Topographical alterations render bacterial biofilms susceptible to chemical and mechanical stress†
Carolina
Falcón García
 a,
Felix
Stangl
a,
Alexandra
Götz
b,
Weining
Zhao
c,
Stephan A.
Sieber
a,
Felix
Stangl
a,
Alexandra
Götz
b,
Weining
Zhao
c,
Stephan A.
Sieber
 c,
Madeleine
Opitz
c,
Madeleine
Opitz
 b and
Oliver
Lieleg
b and
Oliver
Lieleg
 *a
*a
aDepartment of Mechanical Engineering and Munich School of Bioengineering, Technical University of Munich, Boltzmannstraße 11, 85748 Garching, Germany. E-mail: oliver.lieleg@TUM.de; Fax: +49 89 289 10801; Tel: +49 89 289 10952
bCenter for NanoScience, Faculty of Physics, Ludwig-Maximilians-Universität München, Munich, Germany
cDepartment of Chemistry, Chair for Organic Chemistry II, Technical University of Munich, Boltzmannstraße 11, 85748 Garching, Germany
First published on 8th November 2018
Abstract
For the inactivation or removal of bacterial biofilms via chemical or physical processes, it is crucial to sufficiently wet the biofilm surface. However, many bacterial biofilms efficiently resist wetting by water, oil or even organic solvents. Here, we demonstrate how exposing the surface of mature biofilm colonies to concentrated ethanol, saline or glucose solutions results in topographical changes that enable their wettability. With this approach, even omniphobic biofilm colonies become wettable towards aqueous solutions and oils. As a result of this reduced liquid repellency, the biofilms become susceptible to erosion by water which allows for their removal from the substrate they have been grown on. Moreover, bacteria within pre-treated biofilms can now be inactivated with antibiotic solutions. Thus, the biofilm treatment strategy presented here presents a new stepping stone for fighting biofilms in either industrial or medical settings.
Introduction
Forming biofilms is a key survival strategy of bacteria: when bacteria attach to a surface, they start to secrete extracellular polymeric substances (EPS) into which they embed themselves to create 3-dimensional structures. Unlike their planktonic counterparts, such surface-attached biofilm bacteria are well-shielded from the environment and protected from both chemical and mechanical hazards. Bacterial biofilms can be beneficial for nature and mankind: certain plants employ a coat of harmless biofilms such as those generated by Bacillus subtilis to protect themselves from pathogenic microorganisms;1,2 industrial processes such as waste water treatment,1,3,4 bioremediation,5,6 and non-toxic leaching of copper from ore7 rely on bacterial biofilms to take effect. However, in most industrial and medical settings, bacterial biofilms have a negative impact on the function of processes and devices, and they can also be a source for inflammation and disease which is difficult to fight.8–10 As a consequence, there is increasing effort to develop efficient methods to eradicate this biomaterial.11–14 Typical biofilm control strategies either aim at preventing bacterial attachment and thus biofilm formation,15–17 chemically inactivating the bacteria within the biofilm or removing the whole biomaterial from surfaces by mechanical forces.For biofilm removal, it is crucial to sufficiently wet the biofilm surface with liquids. However, biofilms formed by certain bacterial strains can efficiently resist wetting by water and oils.18–22 In addition to their mechanical sturdiness,23–26 this hydrophobic or even omniphobic behavior of biofilms contributes to their outstanding resilience. Similar to what has been described for plant leaves, the water repellent properties of bacterial biofilms can be sub-divided into two different variants of hydrophobicity; the first is related to the mechanism employed by lotus leaves and the second to that employed by rose petals.22 Although both variants show a high static contact angle (CA) between the liquid–vapor and biofilm–liquid interface,27 they differ once the wetted surface is tilted: in the first case, the water droplet rolls off; in the second case, the water droplet remains attached when the surface is tilted or turned upside down.
Similar to what is described for lotus leaves and synthetic superhydrophobic materials,28–30 superhydrophobic biofilms generated by B. subtilis bacteria comprise roughness features both on the micro- and nano-scale.22,30 Moreover, lotus-like biofilms possess microscopic air-pockets which separate the biofilm surface from a wetting droplet.22 As a consequence, the water drop “hovers” on the surface, and this mechanism is responsible for negligible contact angle hysteresis.22 In contrast, on rose petal-like surfaces, the water can penetrate into the spaces between the microscopic roughness features of the underlying surface, and this results in high adhesion and thus significant contact angle hysteresis.31–33
We here demonstrate that the existence of lotus-like and rose-like hydrophobicity is not limited to Bacillus subtilis biofilms but also occurs for other biofilm-forming strains, specifically when cultivated on semi-solid media exposed to air. Quantifying the biofilm surface roughness by a metrological parameter allows us the creation of a phase diagram which separates lotus-like biofilms from rose-like biofilms. Moreover, we show how treating hydrophobic biofilms with concentrated ethanol, saline or glucose solutions can reduce the micro-roughness of the biofilm surfaces. In parallel to this topographical alteration, the wetting properties of the biofilms change, and initially superhydrophobic/omniphobic biofilms can be rendered hydrophilic. As a result of these changes, the treated biofilms are more sensitive to mechanical erosion by dripping or flowing water. At the same time, bacteria within the treated biofilms can be targeted more efficiently by aqueous antibiotic solutions. Modifying the wetting resistance of biofilms as we demonstrate it here should considerably improve existing biofilm control strategies and thus facilitate biofilm removal from surfaces in industrial or medical settings.
Experimental
Bacterial strains
Bacillus subtilis natto (27E3) was obtained from the Bacillus Genetic Stock Center (BGSC) and both Pseudomonas putida (mt-2 KT2440) and Burkholderia thailandensis (E264) were obtained from the Leibniz-Institut DSMZ GmbH. See Acknowledgments for information on Bacillus subtilis NCIB 3610 and B-1 strains.Biofilm cultivation
Liquid cultures were prepared as specified next and incubated overnight at 37 °C in a shaking incubator (Sartorius, Göttingen, Germany) set to 100 rpm. For cultivation of the bacterial species Bacillus subtilis and Pseudomonas, 10 mL of sterile 2.5% Luria/Miller or “LB” medium (Carl-Roth, Karlsruhe, Germany) were inoculated with a frozen bacterial/glycerol stock. For cultivation of the bacterial species Burkholderia thailandensis, CASO broth (1.5% peptone from casein, 0.5% peptone from soymeal, 0.5% NaCl) was used.Biofilm colonies were obtained by cultivating all bacterial strains in three different substrates, each containing different media as nutrient sources. To generate solid nutrient substrates for biofilm formation, the different media were mixed with 1.5% Agar–Agar (Carl-Roth, Karlsruhe, Germany) and poured sterile into petri dishes. The nutrient sources consisted of: standard 2.5% Luria/Miller or “LB” medium, “LBGM” medium (LB enriched with 1% glycerol and 100 μM MnSO4)34 and “MSgg” minimal medium (adapted from Branda et al.:35 5 mM potassium phosphate, 100 mM Mops, 2 mM MgCl2, 700 μM CaCl2, 50 μM MnCl2, 50 μM FeCl3, 1 μM ZnCl2, 2 μM thiamine, 0.5% glycerol, 0.5% glutamate, 50 μg mL−1 tryptophan, 50 μg mL−1 phenylalanine, and 50 μg mL−1 threonine). Alternatively, for the growth of Burkholderia thailandensis biofilm colonies, CASO medium was used as a fourth nutrient source for agar enrichment. To obtain individual bacterial biofilm colonies, three separate 5 μL drops of bacterial liquid culture were pipetted onto each petri dish and incubated as follows: for obtaining the data shown in Fig. 1, all species were cultured at 30 °C for 24 and 48 h; for the rest of the data shown in the manuscript, biofilms were cultivated at 30 °C for 24 h. We also tested the influence of two different levels of humidity during biofilm cultivation: low (∼22%) and high (>80%). Moreover, the low humidity condition was studied in two different ways: first, the petri dishes containing the samples were placed directly into the incubator, and second, the samples were grown inside closed bags (without air exchange with the incubator environment). For simplicity, the biofilms grown on the different agar variants are referred to as “LB biofilm”, “LBGM biofilm”, and “MSgg biofilm” throughout this work.
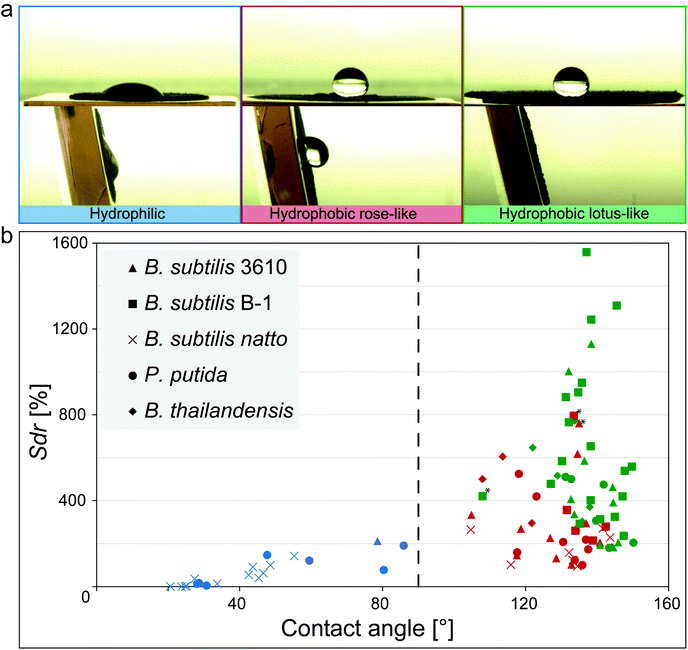 | ||
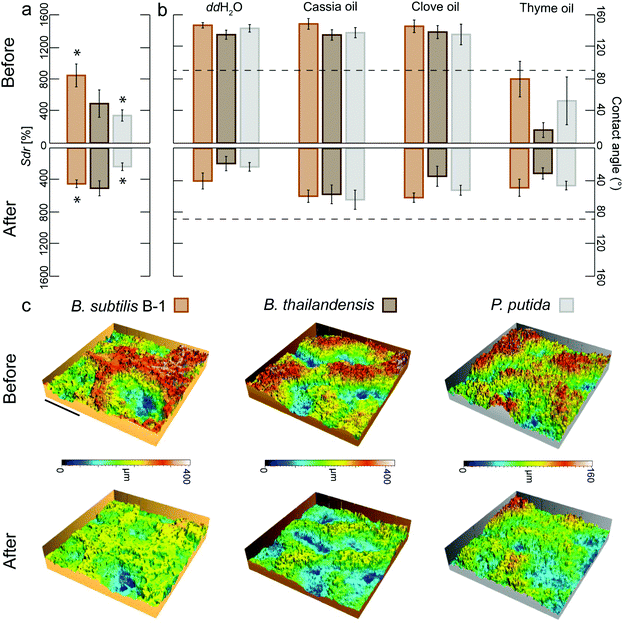
| Fig. 1 Relationship between wetting behavior and surface micro-topography for different biofilm variants. (a) Images of a ddH2O droplet on different biofilm surfaces before (upper row) and after tilt (lower row) illustrating the different wetting behaviors identified in the phase diagram in (b). (b) Data points represent the average of three replicates from a specific growth condition (Fig. S7 and S8, ESI†). The vertical line separates hydrophilic (blue) from hydrophobic samples (green, red). Green symbols denote lotus-like hydrophobic biofilms, whereas red symbols represent rose petal-like. Samples marked with an asterisk might be a result of technical measurement errors (Fig. S9, ESI†). | ||
For osmotic treatment of biofilms (ethanol, salts and sugar), B. subtilis B-1 was cultivated on LB agar, while P. putida and B. thailandensis were cultivated on LBGM agar, producing lotus-like hydrophobic samples in all cases. Additionally, rose-like hydrophobic biofilms were obtained by cultivating P. putida bacteria on MSgg agar.
For the erosion assays, the biofilms were cultivated as continuous layers. Here, 200 μL of bacterial liquid culture was spread across the entire surface of an agar-filled petri dish using a Drigalsky spatula while the petri dish was rotated on a stage, until the bacterial liquid culture was evenly distributed. To obtain robust lotus-like biofilm layers, B. subtilis B-1 biofilm samples were cultivated on MSgg agar, whereas P. putida and B. thailandensis biofilms were cultivated on LBGM agar.
Biofilm treatment
The osmotic treatment was performed on B. subtilis B-1, P. putida and B. thailandensis hydrophobic biofilms only, as follows: a 150–250 μL droplet (depending on the size of the biofilm colony) of each osmotic agent was pipetted onto the biofilm surface while ensuring that the size of the liquid droplet was big enough to cover the entire surface, but small enough so that it would not fall-off during the treatment. Initially, a 60 min ethanol treatment (using an 80% (v/v) aqueous solution) was tested on biofilms generated by the three different bacterial species to ensure that the effects we previously obtained with this solvent were not limited to biofilms of the B. subtilis family (Fig. S1, ESI†).36 Since follow-up experiments showed that a 10 min ethanol treatment is sufficient to observe similar effects, this shorter treatment time was used for the results reported in Fig. 2 and 5. For this treatment procedure with ethanol, only biofilms with initial lotus-like hydrophobicity were used.Biofilm surface treatment with the concentrated osmotic agents 5 M NaCl, 3 M KCl and 1.5 M glucose was performed in the same way as described for ethanol solutions. First, short incubation times of 1 h or 2 h were tested. However, with those exposure times, no visible alteration of the biofilm surface was detected by profilometry (denoted by the Sdr values before and after treatment) nor a change in hydrophobicity was reported by the measured CA (Fig. S2a, ESI†). Thus, longer exposure times of 18 h or 48 h were tested. Here, a clear reduction of the surface features was observed; however, owing to the long duration of this exposure step, partial evaporation or absorption of the osmotic solution by the biofilm surface was observed in some cases (Fig. S2b, ESI†). Thus, to achieve comparable conditions, we only analyzed those combinations of biofilms and osmotic solutions where the treating droplet maintained a stable shape and size throughout the experiment (shaded conditions in Fig. S2b, ESI†). The treatment duration times for the conditions reported in the main paper are 18 h for B. subtilis B-1 and P. putida biofilms, and 48 h for B. thailandensis biofilms. In parallel, the osmotic agents reported are NaCl and KCl, for lotus-like and rose-like P. putida biofilms, respectively; and glucose for the other two bacterial strains.
Contact angle measurements
To probe the wetting behavior of the different biofilm variants, a 10 μL droplet of a particular liquid was placed onto the biofilm surface, and a transversal image of the liquid–solid interface was captured using a high resolution camera (Point Gray Research, Richmond, Canada). The following liquids were used: ddH2O, 50% (v/v) aqueous solutions of 2-propanol, methanol, and acetone (Carl Roth, Karlsruhe, Germany), as well as essential oils from cassia, clove, and thyme (Sigma-Aldrich, St Louis, USA).The static contact angle value was determined using the software Image J and the “drop snake” plug-in. At least three measurements were performed per individual sample, and the standard deviation was used for the calculation of error bars. Afterwards, hydrophobic biofilm samples (i.e., those with static contact angles >90°) were tilted and the response of the liquid droplet was observed to distinguish between rose-petal (high adhesion: droplet sticks) and lotus-like (low adhesion: droplet rolls off) hydrophobicity.
For determining the contact angle hysteresis, the same procedure was applied as for the static contact angle measurement. However, here, the volume of a water droplet was first increased from 5 μL (in increments of 1 μL) to a final volume of 10 μL; afterwards, the volume of the same droplet was gradually decreased back to 5 μL, and the static contact angle was determined at each step. With this procedure, the hysteresis curves shown in Fig. 3 and Fig. S3 (ESI†) were generated. For the contact angle hysteresis values depicted in Fig. 4, only the final advancing (from 9 to 10 μL) and final receding (from 6 to 5 μL) contact angles were evaluated, and the difference between these two contact angle values was calculated to represent the degree of hysteresis.
Topographical characterization
To investigate the topographical changes on the biofilm surfaces, light profilometry images were acquired using a Nanofocus μsurf profilometer (NanoFocus AG, Oberhausen, Germany). Pictures were acquired at 20× magnification producing surface images with an area of 800 × 772 μm. 2 × 2 stitched images were obtained using the software Nanosoft (NanoFocus AG, Oberhausen, Germany) that allowed for a wider view of the surface with a final area of 1.6 × 1.54 mm. For visualizing topographical alterations induced by ethanol, salt or glucose solutions, such locations on the biofilm surface were selected which contained distinguishable surface features; they were imaged before the treatment and their coordinates where registered. Once the solution was placed onto the surface of the biofilms the samples were left undisturbed (but covered to prevent drying). After the incubation time, the solution was removed with a plastic pipette and the samples were exposed to air for 30 min to 2 h to allow for residual liquid to evaporate. Finally, a second profilometric measurement was performed at the exact same coordinates as before the treatment. The topographical data was evaluated with the software μsoft (Version 6.0, NanoFocus AG, Oberhausen, Germany). Only images with a minimum of 60% measured data points were considered for analysis, and missing data points were interpolated. From those topographical profiles z(x,y), the developed interfacial area ratio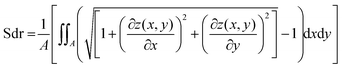 was calculated. This metrological parameter is defined in the ISO 25178 norm, which specifies terms, definitions and parameters for the determination of surface texture by areal methods;37 throughout the text, such parameter is referred to as “Sdr value”. Two further surface parameters were calculated to complement the results observed with Sdr:
was calculated. This metrological parameter is defined in the ISO 25178 norm, which specifies terms, definitions and parameters for the determination of surface texture by areal methods;37 throughout the text, such parameter is referred to as “Sdr value”. Two further surface parameters were calculated to complement the results observed with Sdr: 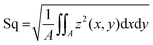 , representing the standard deviation of the height distribution – or root mean square roughness; and Sz, which quantifies the height difference between the highest peak and the deepest valley occurring in the scanned zone. The resolution of the images was 1.56 μm in lateral direction. The step size in z direction was 0.22 μm; however, owing to the peak detection algorithm the profilometer uses, the resolution in z is better than this step size and can – under ideal conditions – be as good as 10 nm with the objective used here.
, representing the standard deviation of the height distribution – or root mean square roughness; and Sz, which quantifies the height difference between the highest peak and the deepest valley occurring in the scanned zone. The resolution of the images was 1.56 μm in lateral direction. The step size in z direction was 0.22 μm; however, owing to the peak detection algorithm the profilometer uses, the resolution in z is better than this step size and can – under ideal conditions – be as good as 10 nm with the objective used here.
The fraction of biofilm material that the profilometer scans using the aforementioned settings corresponds to approximately 20 to 50% of the entire bulk of the material. This was calculated by comparing average Sz values to the entire thickness of the biofilm colonies and is consistent with previous results using the same parameter.38 Throughout the manuscript, features characterized in this fraction of the material are referred to as ‘surface topography’. The topographical changes assessed and reported here correspond only to this upper fraction of the biofilm samples, and any (putatively additional) changes in the bulk of the material have not been assessed here.
Erosion assays
Erosion stability tests were carried out in two modes: first, with dripping water and second, with flowing water. Both set-ups comprised a simple intravenous (IV) system equipment that was adapted for this particular application: the IV-bag served as the water reservoir, the drip chamber was used as inlet and outlet, and the roller clamp was used as a valve for water flow control. In the dripping mode set-up (see Fig. S4a, ESI† for details), the drip chamber served as the outlet, and the biofilm sample was located at a tilt 25° angle. In this configuration, the water droplets fell onto the biofilm surface from a relative height distance of 30 cm at a rate of 2 droplets per second. The flowing mode (see Fig. S4b, ESI† for details) consisted of the same elements, but was configured differently: now, the end of the tubing system served as outlet, the valve was completely opened, and the distance between the biofilm sample and the water outlet is reduced to a few millimeters. Thus, the water was guided directly from the reservoir onto the biofilm surface. In both modes, time-lapse images were taken using a high definition camera (Point Grey Research, Richmond, Canada). For experiments performed in dripping mode images were taken every 15 s, and for flowing mode this was done at different time points depending on the specific sample.The biofilm samples tested here had the size of a standard petri dish (i.e., 90 mm in diameter). To avoid accumulation of water at the edge of the biofilm samples during the erosion experiments, the entire biofilm-covered agar layer was extracted from the petri dish and carefully placed directly onto the stage; ddH2O was used as eroding agent. Each biofilm sample contained both untreated and treated areas that were studied separately. The treated areas were exposed to 700 μL of an 80% (v/v) ethanol aqueous solution and left undisturbed for 10 min. After incubation, the liquid was carefully removed with a pipette and the sample was left to air-dry for 2 h. Image J was used to quantitatively analyze the images obtained from the dripping experiments. A global scale was set using the known diameter of the entire biofilm sample, then each image was segmented into the area of interest and converted to 8-bit. Subsequent “particle analysis” after threshold adjustment of the image allowed for calculating the eroded area.
Antibiotic treatment
Antibiotic efficiency was tested on Pseudomonas putida biofilms; for this, an aqueous antibiotic solution was prepared as follows: piperacillin/tazobactam (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Sigma Aldrich, St Louis, USA) was dissolved in sterile ddH2O at a concentration of 160 μg mL−1.39,40 Antibiotic stock solutions were prepared according to the Clinical and Laboratory Standards Institute,41 kept frozen at −20 °C in aliquots, and thawed when needed. Osmotic treatment of biofilm surfaces was performed as described in “Biofilm treatment” and such samples are referred to as “treated”. To test for the effect of degradation over time, biofilms were left undisturbed for 10 min or 18 h in the presence of oxygen, and those samples are referred to as ‘untreated’.
1) (Sigma Aldrich, St Louis, USA) was dissolved in sterile ddH2O at a concentration of 160 μg mL−1.39,40 Antibiotic stock solutions were prepared according to the Clinical and Laboratory Standards Institute,41 kept frozen at −20 °C in aliquots, and thawed when needed. Osmotic treatment of biofilm surfaces was performed as described in “Biofilm treatment” and such samples are referred to as “treated”. To test for the effect of degradation over time, biofilms were left undisturbed for 10 min or 18 h in the presence of oxygen, and those samples are referred to as ‘untreated’.
Subsequently, both treated and untreated biofilm surfaces were covered with the antibiotic solution for a duration of 2 h, or until the aqueous phase had evaporated and the antibiotic agent had precipitated on the surface (Fig. S5, ESI†). This antibiotic exposure step was performed inside of a biosafety cabinet while keeping the lid of the petri dish slightly open. Afterwards, a fragment of the antibiotic exposed biofilm surface was taken using a disposable inoculation loop and suspended in 100 μL sterile ddH2O (Fig. S5, ESI†). The resulting biofilm suspension was shaken to ensure the presence of free-floating bacteria, and 50 μL of this cell suspension were extracted avoiding biofilm debris.
Viability of the antibiotic exposed biofilm cells was assessed using the LIVE/DEAD BacLight bacterial viability kit L7012 (Thermo Fischer, MA, USA). The dye was prepared following the manufacturer's instructions, added to the biofilm cell suspension at a concentration of 1% v/v and the samples were incubated in the dark for 15 min. The stained bacterial cells were visualized with an inverse fluorescence microscope (Leica Biosystems, Hesse, Germany) using 63× magnification; Texas red and FITC filters were used for visualization of red (propidium iodine) and green (SYTO 9) dyes, respectively. Ibidi chambers were used as sample holders (Ibidi, Planegg, Germany). To allow for subsequent cell counting, a patch of agarose (600 μm in thickness) was placed on top of the stained biofilm cells suspension to stop the bacteria from moving. To assess the accuracy of the stains, we visualized bacteria extracted from a fresh biofilm (‘fresh’ sample, as a control for viable bacterial cells), and biofilm bacteria that had been subjected to treatment with heat at approximately 100 °C for 5 minutes (‘heated’ sample, as a control for dead bacteria) (Fig. S6, ESI†). Although the dyes stained our P. putida samples differently than what is reported for other bacteria (Fig. S6, ESI†), we used green color in the bar plots of Fig. 5 to represent live cells and red to represent dead cells, as this reverse-staining effect has been reported before for Pseudomonas.42
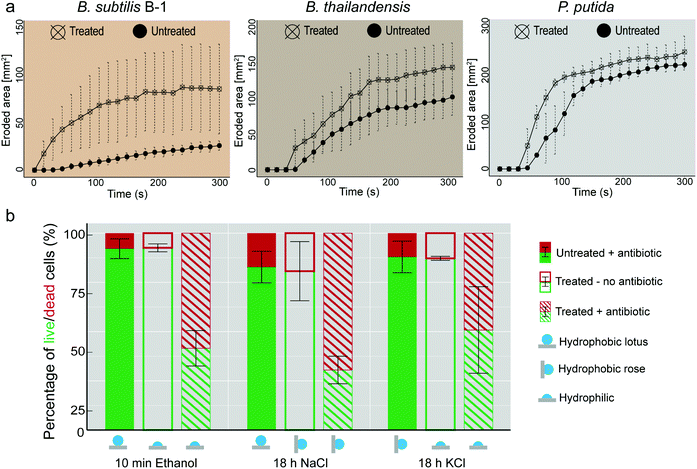 | ||
| Fig. 5 Osmotic treatment renders biofilms susceptible to mechanical and chemical treatment. (a) Erosion process of biofilms formed by three different bacterial variants tested in dripping water mode (see Fig. S4, ESI† and Experimental). (b) Antibiotic treatment of P. putida biofilms. Bar plots show percentage values of live (green) and dead (red) cells. ‘Untreated’ refers to biofilms exposed to air for the same time as their ‘treated’ counterparts. ‘Treated’ refers to samples exposed to an osmotic solution. The surfaces of the samples identified as ‘+antibiotic’ were incubated with piperacillin/tazobactam (see Experimental). Error bars denote standard deviation from N = 3 and n ≥ 2. | ||
Counting of live and dead bacterial cells was performed using Image J. First, the background was removed and the brightness intensities of the red and green channels were equalized. Then, the red and green channels were merged and the composite image was converted to RGB. Finally, red and green cells were identified separately using color threshold and counted using particle analysis.
Mass spectrometry sample preparation, measurement, and analysis
Proteomics analysis of biofilm samples was performed in independent triplicates. The extracellular proteins were extracted from P. putida 160488 bacterial biofilms (24 mg for each sample) grown on different agars (LBGM and MSgg at 30 °C for 24 h). The mass spectrometry sample preparation, measurement and analysis were performed in a label-free mode as previously reported.22 Max Quant (version 1.6.1.2) was used for MS/MS based peptide identification against P. putida 160488 UniProtKB database (April 2018).43Statistics
Sample sizes are described throughout the manuscript as follows: for independent biological replicates the symbol ‘N’ is used, whereas for technical replicates the symbol ‘n’ is used.Statistical significance between the Sdr, Sq, and Sz values measured on untreated and ethanol treated samples shown in Fig. 2 and Fig. S1c, ESI† was calculated by performing paired t-tests assuming an upper-tailed alternative hypothesis (H1). The assumptions of normal distribution and homogeneity of variances were verified using the Shapiro–Wilk test of normality and a Levene test for testing homogeneity of variances, respectively. These significance tests were performed using the software R (Foundation for Statistical Computing), and a p-value of 0.05 was used for statistical significance.
For statistical evaluation of the biofilm composition using mass spectrometry, volcano plots were generated from data obtained from three independent biological replicates (where n ≥ 2 in each) to illustrate differences in protein expression between different biofilm formation conditions, i.e. LBGM and MSgg biofilms either osmotically treated or untreated. In Fig. 3b, the y-axis represents the p-value and the x-axis lists the binary logarithm of the n-fold change in protein expression levels between the different biofilm formation conditions. The solid lines indicate a significance level of p = 0.05, and a required minimum fold change of 2 (s0 = 1) was used as a cut-off for significance. Red dots above the cut-off lines would denote significantly differently expressed proteins, yet such significant differences in protein expression was not detected for the samples analyzed here.
Results and discussion
The micro-roughness of biofilms is related to their wetting behavior
When Bacillus subtilis NCIB 3610 bacteria were cultivated at various growth conditions and on different agar variants, biofilm colonies with three different types of wetting behavior were generated: hydrophilic biofilms and two variants of hydrophobic biofilms (Fig. 1a and Fig. S7, ESI†).The first hydrophobic variant is related to the lotus effect, whereas the second variant is related to the rose petal effect. Also for other biofilm-forming Bacillus strains such as Bacillus subtilis natto or Bacillus subtilis B-1, and for other bacterial species such as Pseudomonas putida or Burkholderia thailandensis, the hydrophobic biofilms could still be subdivided into colonies with lotus-like and rose petal-like properties (Fig. 1; Fig. S7 and S8, ESI†). This demonstrated that the occurrence of lotus-like and rose-like hydrophobicity is not limited to Bacillus subtilis NCIB 3610 biofilms but is a more generic feature.
We previously reported a link between the wetting behavior of biofilms generated by B. subtilis NCIB 3610 and their microscopic surface topography. Furthermore, we also detected that these same biofilms employ similar mechanisms as described for lotus-leaves and rose-petals to resist wetting.22,31 Thus, to test if this relation between microscopic surface roughness and wetting could be extended to the other biofilm variants investigated here, we imaged their topography with white light profilometry and characterized their microscopic surface features by a metrological parameter. The Sdr value, i.e., the developed interfacial area ratio, is a hybrid parameter combining both height and spacing information, which compares the actual surface of a sample with its projected surface and calculates a percentage of surface development. This parameter can successfully differentiate between biofilm topographies with hydrophilic (Sdr < 100%), rose-like (Sdr ∼ 200%) and lotus-like (Sdr > 400%) wetting properties.22 Interestingly, this relation appears to be more generic as we again observed three distinct populations in the contact angle/Sdr phase diagram depicted in Fig. 1b. For almost all biofilm variants investigated here, we found a similar relation between their wettability (as quantified by the contact angle) and their surface topography: the lowest Sdr values occurred for hydrophilic biofilm colonies and the highest Sdr values for biofilms with lotus-like wetting resistance. This finding underscored that the complexity of the surface topography is linked to the wetting properties of the biofilms, and that this relation is relatively independent from the detailed growth conditions we used to cultivate those biofilms, i.e. on semi-solid substrates exposed to air.
We have previously observed superhydrophobic behavior even on biofilm pellicles produced by wild type B. subtilis bacteria.44,45 However, the topographical features of those biofilm pellicles are less pronounced than the ones observed on biofilm colonies formed on semi-solid media. Also there, a relation between surface complexity and wetting could be established, but the absolute roughness values determined on biofilm pellicles differed greatly from the ones reported here. Thus, we did not include data on biofilm pellicles into our phase diagram but limited our analysis to cultivation conditions that produce biofilms with roughness features of similar or at least comparable scales.
A short treatment with ethanol solutions renders omniphobic biofilms omniphilic
Our previous observations motivated the following hypothesis: if the roughness features of a highly complex biofilm surface could be smoothened, such a biofilm surface should lose its strongly hydrophobic character. The surface roughness of certain B. subtilis biofilms can be decreased by exposing the biofilm to 80% ethanol for 60 min.36 The observed topographical changes are suggested to arise from a dehydration of the biofilm colonies after ethanol exposure. This notion is consistent with the use of such solvent as part of the biological sample preparation procedure for scanning electron microscopy, where biofilm shrinkage has been reported.46 Indeed, the exposure of a hydrated sample to concentrated organic solvents such as ethanol leads to diffusion driven mass transfer, i.e. water will leave the sample and alcohol will enter it until an equilibrium concentration is reached.47,48 Thus, in a next step, we asked if ethanol-induced biofilm dehydration and the ensuing alterations in the biofilm topography would render hydrophobic biofilms wettable.To test this hypothesis, we applied ethanol solutions to the surfaces of those biofilm colonies which showed the most complex topographies (i.e., lotus-like B. subtilis B-1 biofilms with very high Sdr values) and tested the effect of different exposure times (see Experimental). For a 60 min exposure of B-1 biofilm surfaces to 80% ethanol, we found similarly strong topographical alterations as reported before36 and the biofilms were rendered hydrophilic (Fig. S1a, ESI†). Interestingly, also short exposure times as low as 10 min caused similar effects: treated B-1 biofilm samples lost their hydrophobic character and showed both contact angles towards water in the order of ∼40° (Fig. 2b) and a clear reduction in Sdr values of ∼50% (Fig. 2a).
It is important to note that, without the application of such an ethanol treatment, those B. subtilis B-1 biofilms not only repel water but also organic solvents and oils, i.e. they behave omniphobic (Table S1 and Fig. S1b, ESI†). In terms of biofilm treatment, this oil-repellency is a problematic property as selected essential oils from plants or fruits possess strong anti-bacterial properties;49,50 however, the benefits of such essential oils are difficult to harness if the biofilm surface repels them. Indeed, we found that lotus-like B-1 biofilms initially repelled essential oils from both cassia and clove; yet, after an ethanol-induced topographical ‘smoothening’ (Fig. 2c), these biofilms became wettable towards these oils (Fig. 2b and Fig. S1b, ESI†). This finding underscored the efficiency of the topographical alteration and suggested that the ethanol exposure primarily affected the topographical properties of the biofilm surface rather than its chemical properties.
Similarly, when the surface of biofilm colonies generated by Pseudomonas putida were exposed to ethanol in the same way as described before for B. subtilis B-1 biofilms, a clear deflation of their surface structures was also observed (Fig. 2c). As a consequence of a 10 min exposure to ethanol, the Sdr values obtained from the treated biofilms showed a decrease of ∼30% (Fig. 2a) and the biofilms were rendered wettable (Fig. 2b). Also biofilms generated from B. thailandensis lost their omniphobic properties by the ethanol treatment (Fig. 2b); however, here, we only detected a measurable decrease in Sdr values for longer treatment times (Fig. S1a, ESI†). We speculate that a short exposure to ethanol might cause topographical alterations which are sufficient to alter the biofilm wetting properties but too small to lead to measurable changes in the Sdr parameter as determined by optical profilometry. However, significant differences before and after ethanol treatment were observed on two additional parameters that characterize the biofilm surface topography: the root mean square surface roughness (Sq) and the height difference of the surface features (Sz) on all biofilms types (Fig. S1c, ESI†).
In general, ethanol contact with the biofilm could cause conformational changes in one or more of the matrix components (e.g., protein denaturation) even at such short exposure times. This could affect the surface chemistry of the biofilm surface and, in turn, its surface tension thus affecting the wetting behavior as quantified by the reported contact angle values. In the case of Bacillus subtilis biofilms, the surface layer hydrophobin BslA, e.g., loses its function as hydrophobic coat when this protein is present in its monomeric form, and it does not assemble on the biofilm surface when internal disulfide bonds are formed incorrectly.51 It is possible that some of the treatments conducted here affect the conformation and thus activity of this B. subtilis surface layer protein as well as other biofilm matrix components, which could affect biofilm wetting behavior without causing changes in the biofilm surface roughness.
Also concentrated salt and sugar solutions can decrease the biofilm wetting resistance
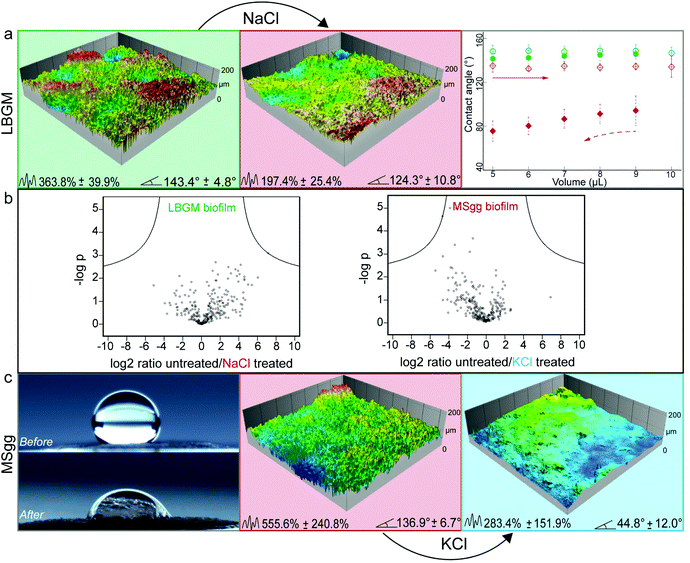
Although the efficiency of the ethanol treatment is remarkable, using concentrated ethanol solutions for altering the surface topography of biofilms could cause negative side effects on the surfaces the biofilm grows on – at least in certain medical or industrial settings. Thus, in a next step, we explored if the same effect could be obtained by using less aggressive chemicals. When looking for alternative biofilm treatments that could induce a similar topographical ‘smoothening’, we hypothesized that an osmotic pressure acting on the biofilm surface might be responsible for the observed effects. Indeed, osmotic dehydration is a typical fruit and vegetable preservation method in the food industry, where water is partially removed from compartments with lower solute concentration and transported into compartments of higher solute concentration.47,52 Different osmotic agents including salts and sugars are currently used in this process, and typical exposure times range from a few hours to days.52 Inspired by this food preservation process, we here tried to induce osmotic dehydration of biofilms by applying concentrated solutions of NaCl, KCl, and glucose (see Experimental).Putative topographical changes were assessed by imaging the surface of each biofilm sample in situ before and after treatment with the osmotic solutions (see Experimental). Treatment of lotus-like P. putida biofilms with NaCl solutions showed the strongest topographical alteration: here, the Sdr value calculated from the biofilm samples was reduced by up to 46% (Fig. 3a). As a consequence of this NaCl treatment and the ensuing strong topographical changes, the surface of these lotus-like biofilm colonies became highly adhesive towards water, i.e. it acquired rose-like behavior as demonstrated by contact angle hysteresis measurements (Fig. 3a).
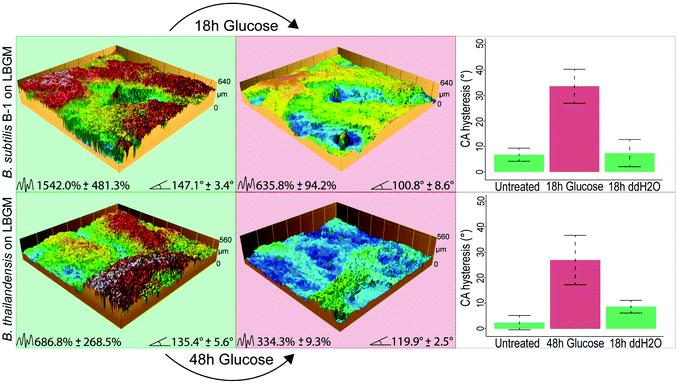
For lotus-like biofilm samples generated by B. subtilis B-1 or B. thailandensis, we obtained similar results when those biofilm samples were treated with a glucose solution for 18 and 48 h, respectively. Due to the initially higher surface complexity of both of these biofilms – as compared to Putida, the deflation effects can be strongly observed on the profilometric images taken after the treatment. As a consequence of these topographical alterations, a switch from lotus- to rose-like wetting behavior was measured in both cases, as denoted by the higher CA hysteresis after glucose exposure (Fig. 4).
Next, we asked if a similar treatment could yield a hydrophilic biofilm when a hydrophobic biofilm surface with intermediate complexity is chosen. To test this, biofilm colonies generated by P. putida grown on MSgg agar (which exhibit rose-like surfaces) were selected and treated with all osmotic solutions described before. Here, the strongest effect was obtained by exposing the biofilms to a KCl solution: we measured a decrease in Sdr of ∼50% and a final contact angle of ∼45° (Fig. 3c).
Control experiments were performed with ddH2O on both, lotus-like and rose-like putida biofilms (Fig. S3, ESI†) as well as on lotus-like B-1 and thailandensis biofilms (Fig. 4). In all cases, the samples retained their initial hydrophobic character, demonstrating that the effects described above indeed result from the high concentrations of osmotic agents present in the aqueous solutions. Furthermore, a proteomics analysis of osmotically treated P. putida biofilm samples revealed no significant differences in protein expression compared to untreated biofilms (Fig. 3b). These results suggest that the topographical “smoothening” effect achieved with the osmotic solutions might be a physical mechanism, which is relatively independent of the type of bacteria or the cultivation conditions.
Treated biofilms are more sensitive to erosion by water
When trying to remove biofilms from surfaces with mechanical forces induced by flowing or dripping water, strongly hydrophobic surface properties can be a factor that contributes to the high resilience of a biofilm. Especially when exhibiting lotus-like behavior, the biofilm surface can efficiently avoid contact with an aqueous solution – at least on a nano- and microscopic length scale. Thus, one would expect that hydrophobic, lotus-like biofilms can resist erosion by water more efficiently than hydrophilic biofilms.We tested this hypothesis by comparing the erosion behavior of biofilms generated by B. subtilis B-1, B. thailandensis, and P. putida in their untreated (i.e., lotus-like hydrophobic) and ethanol treated (i.e., hydrophilic) state. We first exposed the biofilms to dripping water and determined the eroded biofilm area as a function of time (see Experimental and Fig. S4a†). Indeed, as depicted in Fig. 5a, all three biofilm variants became more sensitive towards erosion by dripping water when their surfaces were rendered hydrophilic by a short ethanol treatment. The strongest difference between untreated and ethanol treated samples was obtained for B. subtilis B-1 biofilms. However, this is not surprising considering that, among the three biofilm variants tested, this biofilm type resisted erosion most efficiently. After 5 min of continuous water dripping, the eroded area of untreated B-1 biofilms was 5–10 times smaller than what we obtained for B. thailandensis and P. putida biofilms, respectively.
Such a dripping water erosion test with impacting water droplets on the biofilm surface does not only probe the surface polarity of a biofilm but also its mechanical sturdiness. To reduce the contribution of the latter, which can differ among the biofilm variants, we performed a second type of erosion experiment where the biofilm surfaces were exposed to a continuous stream of flowing water (Fig. S4b, ESI†). In this particular setup, all biofilm variants withstood erosion for much longer time intervals than in the dripping water tests (Fig. S4c, ESI†). However, also here, the ethanol treated biofilms became more susceptible to erosion as the onset of biofilm removal was clearly shifted to earlier time points compared to untreated samples.
A decreased biofilm liquid repellency increases the efficiency of antibiotic solutions
Having established that altering the wetting properties of biofilms from lotus-like hydrophobic to hydrophilic enhances biofilm erosion by water, we asked if weakening the wetting resistance of biofilm colonies would also increase the efficiency of anti-microbial solutions. As a model for an anti-microbial liquid, we chose an aqueous solution containing an antibiotic. We first studied lotus-like P. putida biofilms and exposed those biofilms to a solution containing 160 μg mL−1 of the clinically used broad-spectrum β-lactam antibiotic piperacillin. After a 2 h incubation with this antibiotic solution, the percentage of live cells remained well above 80% (filled bars in Fig. 5b) and thus comparable to control samples that were not exposed to the antibiotic (Fig. S6, ESI†). This demonstrates the poor efficiency of the antibiotic solution towards biofilm bacteria, even when such is suitable to kill planktonic P. putida bacteria.39 Interestingly, virtually identical results were obtained when P. putida biofilms with rose-like hydrophobicity were treated with the antibiotic solution (filled bars in Fig. 5b).However, for biofilms that were osmotically treated before they were exposed to the antibiotic, the percentage of dead cells was strongly increased (dashed bars in Fig. 5b). When a hydrophobic biofilm sample was rendered hydrophilic, either by a short ethanol treatment or a long KCl treatment, the percentage of dead cells was increased from 10 to 60% and from 15 to 50%, respectively.
A similarly strong increase in the bacteria susceptibility to the antibiotic occurred when a lotus-like biofilm was converted into a rose-like biofilm, e.g. by exposure of the biofilm surface to a concentrated NaCl solution. Even though the biofilm surface remained hydrophobic in this case, the change of its wetting mode alone was sufficient to obtain an increase of dead bacterial cells from ∼20 to 70% compared to the control (filled bars in Fig. 5b).
To test for the effect of the individual osmotic agents on cell viability, the osmotic treatment was performed without subsequent antibiotic exposure. Here, the fraction of dead cells was similar for all solutions as for the control (open bars in Fig. 5b), demonstrating that the increased killing rate indeed originated from the antibiotic, and not from the osmotic agents. It is important to note that the high prevalence of live cells applies also for ethanol treated biofilms, underscoring biofilm resistance to disinfectants.53 Such results suggest that the efficiency of the antibiotic solutions is linked to the detailed mode of wetting of the biofilm surfaces.
Conclusion
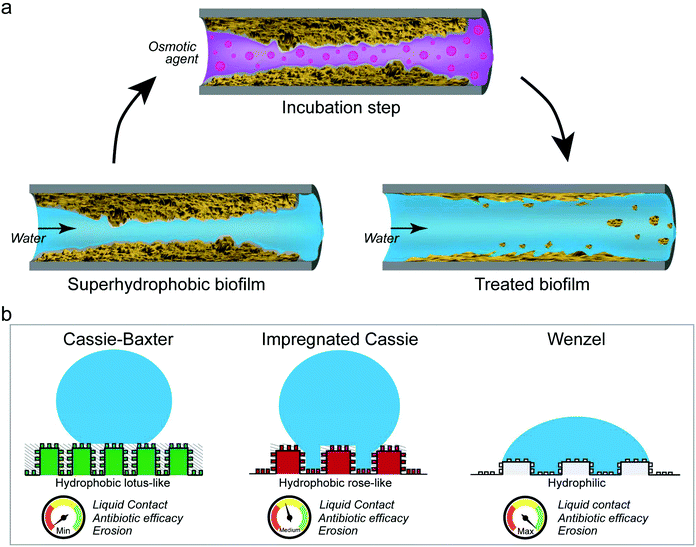
We here have shown that the wetting resistance of omniphobic biofilm colonies can be altered by incubating the biofilm surface with either ethanol solutions or concentrated salt or sugar solutions. In parallel to the observed wetting alteration, we detected topographical changes on the biofilm surfaces, suggesting that mostly physical and not chemical mechanisms are responsible for the effects described here. As a result of these alterations, the erosion sensitivity and antibiotic efficiency of the tested biofilms was increased (Fig. 6a).Although the particular wetting state obtained on a biofilm surface after the osmotic treatment depended both on the solution and the exposure time, the physical nature responsible for this effect suggests that such a treatment could be applicable to a broad range of hydrophobic biofilms. Furthermore, as demonstrated by our experiments with antibiotic solutions, already converting a lotus-like into a rose-like hydrophobic surface can be highly beneficial. We interpret this result such that removing the ‘air cushion’ between the biofilm and the water phase (i.e. eliminating the Cassie-Baxter non-wetting state) and turning the wetting mode into an impregnated Cassie state, allows aqueous solutions to reach the biofilm surface on a microscopic level (Fig. 6b). With such an approach, a treatment of biofilms with harmless solutions as presented here, will aid with the inactivation and removal of such materials from contaminated surfaces in both industrial and medical settings.
Author contributions
OL and CFG conceived the study and planned the experiments. CFG, FS, AG and WZ performed the experiments and analyzed data. MO and SS supervised a subset of experiments and contributed to the discussion. The manuscript was written through contributions of all authors.Conflicts of interest
There are no conflict of interests to declare.Acknowledgements
We thank Roberto Kolter and Masaaki Morikawa for the Bacillus subtilis strains NCIB 3610 and B-1, respectively. CFG acknowledges a CONACYT fellowship granted by the Mexican government. This project was supported by the Deutsche Forschungsgemeinschaft (DFG) through project B11 in the framework of SFB863.References
- M. Morikawa, J. Biosci. Bioeng., 2006, 101, 1–8 CrossRef CAS PubMed.
- R. Hayat, S. Ali, U. Amara, R. Khalid and I. Ahmed, Ann. Microbiol., 2010, 60, 579–598 CrossRef.
- G.-P. Sheng, H.-Q. Yu and X.-Y. Li, Biotechnol. Adv., 2010, 28, 882–894 CrossRef CAS PubMed.
- Biofilms in Wastewater Treatment, ed. S. Wuertz, P. L. Bishop and P. A. Wilderer, IWA Publishing, London, UK, 2003 Search PubMed.
- B. Halan, K. Buehler and A. Schmid, Trends Biotechnol., 2012, 30, 453–465 CrossRef CAS PubMed.
- Biofilms in Bioremediation, ed. G. Lear, Caister Academic Press, Norfolk, UK, 2016 Search PubMed.
- S. K. Behera, M. Manjaiah, S. Sekar, S. K. Panda, M. Vuyo and A. F. Mulaba-Bafubiandi, Geomicrobiol. J., 2017, 447–459 Search PubMed.
- L. Hall-Stoodley, J. W. Costerton and P. Stoodley, Nat. Rev. Microbiol., 2014, 2, 95–108 CrossRef PubMed.
- Biofilms in Medicine, Industry and Environmental Biotechnology, ed. P. Lens, V. O'Flaherty, A. P. Moran, P. Stoodley and T. Mahony, IWA Publishing, London, UK, 2003 Search PubMed.
- C. R. Arciola, D. Campoccia and L. Montanaro, Nat. Rev. Microbiol., 2018, 16, 397–409 CrossRef CAS PubMed.
- D. McDougald, S. A. Rice and S. Kjelleberg, Nat. Rev. Microbiol., 2012, 10, 39–50 CrossRef CAS PubMed.
- M. Simoes, L. C. Simoes and M. J. Vieira, LWT – Food Sci. Technol., 2010, 43, 573–583 CrossRef CAS.
- J. B. Kaplan, J. Dent. Res., 2010, 89, 205–218 CrossRef CAS PubMed.
- H. Koo, R. N. Allan, R. P. Howlin, P. Stoodley and L. Hall-Stoodley, Nat. Rev. Microbiol., 2017, 15, 740–755 CrossRef CAS PubMed.
- X. Zhu and X. J. Loh, Biomater. Sci., 2015, 12, 1505–1518 RSC.
- G. Hazell, P. W. May, P. Taylor, A. H. Nobbs, C. C. Welchc and B. Sua, Biomater. Sci., 2018, 6, 1424–1432 RSC.
- S. Bierbaum, S. Mulansky, E. Bognár, I. Kientzl, P. Nagy, N. E. Vrana, M. Weszl, E. Boschke, D. Scharnwebera and C. Wolf-Brandstetter, Biomater. Sci., 2018, 6, 1390–1402 RSC.
- A. K. Epstein, B. Pokroy, A. Seminara and J. Aizenberg, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 995–1000 CrossRef CAS PubMed.
- K. Kobayashi and M. Iwano, Mol. Microbiol., 2012, 85, 51–66 CrossRef CAS PubMed.
- S. Arnaouteli, C. E. MacPhee and N. R. Stanley-Wall, Curr. Opin. Microbiol., 2016, 34, 7–12 CrossRef CAS PubMed.
- Á. T. Kovàcs, J. van Gestel and O. P. Kuipers, Mol. Microbiol., 2012, 85, 8–11 CrossRef PubMed.
- M. Werb, C. Falcón García, N. C. Bach, S. Grumbein, S. A. Sieber, M. Opitz and O. Lieleg, NPJ Biofilms Microbiomes, 2017, 3, 11 CrossRef PubMed.
- P. S. Stewart, Pathog. Dis., 2014, 70, 212–218 CrossRef CAS PubMed.
- O. Lieleg, M. Caldara, R. Baumgärtel and K. Ribbeck, Soft Matter, 2011, 7, 3307–3314 RSC.
- P. Stoodley, R. Cargo, C. Rupp, S. Wilson and I. Klapper, J. Ind. Microbiol., 2002, 29, 361–367 CrossRef CAS PubMed.
- M. Tallawi, M. Opitz and O. Lieleg, Biomater. Sci., 2017, 5, 887–900 RSC.
- E. L. Decker, B. Frank, Y. Suo and S. Garoff, Colloids Surf., A, 1999, 156, 177–189 CrossRef CAS.
- A. Marmur, Langmuir, 2004, 20, 3517–3519 CrossRef CAS PubMed.
- L. Gao and T. J. McCarthy, Langmuir, 2006, 22, 2966–2967 CrossRef CAS PubMed.
- M. Nosonovsky and B. Bhushan, Microelectron. Eng., 2007, 84, 382–386 CrossRef.
- M. Nosonovsky and B. Bhushan, Lotus Versus Rose: Biomimetic Surface Effects, Springer, Berlin, Heidelberg, 2012 Search PubMed.
- B. Bhushan and M. Nosonovsky, Philos. Trans. R. Soc., A, 2010, 368, 4713–4728 CrossRef CAS PubMed.
- L. Feng, Y. Zhang, J. Xi, Y. Zhu, N. Wang, F. Xia and L. Jiang, Langmuir, 2008, 24, 4114–4119 CrossRef CAS PubMed.
- M. Shemesh and Y. Chai, J. Bacteriol., 2013, 195, 2747–2754 CrossRef CAS PubMed.
- S. S. Branda, J. E. González-Pastor, S. Ben-Yehuda, R. Losick and R. Kolter, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 11621–11626 CrossRef CAS PubMed.
- S. Kesel, S. Grumbein, I. Gümperlein, M. Tallawi, A.-K. Marel, O. Lieleg and M. Opitz, Appl. Environ. Microbiol., 2016, 81, 2424–2432 CrossRef PubMed.
- ISO, ISO 25178-2:2012(en), https://www.iso.org/obp/ui/#iso:std:iso:25178:-2:ed-1:v1:en, 2018).
- S. Kesel, B. von Bronk, C. Falcón García, A. Götz, O. Lieleg and M. Opitz, RSC Adv., 2017, 7, 31886–31898 RSC.
- D. M. Johnson, D. J. Biedenbach and R. N. Jones, Diagn. Microbiol. Infect. Dis., 2002, 43, 49–60 CrossRef CAS PubMed.
- O. Ciofu, E. Rojo-Molinero, M. D. Macià and A. Oliver, APMIS, 2017, 125, 304–319 CrossRef PubMed.
- CLSI, 2015, M07-A10, 112pp., ISBN: 1-56238-836-3.
- P. Stiefel, S. Schmidt-Emrich, K. Maniura-Weber and K. Ren, BMC Microbiol., 2015, 15, 36 CrossRef PubMed.
- J. Cox and M. Mann, Nat. Biotechnol., 2008, 26, 1367–1372 CrossRef CAS PubMed.
- A. Dragoš, N. Lakshmanan, M. Martin, B. Horváth, G. Maróti, C. Falcón García, O. Lieleg and Á. T. Kovács, FEMS Microbiol. Ecol., 2018, 94, fix155–fix155 CrossRef PubMed.
- A. Dragoš, M. Martin, C. Falcón Garcia, L. Kricks, P. Pausch, T. Heimerl, B. Bálint, G. Maróti, G. Bange, D. López, O. Lieleg and Á. T. Kovács, Nat. Microbiol., 2018 Search PubMed , ISSN: 2058–5276.
- S. B. Surman, J. T. Walkerb, W. Weaver, A. Skinnere, D. T. Goddardc, K. Hansonf, L. H. G. Mortond, D. Caldwellf, C. W. Keevilb and J. Kurtz, J. Microbiol. Methods, 1996, 25, 57–70 CrossRef.
- R. N. Biswal and N. L. Maguer, J. Food Process Eng., 1989, 11, 159–176 CrossRef.
- M. P. Herrling, J. Weisbrodt, C. M. Kirkland, N. H. Williamsone, S. Lacknera, S. L. Codd, J. D. Seymour, G. Guthausen and H. Horn, Biotechnol. Bioeng., 2017, 12, 2857–2867 CrossRef PubMed.
- N. L. Kavanaugh and K. Ribbeck, Appl. Environ. Microbiol., 2012, 78, 4057–4061 CrossRef CAS PubMed.
- O. Borugă, C. Jianu, C. Mişcă, I. Goleţ, A. Gruia and F. Horhat, J. Med. Life, 2014, 7, 56–60 Search PubMed.
- S. Arnaouteli, A. S. Ferreira, M. Schor, R. J. Morris, K. M. Bromley, J. Jo, K. L. Cortez, T. Sukhodub, A. R. Prescott, L. E. P. Dietrich, C. E. MacPhee and N. R. Stanley-Wall, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, E6184–E6191 CrossRef CAS PubMed.
- A. K. Yadav and S. V. Singh, J. Food Sci. Technol., 2014, 51, 1654–1673 CrossRef PubMed.
- A. Bridier, R. Briandet, V. Thomas and F. Dubois-Brissonnet, Biofouling, 2011, 27, 1017–1032 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8bm00987b |
| This journal is © The Royal Society of Chemistry 2019 |