soaking M + D microspheres in 5SBF for 12 h at 37 °C, and the resulting microspheres were termed M + D + B.Characterization
The chemical composition of the PLLA-PEG-PLLA tri-copolymer was evaluated by 1H nuclear magnetic resonance (NMR, Bruker AV600, USA). Raman spectroscopy (Renishaw-inVia Reflex, UK) was performed to characterize the coating of polydopamine. Mineral deposition was analyzed by X-ray diffraction (XRD, Rigaku, Japan) to identify the crystalline structure, and by thermogravimetric analysis (TGA, TA Instruments, USA) in air at a heating rate of 20 °C min−1 in the range of R.T. to 600 °C. X-ray photoelectron spectroscopy (XPS) was performed using an ESCALAB 250Xi electron spectrometer from VG Scientific using monochromatic Al Kα radiation (1486.6 eV) as the excitation source, and spectra were acquired at a pass energy of 20 eV with the anode operated at 300 W under 3 × 10−9 mbar. The microsphere morphology was observed using a scanning electron microscope (SEM, JSM-7500F, Japan) after samples were sputter-coated with platinum (30 mA, 30 s) using a sputter-coater (Polaron E5600, USA). Elemental mapping was performed under the same parameters as SEM observation with an exposure time of 180 s. X-ray energy-dispersive spectroscopy (EDS, Inca X-Max, UK) analysis was conducted and spectra were recorded at 15 kV.Silver ion release
Release behaviors of silver ions from M + D + Ag and M + D + Ag + B microspheres were determined using an inductively coupled plasma optical emission spectrometer (ICP-OES; ICPS-7500, Shimadzu, Japan). In brief, microspheres (0.03 g) were placed in 10 mL of PBS buffer (pH 7.2) and incubated at 37 °C for 28 days under continuous agitation (50 rpm). Total liquids were collected at pre-determined time points, and subjected to ICP measurements. At the same time, fresh PBS buffer (10 mL) was replenished to continue the release experiment. Three parallel measurements were conducted for averaging. For comparison, the release behaviors of silver and calcium ions from M + D + Ag + B microspheres were further conducted under acidic conditions (pH = 5 or 3) and determined similarly using ICP.In vitro antibacterial tests
Antibacterial analysis of M + D + Ag microspheres was evaluated using Escherichia coli (E. coli) and S. aureus by both a live/dead staining assay and an inhibition zone test. E. coli and S. aureus were incubated in brain heart infusion (BHI, Solarbio) broth at 37 °C for 24 h, and then their densities were diluted to 107 colony-forming units (CFU) per milliliter, based on the absorbance measured at a wavelength of 600 nm using an established standard curve. To each well of a 96-well tissue culture plate, M + D + Ag microspheres (5 mg) were added, followed by 200 μL of E. coli or S. aureus suspension being added. After the systems were incubated at 37 °C for 24 h, acridine orange/ethidium bromide (AO/EB) staining was performed. Fluorescence images were captured using a confocal laser scanning microscope (CLSM, TCS SP8, Leica) to judge the live/dead state of E. coli or S. aureus. To conduct the inhibition zone test, microspheres (50 mg) were compressed into circular discs, (ϕ = 13 mm, h = 1 mm). These discs were laid on the surface of 1.5% agarose gel containing approximately 1 × 107E. coli or S. aureus per milliliter, followed by 24 h incubation at 37 °C.Cell culture
Sprague Dawley (SD) rat BMSCs were purchased from Cyagen Biosciences (Guangzhou, China). The cells were cultured at 37 °C in α-MEM (Hyclone) supplemented with 10% fetal bovine serum (FBS, AusGenex), 100 IU mL−1 penicillin (Sigma) and 100 μg mL−1 streptomycin (Sigma) with 5% CO2 and saturated humidity. When BMSCs grew to 80% confluence, they were dissociated by 0.25% trypsin (Gibco) and 0.02% ethylene diamine tetraacetic acid (EDTA) for further use. BMSCs at passages 3–4 were used in this study.Cell viability assay
Microspheres were soaked in 75% ethanol and exposed to ultraviolet light for 4 h. After the sterilization, the microspheres were washed three times with PBS, followed by immersing in culture medium overnight. Then the liquid was discarded and the microspheres were ready for cell seeding. Cell proliferation was performed in two ways, i.e., BMSCs being seeded onto microspheres in 96-well cell culture plates (contact mode) or BMSCs being cultured in transwell chambers (0.4 μm, Costar) in the presence of microspheres (non-contact mode). In both cases, 1 mL BMSC suspension at a density of 1 × 104 cell per mL was added and cell culture was continued for 7 days using BMSCs cultured on tissue culture polystyrene (TCPS) as the control. The culture medium was refreshed every other day. At 1, 3, 5 and 7 days after cell seeding, the culture medium was removed and each well was refilled with 1 mL of serum-free culture medium containing 10% Alamar blue stock solution (Invitrogen, USA). The plates were then incubated at 37 °C for 3 h. An aliquot of 100 μL from each well was transferred to another 96-well plate, and the fluorescence density was read at the excitation and emission wavelengths of λex = 530 nm and λem = 590 nm, respectively, on a fluorescence microplate reader (EnSpire 2300, PerkinElmer, USA). At the same time, the remaining Alamar blue working solution in the cell containing 24-well plates was removed completely with fresh culture medium being added, followed by further incubation. After 7 days of culture, calcein-AM/PI (propidium iodide) double staining kit (Thermo Fisher Scientific, USA) was applied for live/dead assay. For cell morphology observation, both SEM observation and cytoskeleton staining were performed. The cell/microsphere complexes retrieved after co-culture were fixed with 2.5% glutaraldehyde (Beijing Chemical Plant, China), dehydrated with gradient ethanol solutions, and subjected to SEM observation. Cytoskeleton staining was conducted using phalloidin for F-actin staining and Hoechst 33528 for nuclei staining, and fluorescence images were captured using CLSM.A cytotoxicity assay was further performed in the following way. BMSCs (3 × 103) and microspheres (5 mg) were co-cultured in 96-well plates. A lactate dehydrogenase (LDH, Beyotime) assay was performed at 24 h after cell seeding according to the manufacturer's guidance. Briefly, BMSCs were seeded into culture plates and incubated for 24 h, allowing for cell attachment, followed by refreshing the media to introduce different microspheres. After 24 h of incubation, the medium in each well was collected, into which, the LDH working solution was added and allowed to react at R.T. for 30 min. After the addition of LDH stop solution, optical density (OD) values of the samples were measured at a wavelength of 490 nm on a microplate reader; they were proportional to the concentrations of LDH. A positive control was obtained by adding lysis buffer 30 min before the media were collected.
In vitro osteogenic differentiation
To induce osteogenic differentiation of BMSCs seeded on microspheres, 50 μM ascorbate, 100 nM dexamethasone and 10 mM β-glycerophosphate were added to culture medium. In a 24-well culture plate, microspheres were incubated with 1 × 105 BMSCs and 1 mL osteogenic inductive medium. The medium was refreshed every 3 days. After the systems were incubated for 3, 7, 14 and 21 days, the expressions of alkaline phosphatase (ALP) and collagen type I (COL-I) were determined. Cell lysates for quantitative analysis were prepared by adding 1 mL cell lysis buffer containing 1% Triton X-100, 20 mmol L−1 Tris (pH 7.5) and 150 mM NaCl in sequence, followed by freezing–thawing three times and centrifugation. An ALP assay kit (Thermo Fisher) and a COL-I ELISA kit (Thermo Fisher) were used following the manufacturers’ protocols. All data were normalized to the total protein content, which was determined using a BCA protein assay kit (Thermo Fisher, USA) using bovine serum albumin (BSA) as a standard.The expression patterns of osteopontin (OPN) and osteocalcin (OCN) of BMSCs were illustrated by immunofluorescence staining. After 14 days of inductive culture, cells were fixed with 4% paraformaldehyde for 60 min, made permeable with 0.1% Triton X-100 for 10 min, blocked with 1% BSA for 30 min, treated with a primary antibody overnight at 4 °C, incubated with a secondary antibody for 1 h at R.T., and then stained with Hoechst 33528 for 5 min. The antibodies used in the study involved: rabbit polyclonal to osteopontin 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 (ab8448, Abcam, UK), mouse monoclonal to osteocalcin 1
1000 (ab8448, Abcam, UK), mouse monoclonal to osteocalcin 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 (ab13420, Abcam, UK), goat anti-rabbit IgG H&L (ab150077, Abcam, UK) and goat anti-mouse IgG H&L (ab150113, Abcam, UK). The stained samples were visually observed using CLSM. For real-time polymerase chain reaction (RT-PCR) analysis, cells on microspheres were treated with liquid nitrogen and crushed. Total RNA was extracted using a Trizol RNA extract kit (Invitrogen, Carlsbad, CA). Four genes in relation to osteogenesis, including runt-related transcription factor 2 (RUNX2), OPN, COL-I and OCN, were selected, and their specific primers were designed as listed in Table S1 (ESI†). Quantitative reverse transcription RT-PCR (qRT-PCR) was performed using SYBR Green Master Mix (Toyobo) and a qTOWER RT-PCR system. All experiments were performed in triplicate, and the amplification signal from the target gene was calculated using the ΔΔCt method after being normalized to the signal of the housekeeping gene 18S-rRNA in the same reaction.
200 (ab13420, Abcam, UK), goat anti-rabbit IgG H&L (ab150077, Abcam, UK) and goat anti-mouse IgG H&L (ab150113, Abcam, UK). The stained samples were visually observed using CLSM. For real-time polymerase chain reaction (RT-PCR) analysis, cells on microspheres were treated with liquid nitrogen and crushed. Total RNA was extracted using a Trizol RNA extract kit (Invitrogen, Carlsbad, CA). Four genes in relation to osteogenesis, including runt-related transcription factor 2 (RUNX2), OPN, COL-I and OCN, were selected, and their specific primers were designed as listed in Table S1 (ESI†). Quantitative reverse transcription RT-PCR (qRT-PCR) was performed using SYBR Green Master Mix (Toyobo) and a qTOWER RT-PCR system. All experiments were performed in triplicate, and the amplification signal from the target gene was calculated using the ΔΔCt method after being normalized to the signal of the housekeeping gene 18S-rRNA in the same reaction.
Generation of infected bone defects
To evaluate the feasibility of the strategy using dual-purpose microspheres for infected bone defect regeneration, a critical-sized circular defect (ϕ = 8 mm) in the rat cranium was created, infected with S. aureus, and microspheres with or without AgNP loading were implanted for 24 weeks. The schematic process of generating infected bone defects is shown in Fig. S1 (ESI†). All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Tianjin Medical University (China) and approved by the Animal Ethics Committee of Tianjin Medical University (China). Briefly, 45 six-week-old SD rats were anesthetized with chloral hydrate (0.5 mL per 250 g), followed by a sagittal incision on the scalp to expose the calvarium. The critical-sized circular defect was created by means of 8 mm-diameter trephine burr on the central region of the calvarium. In the process of surgery, a resorbable collagen sponge (REF 260-509-400, Bicon, USA) pre-soaked with S. aureus suspension (107 CFU in 100 μL of sterile normal saline) was inserted into each defect. Then, the skin was closed with a 4.0 nylon suture. One week later, debridement was performed. The parietal bone was re-exposed using the same incision, and nonviable tissue was removed. During the debridement, any obvious infection was clinically verified. Then microspheres (15–20 mg) were implanted into the infected defects, and the incisions were closed again. At 8, 16 and 24 weeks after the second surgery, samples were collected for assessment. Accordingly, the rats were randomly allocated into three groups: (1) blank infected bone defects (the control group); (2) filled with M + D + B microspheres (the M + D + B group); (3) filled with M + D + Ag + B microspheres prepared from 10 mg mL−1 AgNO3 solution (the M + D + Ag + B group). After the surgery, all the rats were allowed free movement, as well as food and water uptake.Anti-infection evaluation in vivo
Three days after the second surgery, the parietal bone was re-exposed using the same incision, and the formed granulation tissue was collected at a size of about 1 mm3. The granulation tissue was transferred into 10 mL sterile normal saline and vibrated for 5 min. Subsequently, 100 μL liquid was transferred to an agar plate and spread evenly. The agar plate was placed in an incubator at 37 °C for 24 h and bacterial quantity was counted. In another evaluation, the retrieved granulation tissues (1 mm3) were extracted for the qRT-PCR test. The extraction of total RNA and the corresponding test were conducted as described above. The primers designed for genes such as tumour necrosis factor (TNF) and interleukin-6 (IL-6) are listed in Table S1 (ESI†).Evaluation of new bone formation
At 8, 16 and 24 weeks post-operation, 3 rats from each group were euthanized under general anesthesia, and calvaria were collected and fixed in 10% neutral buffered formalin for over 24 h. The harvested calvaria were examined using a micro-CT (Skyscan 1174, Aartselaar, Belgium). The final reconstructed data were converted to three-dimensional images using the micro-CT system software package. Analysis was performed using a 7.8 mm diameter circular region of interest that was placed in the center of the defect area and a total cylindrical volume of interest oriented perpendicular to the outer table of the calvarium was analyzed for each specimen. The new bone volume fraction (BV/TV) and bone mineral density (BMD) were obtained using CTAn software version 1.14 (Bruker micro-CT, Kontich, Belgium). After micro-CT analysis, calvaria were decalcified in a rapid decalcifier (RapidCal. Immuno, ZS-Bio, China) for 48 h at R.T., followed by dehydration and being embedded in paraffin. Sections of 5 μm thickness were prepared for histological and immunohistochemical analyses. Hematoxylin–eosin (H&E) staining and Masson's trichrome (Senbeijia, China) staining were performed separately on consecutive tissue sections, and images were captured with digital slice scanning equipment (Nanozoomer, Hamamatsu, Japan). Immunohistochemical staining was conducted for OPN and OCN to assess the osteogenesis. Briefly, endogenous peroxidase was blocked by incubating the sections with 3% hydrogen peroxide. After being washed with PBS buffer, the sections were treated with 10% horse serum for 10 min to prevent nonspecific binding. Then OPN and OCN were detected by anti-OPN (ab8448, Abcam, UK) and anti-OCN (ab13420, Abcam, UK) primary antibodies in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 diluted solution with an antibody diluent (ZSGB-BIO, China), followed by incubation overnight at 4 °C. Thereafter, sections were rinsed three times with PBS for 5 min each time and labeled using a Polink-1 HRP DAB detection system (GBI, USA), and incubated for 30 min in a moist chamber. Antibody complexes were visualized with 3,3-diaminobenzidine (DAB kit) as a chromogen (ZSGB-BIO, China). As control, sections were parallel treated in a similar way without the incubation step of using the primary antibodies. The sections were dehydrated in ethanol and xylene and mounted using permanent medium. Stained sections were photographed using an optical microscope.
500 diluted solution with an antibody diluent (ZSGB-BIO, China), followed by incubation overnight at 4 °C. Thereafter, sections were rinsed three times with PBS for 5 min each time and labeled using a Polink-1 HRP DAB detection system (GBI, USA), and incubated for 30 min in a moist chamber. Antibody complexes were visualized with 3,3-diaminobenzidine (DAB kit) as a chromogen (ZSGB-BIO, China). As control, sections were parallel treated in a similar way without the incubation step of using the primary antibodies. The sections were dehydrated in ethanol and xylene and mounted using permanent medium. Stained sections were photographed using an optical microscope.
Statistical analysis
All quantitative data were expressed as the mean ± standard deviation (SD) for n ≧ 3. Statistical analysis was carried out using one-way analysis of variance (ANOVA) with Tukey's test. Differences between groups of *p < 0.05 were considered statistically significant and **p < 0.01 was considered highly significant.Results
Physicochemical characterization of the prepared microspheres
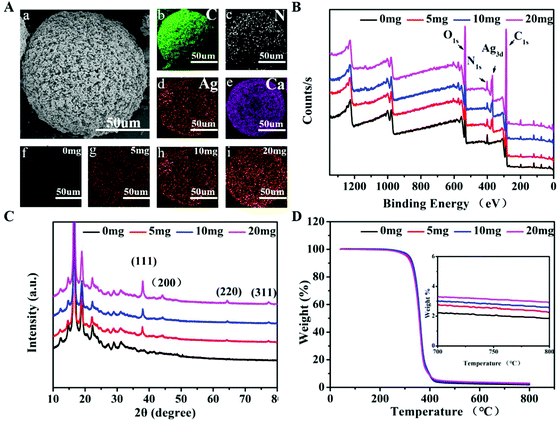
The term M + D + Ag + B was used to represent polymeric microspheres (M) being coated with polydopamine (D), followed by being loaded with AgNPs (Ag) and biomineralized (B). As a result, the prepared M + D + Ag + B microspheres displayed a rough surface as shown in Fig. 1A. The average size of the micropsheres was estimated at ∼150 μm from SEM images (Fig. S2, ESI†), showing a porous structure on the surface ranging from 5 to 10 μm in pore size. M + D + Ag + B microspheres were prepared step by step by coating polydopamine on PLLA-PEG-PLLA microspheres, followed by AgNPs loading and apatite deposition. The coating of polydopamine was confirmed by both the Raman spectrum and the XPS profile for M + D microspheres (Fig. S3, ESI†), in which the stretching vibration (1380 cm−1, 1540 cm−1) of the aromatic component and N 1s signal (400 eV) of polydopamine were identified. Antibacterial component AgNPs were then mounted onto M + D microspheres via the reductive function of polydopamine. In Fig. 1B, Ag 3d signal (∼370 eV) was detected in XPS profiles of M + D + Ag microspheres. And the Ag 3d signal was composed of two individual peaks at 368 eV and 374 eV with a spin–orbit separation of 6.0 eV (Fig. S4, ESI†), which were in accordance with the binding energies of Ag 3d3/2 and Ag 3d5/2 from Ag0 species, respectively. In XRD analysis (Fig. 1C), four additional diffraction peaks at 38°, 44°, 64° and 77° were found for M + D + Ag microspheres in comparison with the XRD pattern of M + D microspheres, which were ascribed to the (1 1 1), (2 0 0), (2 2 0) and (3 1 1) crystal faces of elemental silver. Subsequently, the M + D + Ag microspheres were soaked in 5SBF for different time spans (1–24 h) to induce apatite deposition. As shown in Fig. S5 (ESI†), the deposition amounts of mineral increased along with longer soaking time. The deposition contained calcium and phosphorus elements (Fig. S5B, ESI†), which transformed from amorphous CaP compound into weakly crystallized hydroxyapatite (HA) when the soaking time was longer than 12 h, as verified by the appearance of peaks at 26° (0 0 2) and 32° (2 1 1) in the XRD patterns of mineralized microspheres (Fig. S5D, ESI†). Accordingly, characteristic elements including C, N, Ag and Ca were all identified on the M + D + Ag + B microsphere using elemental mapping analysis (Fig. 1A(b–e)).In vitro antibacterial activity of M + D + Ag microspheres
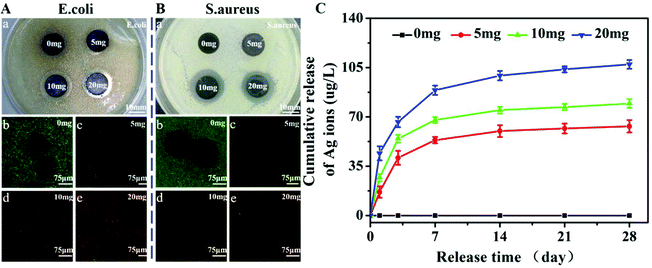
The amount of AgNPs being loading onto microspheres could be controlled by adjusting the feeding ratio of AgNO3 to the microsphere. In Fig. 1, it is demonstrated that the loading content of AgNPs increased as the feeding ratio was increased, and it was estimated to be ∼0.4 wt%, ∼0.8 wt%, and ∼1.0 wt% from TGA analysis, respectively, for M + D + Ag(5), M + D + Ag(10) and M + D + Ag(20) microspheres. These M + D + Ag microspheres were compressed into discs and co-cultured with two bacteria, Gram-negative E. coli and Gram-positive S. aureus, in order to investigate their antibacterial activities. In Fig. 2A(a) and B(a), inhibition zones around sample discs were observed for both the bacteria, showing dependence on the loading amounts of AgNPs. Only the M + D + Ag microspheres prepared at higher AgNO3 concentrations (10 and 20 mg) displayed significant inhibition zones. Accordingly, live/dead staining of E. coli and S. aureus co-cultured with M + D + Ag microsphere suspensions revealed similar results, the density of green dots (i.e., the live bacteria) displayed a clear descending trend along with the increasing amounts of loaded AgNPs. The strength of antibacterial activity was suggested to be closely related to the release behaviors of silver ions from M + D + Ag microspheres. As shown in Fig. 2C, sustained silver ion releases were detected for all the M + D + Ag microspheres, and the released amount of silver ions was apparently higher if the loading content of AgNPs on microspheres was higher.Cytotoxicity assay of M + D + Ag microspheres
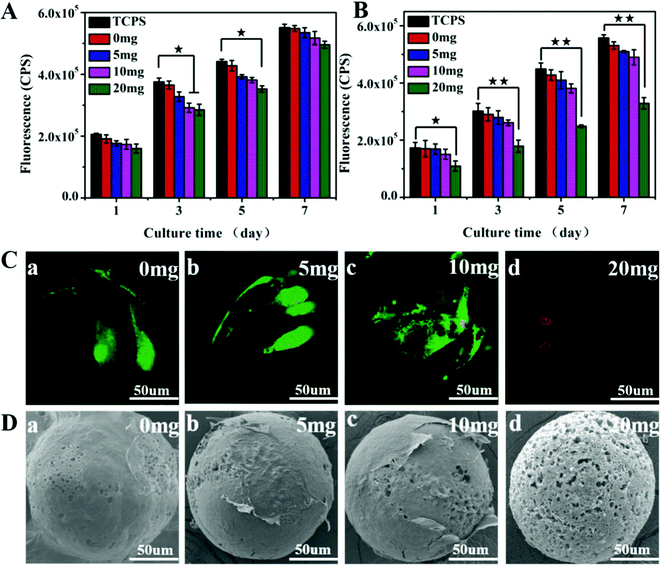
In addition to antibacterial activity, the AgNPs-loaded microspheres should have no significant cytotoxicity for biomedical applications. The viability of BMSCs co-cultured with different M + D + Ag microspheres was checked to evaluate the potential cytotoxicity of these microspheres. When BMSCs were cultured in transwell chambers in the presence of M + D + Ag microspheres, the silver ions released from the microspheres could reach the cells to cause diverse effects on cell proliferation. In Fig. 3A, it was shown that cell growth rate decreases as the release amounts of sliver ions in the media increased (with reference to Fig. 2C), but no significant difference could be identified between groups in this case. When BMSCs were cultured on M + D + Ag microspheres, in comparison with the control group (TCPS), the M + D + Ag(20) microspheres with the highest AgNPs loading showed significant cytotoxicity, while other microspheres displayed comparable cell growth rates (Fig. 3B). Live/dead staining images illustrated that BMSCs had almost all died on M + D + Ag(20) microspheres after being incubated for 3 days, as shown by the red fluorescent dots in Fig. 3C(d). Strong green fluorescence representing live cells was observed on M + D, M + D + Ag(5) and M + D + Ag(10) microspheres (Fig. 3C(a–c)). In SEM images (Fig. 3D), cells were found to firmly attach and widely spread on these microspheres, while sparse cells could be observed on M + D + Ag(20) microspheres. Considering the results of antibacterial activity and cell viability, thus, M + D + Ag(10) microspheres were chosen for the following osteogenic differentiation and in vivo studies.Antibacterial activity and cytotoxicity of mineralized microspheres
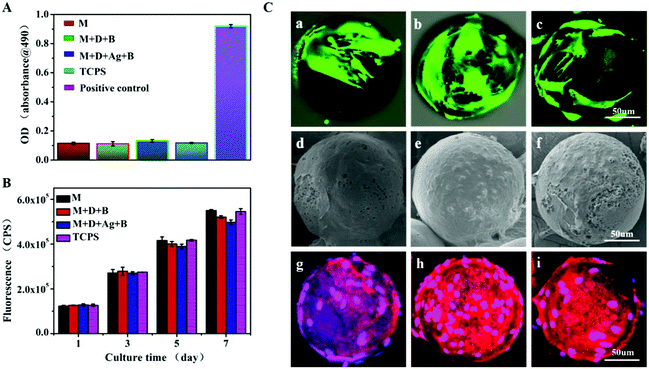
The silver ion release and antibacterial activity of M + D + Ag + B (i.e. mineralized M + D + Ag(10)) microspheres were then investigated. As shown in Fig. S6 (ESI†), the discs made of M + D + Ag + B microspheres still displayed an obvious inhibition zone against S. aureus, together with sustained silver ion release that was comparable with M + D + Ag(10) microspheres in PBS (Fig. 2C). The surface apatite coating did not compromise the antibacterial activity significantly. The control groups such as M + D + B microspheres had no such activity. Considering the acidic feature of the infected situation, release behaviors of silver and calcium ions from M + D + Ag + B microspheres were further studied under different pH values. As shown in Fig. S7 (ESI†), the acidic condition would accelerate the release rates of both ions in comparison with the neutral PBS case, which might be helpful in strengthening both the antibacterial and osteogenic activities when facing infected bone defects. Then BMSCs were cultured on M + D + Ag + B microspheres for assays including LDH leakage, cell proliferation and morphology, using TCPS, M and M + D + B microspheres as controls. In all the cases, no obvious LDH leakage was detected (Fig. 4A). Continuous cell growths were determined (Fig. 4B) in accordance with the live/dead staining results (green fluorescence shown in Fig. 4C). Cells were in normal morphology and had grown into a confluent state with an abundant extracellular matrix after 7 days incubation on all the microspheres (Fig. 4D and E). These results suggested that M + D + Ag + B microspheres were noncytotoxic and compatible with BMSCs.Osteogenic differentiation on microspheres
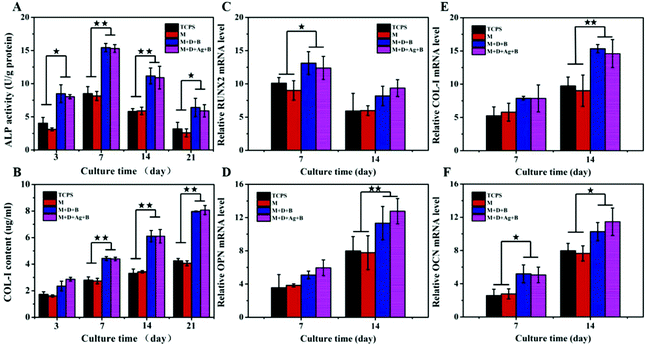
Culturing BMSCs on microspheres and TCPS for 3–21 days, ALP activities and COL-I contents were monitored and presented in Fig. 5A and B. In all the cases, in the figures, it was shown that ALP activities were reaching the maximum at day 7 and COL-I contents were increasing gradually until day 21. At the same time points, the levels of ALP activity and COL-I content were found to be significantly higher in the two groups of mineralized microspheres (M + D + B, M + D + Ag + B) than in the cases of M microspheres and TCPS control, while no significant difference was detected between the former and the latter two groups. For BMSCs being cultured 14 days on M + D + B and M + D + Ag + B microspheres, immunofluorescent staining to show the expressions of OPN and OCN was conducted. As expected, abundant OPN and OCN expressions were illustrated by strong fluorescence strengths (Fig. S8, ESI†). PCR analyses of gene expressions are presented in Fig. 5(C–F). In each case, the expression of the RUNX2 gene reached a high value at day 7 and decreased thereafter; the expressions of the other three genes (OPN, COL-I and OCN) increased gradually from day 7 to day 14. Similarly, the genes were expressed at significantly higher levels in both the groups of mineralized microspheres than in the other two groups. These results revealed that the loading of antibacterial AgNPs did not cause an adverse effect on differentiation of BMSCs; on the other hand, the deposition of apatite would enhance the osteogenic differentiation potential of BMSCs efficiently. Thus, M + D + Ag + B microspheres were envisioned a kind of dual-purpose repairing material targeting infected bone regeneration.In vivo antibacterial activity
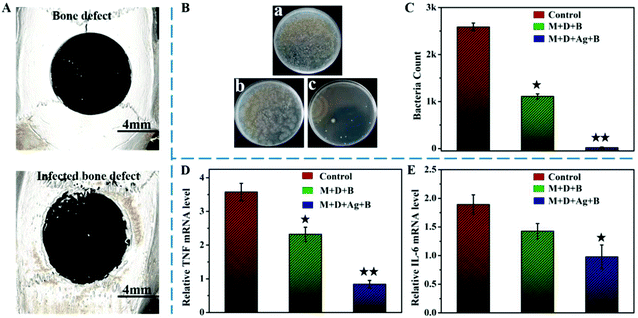
In the central region of the rat cranium, as shown in Fig. 6A, a critical-sized bone defect was created with a smooth contour. After being infected with S. aureus for one week, the contour of the defect could be seen to have been etched, leaving uneven marks along the defect edge. At the second surgery, M + D + Ag + B microspheres were implanted into the defect area after careful debridement; in parallel, M + D + B microspheres were implanted for comparison. Three days later, the defects were re-opened again and tissues were collected for analysis. In Fig. 6B and C, bacterial clones could be seen significantly forming if the tissues were retrieved from the no-filling defect (control) and the defect filled with M + D + B microspheres. However, the filling of M + D + Ag + B microspheres had significantly reduced the survival of S. aureus in the defect site. As verified by qRT-PCR analysis (Fig. 6D and E), the expressions of both TNF and IL-6 genes, which are common indicators for ongoing infections, decreased remarkably with the implantation of M + D + Ag + B microspheres in comparison with the other two groups, and the difference was highly significant (p < 0.01). Photos to show defect areas for rats after 4 week post-operation are presented in Fig. S9 (ESI†). Clearly, a large number of abscesses were visible in the control group, followed by the M + D + B filled group, while no obvious abscesses could be identified in the M + D + Ag + B filled group. These photos not only confirm the long existence of infection if no treatment was applied, but also revealed the antibacterial activity of infilled M + D + Ag + B microspheres. These results confirmed the high efficiency of M + D + Ag + B microspheres in antibacterial activity in vivo. In comparison with the blank group, interestingly, M + D + B microspheres themselves demonstrated some ability to kill S. aureus in vivo.Regeneration of infected cranial defects
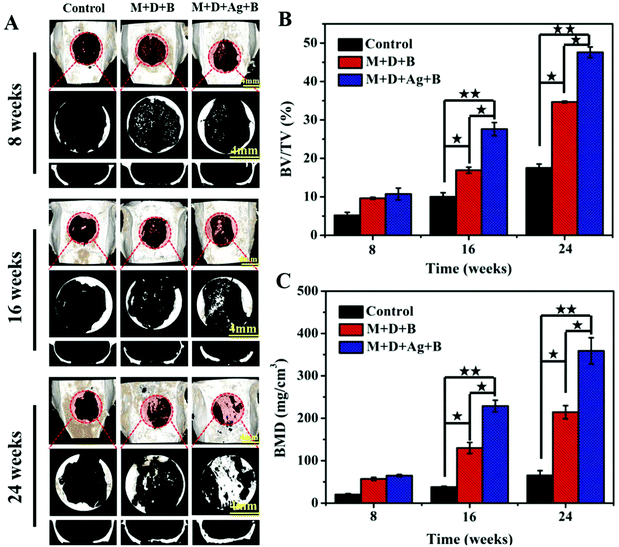
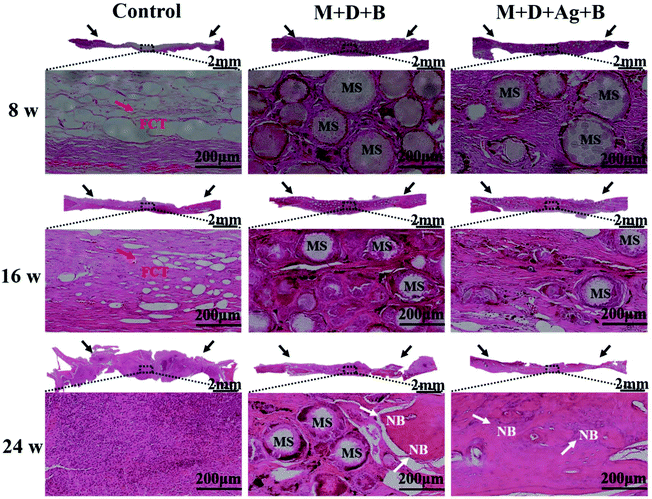
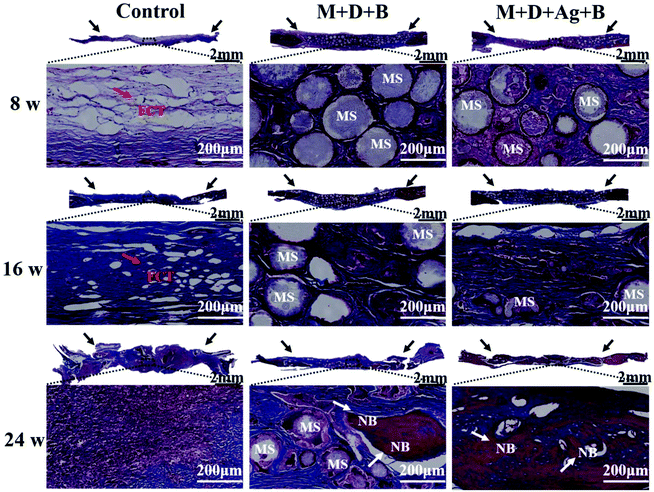
In Fig. 7A, micro-CT images demonstrated new bone formation developing from the defect edge to the center gradually in all the cases along with longer time post-operation, while the rate of osteogenesis displayed significant dependence on how the infected defect was treated (Fig. 7B and C). Twenty-four weeks post-operation, the control group was found having the lowest BV/TV (17.52 ± 0.99%) and BMD (65.41 ± 11.21 mg cm−3) among the three groups, while the infected defect filled with M + D + Ag + B microspheres achieved BV/TV and BMD values as high as 47.5 ± 1.39% and 359.05 ± 30.99 mg cm−3 at the same time point, showing highly significant difference (p < 0.01). The M + D + B group displayed better outcomes than the control group, but was inferior to the M + D + Ag + B group.Histological analyses using both H&E and Masson's trichrome staining were conducted to evaluate the infiltration of fibrous connective tissue and the newly formed bone tissues. As shown in Fig. 8 and 9, the defect area in the control group was dominantly filled with fibrous tissues with no obvious hint of new bone formation. In both the microsphere-filled groups, however, rich collagen components were identified along with the locations of microspheres being clearly presented. From 8 weeks to 24 weeks post-operation, both the M + D + B and M + D + Ag + B microspheres degraded gradually, and in the meantime, osteogenesis occurred to induce new bone formation. Up to 24 weeks, the stained areas of the newly formed bone tissues were apparently larger in the case of the M + D + Ag + B microspheres being implanted than in the M + D + B case. In the former case, the contour between the new bone and the native bone gradually became distinguishable. Alongside this, the degradation of M + D + Ag + B microspheres was found to be faster than that of M + D + B microspheres, which might be helpful in providing space for new bone formation.
Expressions of OCN and OPN in defect areas were evaluated with immunohistochemical staining. As shown in Fig. S10 (ESI†), OPN was intensively expressed at 8 weeks post-operation in both the groups when the microspheres were implanted, and its expression became weaker as the time lapsed, especially in the M + D + Ag + B group. In contrast, no obvious OPN staining was observed in the control group until 24 weeks post-operation. The expression of OCN was different from the case of OPN. In Fig. S11 (ESI†), it is displayed that OCN was highly expressed in mature bone tissue. Therefore, the expression of OCN was weak at 8 weeks post-operation, and became high in the newly formed bone tissue at 24 weeks post-operation. Accordingly, the M + D + Ag + B group presented higher OCN expression than the M + D + B group due to the faster bone regeneration rate in the former case. In the control group, nevertheless, no obvious expression of OCN was identified for all the time points.
Discussion
Organic–inorganic composites based on aliphatic polyesters and calcium phosphate compounds are widely used in bone defect repair.3,45 In many cases, the composites display promising outcomes in promoting bone regeneration at various forms including solid scaffolds, fibrous networks and microspheres.46–49 Among them, microspheres are attractive, benefiting from their injectability to meet requirements for minimally invasive manipulation and allow perfect filling into irregular shaped defects.15–17,50 In addition, microspheres with rough surfaces are more favored than those with smooth surfaces in enhancing cell attachment and proliferation.51 In this study, thus, biodegradable microspheres with porous surfaces were fabricated from the PLLA-PEG-PLLA tri-block copolymer via the W1/O/W2 emulsion method. During the solvent evaporation in aqueous solution, the solidified microspheres actually absorbed a certain amount of water because of the hydrophilic PEG segment. A porous structure was formed in the following lyophilization due to phase separation.52 The average size of the prepared porous microspheres was controlled at 100–200 μm, which was similar to the commercialized microcarriers for cell culture.53In view of possible infection occurring in the defect area, which will delay tissue regeneration significantly, antibacterial scaffold materials are put forward to meet the need.54 Antibiotics such as vancomycin have been encapsulated in microspheres, while their loading efficiency and release behavior are not so satisfactory due to the limitations of the W1/O/W2 emulsion method, especially, when the polymer contains a hydrophilic component.55 As is well known, nano-silver is broadly applied in biomedical applications as a kind of broad-spectrum antibacterial species. Researchers have incorporated them into hydrogels for infected bone regeneration in a rat model.12 In our previous study, AgNPs were loaded onto polydopamine-coated HA nanorods and blended with PLLA to test cell compatibility, osteogenesis performance and antibacterial activity against E. coli in vitro.56 It was found the composite was dual-functional, showing both strong antibacterial activity and osteogenic enhancement, if a proper amount of AgNPs was incorporated. In this study, thereby, the produced PLLA-PEG-PLLA microspheres were surface coated with polydopamine and loaded with AgNPs in various amounts via the reduction effect of catechol groups in polydopamine.29,30 By controlling the feeding ratio of Ag+ ions to microspheres, a proper loading amount of AgNPs was identified by in vitro evaluations using both bacteria and BMSCs culture. Under the optimized loading amount of AgNPs, the M + D + Ag(10) microspheres demonstrated effective activity against both E. coli and S. aureus, while they showed no significant cytotoxicity.
Biomineralization was then carried out on the selected M + D + Ag(10) microspheres using the SBF soaking method in order to introduce inorganic apatite, in which functional groups in the polydopamine surface layer provided nucleation sites for mineral deposition.41 The soaking time in 5SBF was controlled at 12 h to avoid apatite over-depositing, which might damage the porous structure of microspheres. Although the release of Ag+ ions from M + D + Ag + B microspheres was slightly slower than that from the corresponding M + D + Ag microspheres with the surface mineral covering, the M + D + Ag + B microspheres still demonstrated strong antibacterial activity in vitro. At the same time, the M + D + Ag + B microspheres could promote osteogenic differentiation of BMSCs significantly in comparison with the control and the as-prepared M microspheres, displaying a similar enhancement on osteogenic differentiation of BMSCs with M + D + B microspheres. These in vitro results revealed that the incorporated AgNPs did not compromise the osteocompatibility of biomineralized microspheres. With all these approaches, infected bone defects were created in the rat cranium. On the one hand, the infected model was created successful by observing the abscess formation and the etching marks around the defect contour. On the other hand, the in vitro culture of retrieved granulation tissue from the defect sites confirmed that the infected group with no material being filled displayed significant bacterial growth. And the expressions of both TNF and IL-6 genes, which are common indicators of ongoing infections, were quite high. In the M + D + Ag + B group, however, the infection in the defect site was significantly inhibited after the microspheres were implanted for 3 days. Apparently, Ag+ ions released from the microspheres had taken up their role in fighting against infection. It was interesting to find that M + D + B microspheres also had some effect against inflammatory activity. The reason was suggested to be the alkaline feature of their surface mineral layer, which might have interfered with the reproduction of bacteria, which preferred an acidic environment.
The 8 mm critical-sized defect in the rat cranium could not be self-healed in the normal case; the occurrence of infection would further impede tissue regeneration because it often causes limited blood supply in the defect site.7 Accordingly, the defect in the control group was still left blank at 24 weeks post-operation. With the filling of M + D + B microspheres, the situation was improved, displaying significantly higher BV/TV and BMD values than those in the control group. Results of histological and immunohistogical staining revealed the same trend, and stained new bone tissue could be observed at 24 weeks post-operation. This should be ascribed to the partly inherent antibacterial activity of M + D + B microspheres, as well as to the enhancement effect of inorganic CaP apatite on osteogenesis. However, the fastest regeneration for the infected bone defects was identified in the M + D + Ag + B group. At 24 weeks post-operation, the BV/TV value was close to 50% in the M + D + Ag + B group, which was significantly higher than that in the M + D + B group (∼35%). Amounts of calcified bone tissue could be seen across the defect area. Silver and calcium ions both were detected to be released faster from M + D + Ag + B microspheres under acidic conditions than under neutral conditions, considering the acidic features of the infected situation;57–60 this might have contributed to the regeneration of bone defects with the microspheres being filled. Along with new bone formation, degradation of microspheres had been clearly detected, providing spaces for the newly formed bone. It seemed that the microspheres degraded faster in the M + D + Ag + B group than in the M + D + B group, but the reason was unclear. It was suggested that the infectious micro-environment might have some effect on polymer degradation.61
To the best of our knowledge, this is the first study to explore bone repair in an 8 mm skull infected defect using a rat model. In many reports, the common cranium defect in rats was 5 mm.46,62–64 Our results provided solid evidence that injectable biodegradable microspheres were good filling materials for bone defects, and proper loading of both antibacterial AgNPs and osteoactive CaP mineral would greatly help in the reconstruction of infected bone defects. Dual-purpose repairing materials would find their promising applications in tissue engineering, especially when facing an infection crisis, to effectively improve regeneration efficiency. One thing the authors would like to point out is the in vivo introduction amount of AgNPs in treating the infection. Though the M + D + Ag + B(10) microspheres tested had shown a desirable outcome, it did not mean that it was the optimum content for in vivo application. Theoretically, the antibacterial activity of infilled materials should be compatible with the severity of infection to avoid overdose or being inefficient. There might be a proper AgNP amount in treating different cases. Further in vivo studies with incremental amounts of M + D + Ag + B microspheres might be helpful to understand the issue.
Conclusions
For the regeneration of infected bone defects, dual-purpose scaffolds that have both antibacterial activity and osteocompatibility are expected. In the present study, the microspheres were prepared from the amphiphilic PLLA-PEG-PLLA block copolymer displaying a porous-structured surface, which resulted from the W1/O/W2 double emulsion technique. The rough-surface microspheres were good substrates for polydopamine coating, AgNP loading and apatite depositing and, additionally, for attachment, proliferation and osteogenic differentiation of BMSCs if the loading amount of AgNPs was optimized. With strong antibacterial activity both in vitro and in vivo, the obtained dual-purpose microspheres were able to enhance the osteogenesis in infected bone defects efficiently along with their degradation in vivo. Therefore, the developed microspheres were biodegradable, dual-purpose, and potentially injectable, targeting infected bone defects to meet well the goal of satisfactory bone regeneration.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge financial support from the National Key R&D Program of China (2017YFC1104302/4300), National Natural Science Foundation of China (51473016 and 51873018), Beijing Municipal Natural Science Foundation (7182068 and 7161001), and National Key R&D Program of China (2017YFC1104302/4300).References
- R. Cancedda, P. Giannoni and M. Mastrogiacomo, Biomaterials, 2007, 28, 4240–4250 CrossRef CAS PubMed.
- D. Tang, R. S. Tare, L. Y. Yang, D. F. Williams, K. L. Ou and R. O. Oreffo, Biomaterials, 2016, 83, 363–382 CrossRef CAS PubMed.
- K. Rezwan, Q. Z. Chen, J. J. Blaker and A. R. Boccaccini, Biomaterials, 2006, 27, 3413–3431 CrossRef CAS PubMed.
- M. A. Fernandez-Yague, S. A. Abbah, L. McNamara, D. I. Zeugolis, A. Pandit and M. J. Biggs, Adv. Drug Delivery Rev., 2015, 84, 1–29 CrossRef CAS PubMed.
- D. Puppi, F. Chiellini, A. M. Piras and E. Chiellini, Prog. Polym. Sci., 2010, 35, 403–440 CrossRef CAS.
- K. Jahan and M. Tabrizian, Biomater. Sci., 2016, 4, 25–39 RSC.
- W. T. Jia, Q. Fu, W. H. Huang, C. Q. Zhang and M. N. Rahaman, Antimicrob. Agents Chemother., 2015, 59, 7571–7580 CrossRef CAS PubMed.
- J. Li, L. Tan, X. Liu, Z. Cui, X. Yang, K. W. K. Yeung, P. K. Chu and S. Wu, ACS Nano, 2017, 11, 11250–11263 CrossRef CAS PubMed.
- C. Vitale-Brovarone, M. Miola, C. Balagna and E. Verne, Chem. Eng. J., 2008, 137, 129–136 CrossRef CAS.
- H. Cheng, L. Mao, X. Xu, Y. Zeng, D. Lan, H. Hu, X. Wu, H. You, X. Yang, R. Li and Z. Zhu, Biomater. Sci., 2015, 3, 665–680 RSC.
- A. Nasajpour, S. Ansari, C. Rinoldi, A. S. Rad, T. Aghaloo, S. R. Shin, Y. K. Mishra, R. Adelung, W. Swieszkowski, N. Annabi, A. Khademhosseini, A. Moshaverinia and A. Tamayol, Adv. Funct. Mater., 2018, 28, 1703437 CrossRef.
- S. Zhang, Y. Guo, Y. Dong, Y. Wu, L. Cheng, Y. Wang, M. Xing and Q. Yuan, ACS Appl. Mater. Interfaces, 2016, 8, 13242–13250 CrossRef CAS PubMed.
- H. Y. Cheung, K. T. Lau, T. P. Lu and D. Hui, Composites, Part B, 2007, 38, 291–300 CrossRef.
- A. Jain, K. R. Kunduru, A. Basu, B. Mizrahi, A. J. Domb and W. Khan, Adv. Drug Delivery Rev., 2016, 107, 213–227 CrossRef CAS PubMed.
- I. Garzon, M. A. Martin-Piedra, V. Carriel, M. Alaminos, X. Liu and R. N. D'Souza, J. Tissue Eng. Regener. Med., 2018, 12, 204–216 CrossRef CAS PubMed.
- X. Liu, X. Jin and P. X. Ma, Nat. Mater., 2011, 10, 398–406 CrossRef CAS PubMed.
- Z. Zhang, M. J. Gupte, X. Jin and P. X. Ma, Adv. Funct. Mater., 2015, 25, 350–360 CrossRef CAS PubMed.
- P. Tayalia and D. J. Mooney, Adv. Mater., 2009, 21, 3269–3285 CrossRef CAS PubMed.
- P. Yuan, X. Qiu, R. Jin, Y. Bai, S. Liu and X. Chen, Biomater. Sci., 2018, 6, 820–826 RSC.
- B. J. Zhang, L. He, Z. W. Han, X. G. Li, W. Zhi, W. Zheng, Y. D. Mu and J. Weng, J. Mater. Chem. B, 2017, 5, 8238–8253 RSC.
- S. E. Kim, Y. P. Yun, K. S. Shim, K. Park, S. W. Choi, D. H. Shin and D. H. Suh, Colloids Surf., B, 2015, 134, 453–460 CrossRef CAS PubMed.
- F. B. Basmanav, G. T. Kose and V. Hasirci, Biomaterials, 2008, 29, 4195–4204 CrossRef PubMed.
- B. J. Zhang, Z. W. Han, K. Duan, Y. D. Mu and J. Weng, J. Biomed. Mater. Res., Part A, 2018, 106, 95–105 CrossRef CAS PubMed.
- Z. Y. Mao, Y. Li, Y. Q. Yang, Z. W. Fang, X. Chen, Y. G. Wang, J. Kang, X. H. Qu, W. E. Yuan, K. R. Dai and B. Yue, Front. Pharmacol., 2018, 9, 368 CrossRef PubMed.
- F. Martinez-Gutierrez, E. P. Thi, J. M. Silverman, C. C. de Oliveira, S. L. Svensson, A. V. Hoek, E. M. Sanchez, N. E. Reiner, E. C. Gaynor, E. L. G. Pryzdial, E. M. Conway, E. Orrantia, F. Ruiz, Y. Av-Gay and H. Bach, Nanomedicine, 2012, 8, 328–336 CrossRef CAS PubMed.
- S. Hoover, S. Tarafder, A. Bandyopadhyay and S. Bose, Mater. Sci. Eng., C, 2017, 79, 763–769 CrossRef CAS PubMed.
- S. Q. Wang, H. Zhao, Y. Wang, C. M. Li, Z. H. Chen and V. Paulose, Appl. Phys. B: Lasers Opt., 2008, 92, 49–52 CrossRef CAS.
- M. Yazdimamaghani, D. Vashaee, S. Assefa, M. Shabrangharehdasht, A. T. Rad, M. A. Eastman, K. J. Walker, S. V. Madihally, G. A. Kohler and L. Tayebi, Mater. Sci. Eng., C, 2014, 39, 235–244 CrossRef CAS PubMed.
- Y. Liu, K. Ai and L. Lu, Chem. Rev., 2014, 114, 5057–5115 CrossRef CAS PubMed.
- S. Hong, Y. Wang, S. Y. Park and H. Lee, Sci. Adv., 2018, 4, eaat7457 CrossRef PubMed.
- S. K. Madhurakkat Perikamana, J. Lee, Y. B. Lee, Y. M. Shin, E. J. Lee, A. G. Mikos and H. Shin, Biomacromolecules, 2015, 16, 2541–2555 CrossRef CAS PubMed.
- T. S. J. Kashi, S. Eskandarion, M. Esfandyari-Manesh, S. M. A. Marashi, N. Samadi, S. M. Fatemi, F. Atyabi, S. Eshraghi and R. Dinarvand, Int. J. Nanomed., 2012, 7, 221–234 Search PubMed.
- C. W. M. Yuen, J. Yip, L. W. Liu, K. Cheuk, C. W. Kan, H. C. Cheung and S. Y. Cheng, Carbohydr. Polym., 2012, 89, 795–801 CrossRef CAS PubMed.
- J. Song, E. Saiz and C. R. Bertozzi, J. Am. Chem. Soc., 2003, 125, 1236–1243 CrossRef CAS PubMed.
- D. Depan, T. C. Pesacreta and R. D. K. Misra, Biomater. Sci., 2014, 2, 264–274 RSC.
- X. Li, J. Xie, X. Yuan and Y. Xia, Langmuir, 2008, 24, 14145–14150 CrossRef CAS PubMed.
- A. Nandakumar, L. Yang, P. Habibovic and C. van Blitterswijk, Langmuir, 2010, 26, 7380–7387 CrossRef CAS PubMed.
- J. Li, A. Beaussart, Y. Chen and A. F. Mak, J. Biomed. Mater. Res., Part A, 2007, 80, 226–233 CrossRef PubMed.
- H. Shen, X. Hu, F. Yang, J. Bei and S. Wang, Biomaterials, 2007, 28, 4219–4230 CrossRef CAS PubMed.
- X. Shi, J. Jiang, L. Sun and Z. Gan, Colloids Surf., B, 2011, 85, 73–80 CrossRef CAS PubMed.
- M. Mabrouk, D. Bijukumar, J. A. S. Mulla, D. R. Chejara, R. V. Badhe, Y. E. Choonara, P. Kumar, L. C. du Toit and V. Pillay, Mater. Lett., 2015, 161, 503–507 CrossRef CAS.
- J. Ryu, S. H. Ku, H. Lee and C. B. Park, Adv. Funct. Mater., 2010, 20, 2132–2139 CrossRef CAS.
- Q. Cai, J. Z. Bei and S. G. Wang, Polymer, 2002, 43, 3585–3591 CrossRef CAS.
- Q. Cai, Q. Q. Xu, Q. F. Feng, X. Y. Cao, X. P. Yang and X. L. Deng, Appl. Surf. Sci., 2011, 257, 10109–10118 CrossRef CAS.
- T. Gao, N. Zhang, Z. Wang, Y. Wang, Y. Liu, Y. Ito and P. Zhang, Macromol. Biosci., 2015, 15, 1070–1080 CrossRef CAS PubMed.
- T. W. Sun, W. L. Yu, Y. J. Zhu, R. L. Yang, Y. Q. Shen, D. Y. Chen, Y. H. He and F. Chen, ACS Appl. Mater. Interfaces, 2017, 9, 16435–16447 CrossRef CAS PubMed.
- X. M. Luo, D. Barbieri, R. Q. Duan, H. P. Yuan and J. D. Bruijn, Acta Biomater., 2015, 26, 331–337 CrossRef CAS PubMed.
- H. Lian and Z. X. Meng, Mater. Sci. Eng., C, 2018, 84, 123–129 CrossRef CAS PubMed.
- L. Gao, Z. Y. Huang, S. F. Yan, K. X. Zhang, S. H. Xu, G. F. Li, L. Cui and J. B. Yin, Biomacromolecules, 2017, 18, 3742–3752 CrossRef CAS PubMed.
- S. W. Kang, O. Jeon and B. S. Kim, Tissue Eng., 2005, 11, 438–447 CrossRef CAS PubMed.
- B. D. Boyan, T. W. Hummert, D. D. Dean and Z. Schwartz, Biomaterials, 1996, 17, 137–146 CrossRef CAS PubMed.
- G. M. Li, Q. Cai, J. Z. Bei and S. G. Wang, Polym. Adv. Technol., 2003, 14, 239–244 CrossRef CAS.
- H. J. Chung, I. K. Kim, T. G. Kim and T. G. Park, Tissue Eng., Part A, 2008, 14, 607–615 CrossRef CAS PubMed.
- Y. Y. Tseng, Y. C. Kao, J. Y. Liao, W. A. Chen and S. J. Liu, ACS Chem. Neurosci., 2013, 4, 1314–1321 CrossRef CAS PubMed.
- J. Zhou, T. Fang, J. Wen, Z. Shao and J. Dong, J. Microencapsulation, 2011, 28, 99–107 CrossRef CAS PubMed.
- S. Q. Zhu, H. Y. Sun, H. J. Geng, D. P. Liu, X. Zhang, Q. Cai and X. P. Yang, RSC Adv., 2016, 6, 91349–91360 RSC.
- H. Chen, Z. Ye, L. Sun, X. Li, S. Shi, J. J. Hu, Y. Y. Jin, Q. W. Xu and B. L. Wang, Carbohydr. Polym., 2018, 189, 65–71 CrossRef CAS PubMed.
- M. Craig, A. Altskar, L. Nordstierna and K. Holmberg, J. Mater. Chem. B, 2016, 4, 672–682 RSC.
- F. Hizal, I. Zhuk, S. Sukhishvili, H. J. Busscher, H. C. van der Mei and C. H. Choi, ACS Appl. Mater. Interfaces, 2015, 7, 20304–20313 CrossRef CAS PubMed.
- G. J. A. ter Boo, D. W. Grijpma, T. E. Moriarty, R. G. Richards and D. Eglin, Biomaterials, 2015, 52, 113–125 CrossRef CAS PubMed.
- V. R. Sinha and A. Trehan, J. Controlled Release, 2003, 90, 261–280 CrossRef CAS PubMed.
- T. Tian, J. Liao, T. Zhou, S. Lin, T. Zhang, S. R. Shi, X. Cai and Y. Lin, ACS Appl. Mater. Interfaces, 2017, 9, 30437–30447 CrossRef CAS PubMed.
- J. A. Kim, H. S. Yun, Y. A. Choi, J. E. Kim, S. Y. Choi, T. G. Kwon, Y. K. Kim, T. Y. Kwon, M. A. Bae, N. J. Kim, Y. C. Bae, H. I. Shin and E. K. Park, Biomaterials, 2018, 157, 51–61 CrossRef CAS PubMed.
- Z. Ren, S. Ma, L. Jin, Z. Liu, D. Liu, X. Zhang, Q. Cai and X. Yang, Biofabrication, 2017, 9, 025036 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional figures. See DOI: 10.1039/c8bm00903a |
| This journal is © The Royal Society of Chemistry 2019 |