DOI:
10.1039/C8BM01093E
(Paper)
Biomater. Sci., 2019,
7, 247-261
Versatile functionalization of surface-tailorable polymer nanohydrogels for drug delivery systems†
Received
6th September 2018
, Accepted 7th November 2018
First published on 22nd November 2018
Abstract
Surface decoration of nanohydrogels with functional molecules as well as nanomaterials offers a facile approach for developing multifunctional drug nanocarriers. Herein, the surface-tailorable polymer nanohydrogels, with the catechol groups as a universal anchor, were prepared by simple reflux-precipitation polymerization for versatile functionalization as drug delivery systems. The resultant polymer nanohydrogels were not only capable of delivering doxorubicin (DOX) through electrostatic interactions, but also exhibited facile conjugation with magnetic Fe3O4 nanoparticles and anticancer drug bortezomib (BTZ) via the versatile catechol-based coupling chemistry. The DOX and Fe3O4 loaded nanohydrogels (DOX-Fe3O4@NG) exhibited high DOX loading capability and triggered drug release behaviors in the acidic and redox environment. Furthermore, the DOX-Fe3O4@NG achieved improved cellular uptake in the presence of external magnetic field due to the active magnetic targeting properties. As for the dual drug delivery system (DOX-BTZ@NG), the DOX-BTZ@NG also released the drugs in response to the external stimuli including low pH and GSH presence, indicating their intelligent drug delivery properties. In particular, the DOX-BTZ@NG showed higher antiproliferation efficacy to cancer cells in comparison with the single drug loaded nanohydrogels, suggesting a synergistic effect of the dual drug combination therapy. The degradable poly(AA-co-DMA) nanohydrogels with surface-tailorable functionalities are thus a promising versatile platform for conjugation with both nanomaterials and drug molecules.
1. Introduction
As a unique category of hydrogels, nanohydrogels have attracted extensive interest as a superior candidate for drug delivery systems due to their hydrophilicity, flexibility and biocompatibility.1–5 A variety of stimuli-responsive polymer nanohydrogels have been fabricated as intelligent drug nanocarriers for cancer therapies.6–10 When designing the ideal drug delivery systems, surface decoration of the nanohydrogels offers a versatile approach for the preparation of multifunctional drug nanocarriers. Various functional molecules, such as targeting ligands,11,12 fluorescent probes6 and drug molecules,13,14 have been attached to the nanohydrogels for introducing multifunctionalities and improving the efficacies. In addition, the combination of the magnetic Fe3O4 nanoparticles and the drug nanocarriers allows the directional manipulation which significantly improves the cellular uptake efficiency and therapeutic efficacy.15,16 Therefore, it would be quite favorable to design stimuli-responsive nanohydrogels with surface-tailorable properties to serve as a universal platform for conjugation of both functional molecules and nanomaterials.
Usually, attachment of the above two different components (functional molecules and nanomaterials) to nanohydrogels employs either physical adsorption/encapsulation or chemical linkages. In contrast to the physical methods, carbodiimide-mediated amide coupling is a popular chemical approach for conjugation with high affinities and stabilities.17,18 Nevertheless, the carbodiimide activation process either yields a number of side products or involves sophisticated reactions with additives.17,18 Therefore, designing of surface-tailorable nanohydrogels for coupling of both functional molecules and nanomaterials through simple chemical conjugation is very desirable. As a universal biomimetic anchor, the catechol groups, inspired by the mussel adhesives, exhibit strong binding affinities to metals and metal oxides as well as high reactivities to organic molecules.19–21 The dopamine-modified Fe3O4 nanoparticles have been readily fabricated and applied in various fields.13,22,23 In addition, the catechol moieties have also been proved for their high reactivity to a variety of amine- and mercapto-functionalized molecules.19,24 Recently, their complexation with boronic acid groups has received special attention due to the dynamic nature of boronate ester linkage in different pH environments.25,26 Interestingly, the anticancer drug bortezomib (BTZ), a boronic acid analogue, suffers from rapid hepatic clearance in blood and limited therapeutic efficacy.25,27 The functional catechol groups are able to couple with BTZ via the boronate ester bonds to facilitate its delivery to increase the efficacy. Therefore, the surface-tailorable nanohydrogels with the versatile catechol groups could serve as a universal platform for versatile functionalization via the catechol-based coupling chemistry for magnetic targeting systems and dual drug combination therapy.
In recent years, the reflux precipitation polymerization (RPP) has emerged as a facile approach for the synthesis of monodisperse nanohydrogels because of its merits of high efficiency, excellent repeatability and easy operation without any surfactant or stabilizer.28,29 Moreover, it is very easy to impart desirable functionalities to the nanohydrogels by selection of different functional monomers. Herein, the dual stimuli-responsive nanohydrogels containing versatile catechol groups are prepared by one-pot RPP of acrylic acid (AA), N-(3,4-dihydroxyphenethyl) methacrylamide (DMA) and disulfide containing cross-linker bis(2-methacryloyl)oxyethyl disulfide (BMOD). The resultant nanohydrogels are not only employed as intelligent drug nanocarriers for delivering doxorubicin (DOX), but also exhibit facile conjugation abilities with magnetic Fe3O4 nanoparticles and anticancer drug bortezomib (BTZ). Finally, the therapeutic efficacies of the DOX-loaded nanohydrogels with magnetic targeting properties and the dual drug-loaded nanohydrogels (DOX and BTZ) have been evaluated.
2. Materials and methods
2.1 Materials
Acrylic acid (AA, 99%), dopamine hydrochloride (99%), methacrylic anhydride (94%), glutathione (GSH, 98%), albumin human (rHSA, >99%), iron oxide(II,III) magnetic nanoparticle solution (average particle size: 10 nm) and bis(2-methacryloyl)oxyethyl disulfide (BMOD) were purchased from Sigma-Aldrich (St Louis, MO, USA). Doxorubicin (DOX, 99%), in the form of hydrochloride salt, was supplied by Aladdin Chemistry (Shanghai, China). Bortezomib was purchased from MedChemExpress (USA). 2,2-Azobisisobutyronitrile (AIBN, 98%), glucose, FeCl3 and other reagents were obtained from Sinopharm Chemical Reagent (Shanghai, China). The solvents (analytical grade) were purchased from Nanjing Wanqing Chemical Glassware Instrument Co., Ltd (Nanjing, China) and used without further purification. The inhibitors in monomer AA were removed by distillation under reduced pressure. The initiator AIBN was purified by recrystallization from ethanol. The HeLa and normal 3T3-L1 cells, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium-bromide (MTT) and 4′,6-diamidino-2-phenylindole (DAPI) were purchased from Keygen Biotechnology (Nanjing, China). Deionized water was used in all the experiments.
2.2 Synthesis of the monomer N-(3,4-dihydroxyphenethyl) methacrylamide (DMA)
The monomer N-(3,4-dihydroxyphenethyl) methacrylamide (DMA) was synthesized by the reaction of dopamine and methacrylic anhydride according to the published papers.24,30 In a typical reaction, 10.0 g of Na2B4O7 (26.2 mmol) and 4.0 g of NaHCO3 (47.6 mmol) were dissolved in 100 mL of oxygen-depleted ultrapure water. Then 5.0 g (32.6 mmol) of dopamine hydrochloride was added to the solution and the mixture was stirred under a nitrogen atmosphere. Methacrylic anhydride (4.7 mL) was added to the above solution dropwise and the pH of the reaction mixture was controlled within 8–9. The solution was left stirring overnight at room temperature. Then the reaction solution was extracted with ethyl acetate (EA) and the pH was adjusted to 2. The mixture was extracted with EA (50 mL × 3), dried over MgSO4, concentrated to about 20 mL under reduced pressure and precipitated in hexane at 0 °C. The final product was obtained by recrystallization from boiling EA. A gray powder with a yield of 75% was obtained.
2.3 Synthesis of poly(AA-co-DMA) nanohydrogels (poly(AA-co-DMA) NG)
The multi-functional poly(AA-co-DMA) nanohydrogels were synthesized using the reflux-precipitation polymerization as follows: AA (151.3 mg, 2.1 mmol) and DMA (198.9 mg, 0.9 mmol), AIBN (7 mg, 2 wt%) and crosslinker BMOD (26.1 mg, 3% mmol) were dissolved in 40 mL of acetonitrile in a dried 100 mL three-necked flask with the aid of ultrasound (with a frequency of 37 kHz at 100% power, P30H, Elma, Germany) at room temperature for 5 min to obtain a homogeneous mixture. The flask was then immersed in a heating oil bath and attached with a reflux condenser. Subsequently, the reaction mixture was degassed with nitrogen at room temperature for 20 min to remove oxygen and then heated from ambient temperature to the boiling state within 30 min. The solution became milky white after boiling for about 5 min. Then the reaction was allowed to continue for 1 hour. The resulting poly(AA-co-DMA) nanohydrogels were centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3 min and washed in acetonitrile under ultrasonic bathing three times. Finally, the purified poly(AA-co-DMA) nanohydrogels were uniformly dispersed in deionized water and freeze-dried with a lyophilizer.
000 rpm for 3 min and washed in acetonitrile under ultrasonic bathing three times. Finally, the purified poly(AA-co-DMA) nanohydrogels were uniformly dispersed in deionized water and freeze-dried with a lyophilizer.
2.4 Preparation of DOX and Fe3O4 loaded poly(AA-co-DMA) nanohydrogels (DOX-Fe3O4@NG)
Typically, DOX (1 mg) and iron oxide (II, III) (40 μg, 1 mg mL−1) were added to phosphate buffer solution (PBS) containing 1 mg poly(AA-co-DMA) nanohydrogels. The mixture was kept shaking in an incubator at 37 °C. After 24 h, the DOX-Fe3O4@NG were collected by centrifugation and washed with PBS three times to remove the surface absorbed DOX and Fe3O4. The DOX mass loaded into poly(AA-co-DMA) nanohydrogels was calculated according to the reported method. The drug loading content (DLC) and drug loading efficiency (DLE) were calculated according to eqn (1) and (2).6 A series of DOX-Fe3O4@NG with different DOX concentrations were prepared accordingly.| | 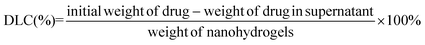 | (1) |
| | 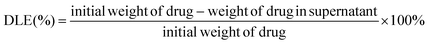 | (2) |
2.5 Preparation of DOX and BTZ loaded poly(AA-co-DMA) nanohydrogels (DOX-BTZ@NG)
DOX and BTZ loaded poly(AA-co-DMA) nanohydrogels (DOX-BTZ@NG) were prepared by the facile conjugation of boronic acid in BTZ to the catechol in poly(AA-co-DMA) nanohydrogels as follows: DOX (1 mg) and BTZ (0.5 mg in 1 mM dimethyl sulfide (DMSO)–water solution (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10)) were added to PBS solution containing 1 mg poly(AA-co-DMA) nanohydrogels. The mixture was kept shaking in an incubator at 37 °C. Afterwards, the DOX-BTZ@NG were collected by centrifugation (10
10)) were added to PBS solution containing 1 mg poly(AA-co-DMA) nanohydrogels. The mixture was kept shaking in an incubator at 37 °C. Afterwards, the DOX-BTZ@NG were collected by centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 5 min) and washed with deionized water three times to remove the unbound BTZ and DOX. The loading content of BTZ was determined by using a UV-vis spectrometer at 270 nm. The DLC and DLE for BTZ were also calculated according to eqn (1) and (2).
000 rpm, 5 min) and washed with deionized water three times to remove the unbound BTZ and DOX. The loading content of BTZ was determined by using a UV-vis spectrometer at 270 nm. The DLC and DLE for BTZ were also calculated according to eqn (1) and (2).
2.6 Characterization
Fourier transform infrared spectra (FT-IR) spectra were recorded on a Spectrum Two IR Spectrometer over potassium bromide pellets. The diffuse reflectance spectra were scanned over the wavenumber range of 400–4000 cm−1. The morphologies of poly(AA-co-DMA) nanohydrogels were characterized by using a transmission electron microscope (TEM, H-7500, Hitachi Co., Tokyo, Japan) operating at an accelerating voltage of 120 kV. The morphologies of nanohydrogels were also analyzed by scanning electron microscopy (SEM, Model S-4800, Hitachi Co., Tokyo, Japan) operating at an accelerating voltage of 5 kV. The average particle size and zeta potentials of poly(AA-co-DMA) nanohydrogels were determined using a zeta-PALS (Brookhaven, USA) with a scattering angle of 90° at 25 °C. A thermogravimetric analyzer (TGA Q500, TA Instruments, USA) was used to obtain the TGA curves of samples. Ultraviolet–visible absorption spectra were recorded with a Shimadzu 3600 Ultraviolet–visible absorption spectrophotometer. The element mapping was characterized by using a high resolution Transmission Electron Microscope (HRTEM). The cells were observed using an FV1000-I × 81 confocal laser-scanning microscope (Olympus, Japan) and the cells were sorted using a flow cytometer (Amnis flowsight, USA).
2.7
In vitro drug release of DOX-Fe3O4@NG and DOX-BTZ@NG
In vitro releases of DOX from DOX-Fe3O4@NG as well as DOX and BTZ from DOX-BTZ@NG were determined by the dialysis method. DOX-Fe3O4@NG were dispersed in 1 mL deionized water and put into a dialysis bag (MWCO: 3500 Da). Then the dialysis bag was incubated in 20 mL different buffer solutions (pH 7.4 and 5.0 with or without 10 mM GSH, respectively) at 37 °C. At various time points, an aliquot solution (2 mL) was obtained from the reservoir and 2 mL of fresh PBS buffer solution was added to maintain a constant volume. The concentration of DOX released from nanohydrogels was determined by using a UV-vis spectrometer. The release profiles of DOX and BTZ from the DOX-BTZ@NG system were measured similarly to that of the DOX-Fe3O4@NG system. Specifically, the DOX-BTZ@NG solution was incubated in 8 mL of PBS solutions at different pH values (pH 7.4 and 5.0) with or without 10 mM GSH, respectively. At different time intervals, 0.5 mL of the solution was obtained and replaced with the same volume of PBS solutions. The contents of DOX and BTZ were determined by using the UV-vis spectrometer. Besides, the buffer solution containing glucose (10 mM), FeCl3 (1.43 μg mL−1) and rHSA (30 μg mL−1) was also used to investigate the release profiles in complicated environments.
2.8 Cell culture
The HeLa and normal 3T3-L1 cells were employed for all the in vitro assays. The HeLa and normal 3T3-L1 cells were grown in Dulbecco's modified Eagle's medium (DMEM), supplemented with 10% heat-inactivated fetal bovine serum, 100 U mL−1 penicillin and 100 U mL−1 streptomycin at 37 °C in 5% CO2. The medium was replaced every two days. All cells were detached when cell confluence reached up to around 70–80% unless otherwise stated.
2.9
In vitro cellular uptake assays of DOX-Fe3O4@NG and DOX-BTZ@NG
The cellular uptake behaviors of the free drugs and drug-loaded nanohydrogels were investigated by confocal laser scanning microscopy (CLSM). The HeLa cells were incubated with DOX-Fe3O4@NG at a DOX concentration of 10 μg mL−1. After incubation for 2, 5 and 8 h, the cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) at room temperature for 15 min and were carefully washed three times with PBS; afterward, the cells were collected and re-suspended in PBS for observation under CLSM. In order to investigate the magnetic targeting effect, the Petri dish containing HeLa cells (5 × 104 cells) with DOX-Fe3O4@NG (10 μg mL−1) was placed on a magnet in the center for 1 h. Then the cellular uptake was observed by CLSM. The fluorescence of DOX in cells was determined using a flow cytometer.
2.10
In vitro apoptosis/necrosis analysis of DOX-BTZ@NG
The apoptosis/necrosis of HeLa cells treated by the blank nanohydrogels and drug loaded nanohydrogels was analyzed with an Annexin V-FITC Apoptosis Detection Kit (Keygen Biotech, Nanjing). Briefly, cells were washed two times with cold PBS and then resuspended in PBS at a concentration of 1 × 106 cells per mL. The cell suspension (100 μL) was then incubated with 5 μL of Annexin V-FITC and 5 μL of PI (propidium iodide) at room temperature for 15 min in the dark. The double-stained HeLa cells were finally sorted using a flow cytometer (Amnis flowsight, USA).
2.11
In vitro cell viability assays of DOX-Fe3O4@NG and DOX-BTZ@NG
The cytotoxicities of pure poly(AA-co-DMA) nanohydrogels and Fe3O4@NG to the normal 3T3-L1 cells were first measured by the MTT test. 3T3-L1 cells were seeded in 96-well plates at a density of 5 × 103 cells per well 24 h prior to the treatment. Cells were treated with poly(AA-co-DMA) nanohydrogels and Fe3O4@NG at various concentrations in triplicate. After 24 h or 48 h of incubation, the culture medium was replaced by a fresh medium containing 100 μL of 5 mg mL−1 MTT and continued culturing in the dark for another 4 h at 37 °C. The resulting formazan crystals were dissolved in 150 μL DMSO. The absorbance was determined using a Microplate Reader (Power Wave XS2, Bio Tek, USA) at 490 nm. Untreated cells served as the control group and the PBS solution as blank. The results were expressed as the cell viability. Secondly, the HeLa cells were used to explore the inhibitory activity of the drug loaded poly(AA-co-DMA) nanohydrogels in a similar way. HeLa Cells were seeded in a 96-well plate at the cell densities of 5 × 103 cells per well. After washing with PBS, the fresh medium containing the poly(AA-co-DMA) NG, Fe3O4@NG, free DOX and DOX-Fe3O4@NG of known concentrations were added into the plates. As for the DOX-BTZ@NG, the cytotoxicities were also studied via MTT assays against HeLa cells. The inhibitory efficacies for free DOX, free BTZ, free DOX and BTZ, DOX@NG, BTZ@NG and DOX-BTZ@NG of known concentrations were investigated. The combination index (CI), calculated from the median-effect analysis proposed by Chou and Talalay,31,32 was used to evaluate the synergistic therapeutic effect of DOX and BTZ.
3. Results and discussion
3.1 Fabrication and functionalization of the surface-tailorable poly(AA-co-DMA) nanohydrogels (poly(AA-co-DMA) NG)
The preparation of the surface-tailorable poly(AA-co-DMA) nanohydrogels and the subsequent functionalization are described in Scheme 1. Firstly, the poly(AA-co-DMA) NG are readily synthesized by one-pot reflux-precipitation polymerization of acrylic acid (AA), N-(3,4-dihydroxyphenethyl) methacrylamide (DMA) and disulfide containing cross-linker bis(2-methacryloyl)oxyethyl disulfide (BMOD). Due to the presence of the carboxyl groups in AA, the poly(AA-co-DMA) NG can load positively charged drugs such as doxorubicin (DOX) through electrostatic interaction.33,34 Because of the pH sensitive nature of electrostatic interaction and the redox responsive disulfide groups in poly(AA-co-DMA) NG, DOX release can be triggered under pH and redox stimuli. More importantly, the versatile catechol groups from DMA, a universal surface anchor, endow the poly(AA-co-DMA) NG with tailorable surface functionalities.20,21 On the one hand, the poly(AA-co-DMA) NG are easily decorated with Fe3O4 nanoparticles through the chemical complexation between catechol groups and iron. The resultant Fe3O4 and DOX loaded poly(AA-co-DMA) NG (DOX-Fe3O4@NG) are further employed as intelligent DOX nanocarriers with magnetic targeting abilities. On the other hand, the anticancer drug bortezomib (BTZ) is attached to poly(AA-co-DMA) NG through catechol-boronic acid conjugation and released because of the dissociation of boronate ester linkages at low pH.22,25 The dual drug loaded poly(AA-co-DMA) NG (DOX-BTZ@NG) are prepared for achieving enhanced treatment effectiveness by overcoming the high dose and easy generation of drug resistance in single drug delivery systems.35,36
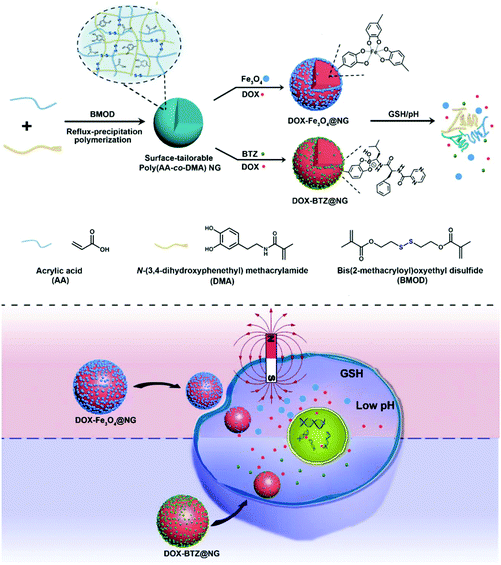 |
| | Scheme 1 Schematic illustration of the preparation and functionalization of surface-tailorable poly(AA-co-DMA) nanohydrogels for drug delivery systems. | |
3.2 Characterization of the surface-tailorable poly(AA-co-DMA) NG
A series of poly(AA-co-DMA) NG were prepared using different monomer ratios (AA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMA) to investigated the effect of the monomer composition on the morphology of nanohydrogels. The typical reaction parameters are listed in Table S1† and the corresponding transmission electron microscopy (TEM) images are shown in Fig. 1. All the nanohydrogels exhibited a spherical shape with good monodispersity. With a decrease of the molar ratio of AA
DMA) to investigated the effect of the monomer composition on the morphology of nanohydrogels. The typical reaction parameters are listed in Table S1† and the corresponding transmission electron microscopy (TEM) images are shown in Fig. 1. All the nanohydrogels exhibited a spherical shape with good monodispersity. With a decrease of the molar ratio of AA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMA from 8
DMA from 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 4
2 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, the diameter of the poly(AA-co-DMA) NG increased from 183 nm to 401 nm, indicating that the size of the nanohydrogels increased as the content of DMA increased. The poly(AA-co-DMA) NG, prepared by the molar ratio of AA and DMA monomers of 7
6, the diameter of the poly(AA-co-DMA) NG increased from 183 nm to 401 nm, indicating that the size of the nanohydrogels increased as the content of DMA increased. The poly(AA-co-DMA) NG, prepared by the molar ratio of AA and DMA monomers of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, were used in all the following experiments. The TEM, scanning electronic microscopy (SEM) and dynamic light scattering (DLS) characterization results are shown in Fig. 2. Obviously, the poly(AA-co-DMA) NG were monodisperse with an average diameter of about 205 nm determined by TEM and around 260 nm determined by DLS (Fig. 2a–c). Due to the high swelling in the aqueous solution, the DLS data of the nanohydrogels were much larger than those of TEM. The FT-IR spectra of the DMA, poly(acrylic acid) (poly(AA) NG) and poly(AA-co-DMA) nanohydrogels are shown in Fig. 2d. The absorption band at a wavenumber of 1715 cm−1, present in the FT-IR spectra of both poly(AA) and poly(AA-co-DMA) NG, was associated with the stretching vibration of the carboxylic acid group in AA.3,6 The absorption bands at a wavenumber of 1651 cm−1 and 1516 cm−1, associated with the amide I and II absorption bands, were present in both DMA and poly(AA-co-DMA) spectra.24 These results indicated the successful preparation of poly(AA-co-DMA) NG for subsequent functionalization.
3, were used in all the following experiments. The TEM, scanning electronic microscopy (SEM) and dynamic light scattering (DLS) characterization results are shown in Fig. 2. Obviously, the poly(AA-co-DMA) NG were monodisperse with an average diameter of about 205 nm determined by TEM and around 260 nm determined by DLS (Fig. 2a–c). Due to the high swelling in the aqueous solution, the DLS data of the nanohydrogels were much larger than those of TEM. The FT-IR spectra of the DMA, poly(acrylic acid) (poly(AA) NG) and poly(AA-co-DMA) nanohydrogels are shown in Fig. 2d. The absorption band at a wavenumber of 1715 cm−1, present in the FT-IR spectra of both poly(AA) and poly(AA-co-DMA) NG, was associated with the stretching vibration of the carboxylic acid group in AA.3,6 The absorption bands at a wavenumber of 1651 cm−1 and 1516 cm−1, associated with the amide I and II absorption bands, were present in both DMA and poly(AA-co-DMA) spectra.24 These results indicated the successful preparation of poly(AA-co-DMA) NG for subsequent functionalization.
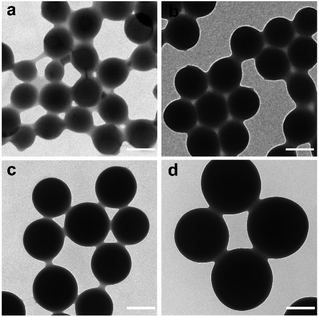 |
| | Fig. 1 TEM images of poly(AA-co-DMA) nanohydrogels with different molar ratio of AA to DMA. (a) 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, (b) 7 2, (b) 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, (c) 6 3, (c) 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and (d) 4 4, and (d) 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (scale bar: 200 nm). 6 (scale bar: 200 nm). | |
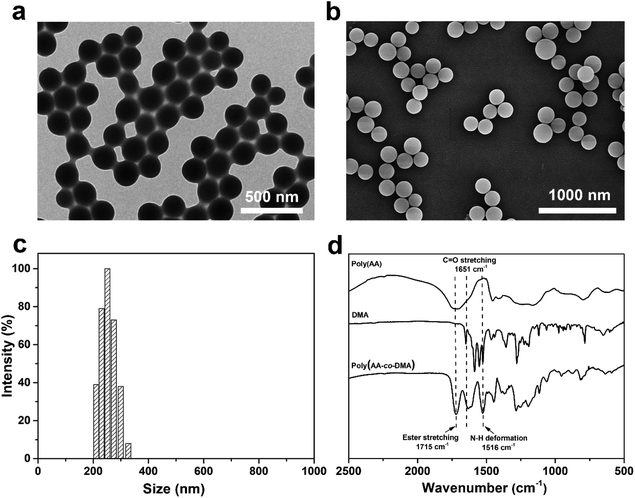 |
| | Fig. 2 (a) Representative TEM images, (b) SEM images and (c) size distribution determined by DLS of the poly(AA-co-DMA) nanohydrogels. (d) FT-IR spectra of monomer DMA, poly(AA) nanohydrogels and poly(AA-co-DMA) nanohydrogels. | |
3.3 Preparation and characterization of the DOX-Fe3O4@NG
The poly(AA-co-DMA) NG can be further functionalized with magnetic nanoparticles to be utilized as DOX nanocarriers with magnetic targeting properties. The large amount of carboxyl groups in NG is capable of loading drug DOX through the strong electrostatic interactions, while the catechol groups in NG can chelate with Fe3O4 through chemical complexation.13,22,37 Besides, the resultant DOX-Fe3O4@NG possess pH and redox stimuli-responsive properties, enabling them to be smart drug nanocarriers with controlled drug release ability and active targeting properties. Firstly, the Fe3O4@NG were characterized by the high resolution TEM (HRTEM) and elemental mapping images (Fig. 3). A number of Fe3O4 nanoparticles were presented on the Fe3O4@NG as shown in Fig. 3a (inset photograph: Fe3O4 nanoparticles). Moreover, the Fe distribution pattern in the elemental mapping images was consistent with that of Fe3O4@NG as observed in the TEM images, indicating the successful loading of Fe3O4 nanoparticles onto poly(AA-co-DMA) NG (Fig. 3b and f). In addition, the uniform distribution of the N element in Fig. 3e also proved the incorporation of the DMA component, providing catechol groups for further surface functionalization.
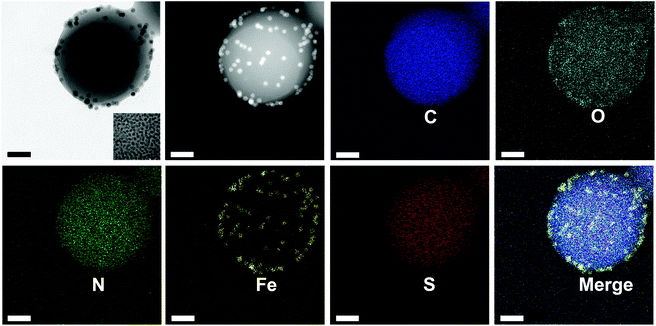 |
| | Fig. 3 HRTEM element mapping image (C, O, N, Fe, S) of Fe3O4@NG (scale bar: 50 nm). | |
The photos in Fig. 4a showed that the Fe3O4@NG were well dispersed in water and became aggregated on the side wall under external magnetic field, suggesting the good magnetic properties of Fe3O4@NG. The zeta potential for all the nanohydrogels was measured to monitor the loading processes (Fig. 4b). Poly(AA-co-DMA) NG exhibited negative charges due to the presence of a large amount of carboxyl acid groups. After loading of the positive DOX and Fe3O4, the zeta potential became neutral in comparison with the poly(AA-co-DMA) NG, indicating the high loading abilities of the nanohydrogels. To quantitatively estimate the loading mass of Fe3O4 nanoparticles onto poly(AA-co-DMA) NG, the thermogravimetric analysis (TGA) of NG, Fe3O4@NG and DOX-Fe3O4@NG was conducted (Fig. 4c); the weight loss was nearly 100 wt% for the NG in the heating process, while the weight loss of Fe3O4@NG (96.5 wt%) and DOX-Fe3O4@NG (97.4 wt%) was mainly attributed to the decomposition of the polymeric component. It suggested that the poly(AA-co-DMA) NG could be employed as promising vehicles for delivering DOX and conjugating Fe3O4 nanoparticles.
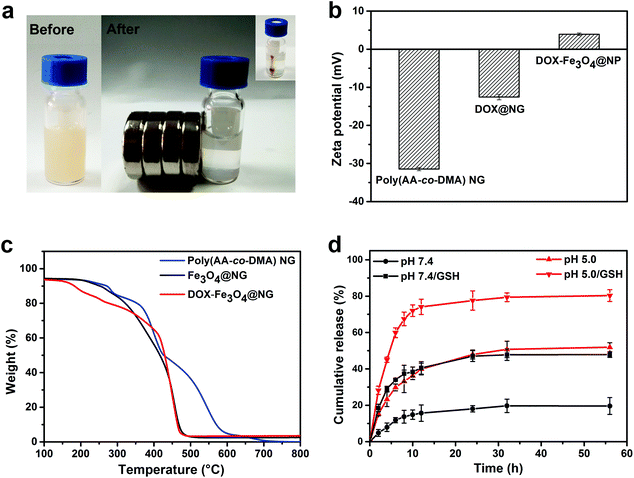 |
| | Fig. 4 (a) The aggregation behaviors of the Fe3O4@NG before and after applying an external magnetic field for 0.5 h. (b) TGA characterization and (c) zeta potentials of the poly(AA-co-DMA) nanohydrogels, Fe3O4@NG and DOX-Fe3O4@NG. (d) DOX release profiles of DOX-Fe3O4@NG in PBS at pH 5.0 and pH 7.4 with/without GSH (10 mM) at 37 °C. | |
3.4 Drug release profiles of DOX in the DOX-Fe3O4@NG
The drug loading capacity (DLC) of DOX in poly(AA-co-DMA) nanohydrogels was 98.3%, while the drug loading efficiency was 96.5%, indicating the great potential of poly(AA-co-DMA) nanohydrogels as nanocarriers with excellent drug loading abilities. Because of the reversible ionization of carboxylic and amino groups, DOX is expected to be released from poly(AA-co-DMA) NG in the acidic tumor areas and cells. Furthermore, the disulfide bonds from BMOD in the poly(AA-co-DMA) nanohydrogels could become dissociated in the reducing environment, leading to the release of DOX and degradation of the nanohydrogels. Accordingly, the DOX release behaviors for DOX-Fe3O4@NG, under low pH and redox external stimuli (high glutathione (GSH) concentration), were explored to evaluate the potential application of poly(AA-co-DMA) NG for controlled drug delivery (Fig. 4d). The DOX-Fe3O4@NG exhibited a slower release of 20.1% without GSH in comparison with that with GSH presence at pH 7.4 in 56 h (47.9%), indicating their redox-responsive properties. Furthermore, the DOX-Fe3O4@NG exhibited a higher release rate at pH 5.0 (51.9%–80.3%) than that at pH 7.4 (20.1%–47.9%) in 56 h. Similarly, the DOX release rate with GSH existence was 80.3% at pH 5.0, which was much higher than the rate without GSH of the value of 51.9% at the same pH. Without GSH and low pH stimuli, the DOX released slowly with the presence of rHSA, FeCl3 and glucose (Fig. S2a†). It confirmed the high stability of DOX-Fe3O4@NG in the simulated human blood plasma, which is preferable for the intelligent stimuli-responsive drug nanocarriers. Thus, the DOX-Fe3O4@NG exhibited different releasing behaviors in response to the different external stimuli, indicating their great potential in controlled drug delivery with high drug loading abilities.
3.5 Cellular uptake studies
The cellular uptake behaviors of the DOX-Fe3O4@NG were explored by the confocal laser scanning microscopy (CLSM) analyses (Fig. 5). After incubation with DOX-Fe3O4@NG of 2 h, the HeLa cells showed a very weak red fluorescence. As the incubation time increased to 5 h and 8 h, the red fluorescence increased gradually, indicating a progressive internalization of DOX-Fe3O4@NG in HeLa cells. This result suggested that the poly(AA-co-DMA) NG could be employed as effective nanocarriers for DOX delivery.
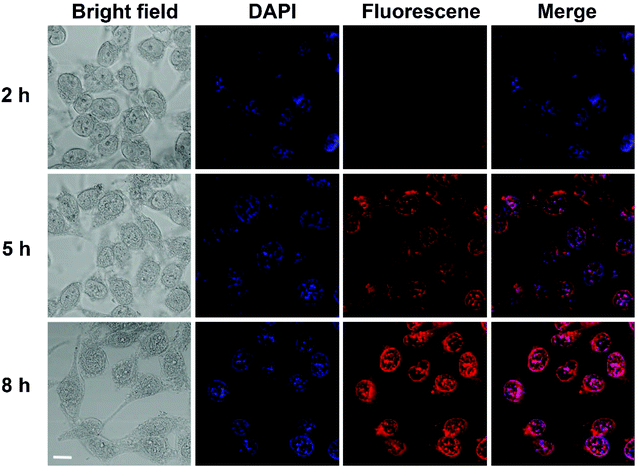 |
| | Fig. 5 CLSM images of HeLa cells incubated with DOX-Fe3O4@NG after 2 h, 5 h, and 8 h (scale bar: 10 μm). | |
The magnetic targeting properties of the Fe3O4-functionalized poly(AA-co-DMA) NG were explored by investigation of cellular uptake behaviors under magnetic field using CLSM (Fig. 6). A strong fluorescence in cells was observed above the magnet (position 1) in the whole vision, while the cells outside the magnet (position 3) showed a very dim fluorescence intensity. At the edge of the magnet (position 2), the red fluorescence of released DOX exhibited a demarcation in the half of DOX-Fe3O4@NG with/without the presence of external vision due to the different magnetic field strength. To further confirm magnetic targeting properties, the cellular uptake rates of magnetic field were explored by the flow cytometric histograms (Fig. 7). The HeLa cells without any treatment were used as a control group. In contrast with the control group, the cells incubated with DOX-Fe3O4@NG showed stronger fluorescence intensities. More importantly, the HeLa cells incubated with DOX-Fe3O4@NG under magnetic field (m-DOX-Fe3O4@NG) exhibited the highest DOX fluorescence intensities, indicating their good magnetic targeting abilities. The above results demonstrated the promising potential of DOX-Fe3O4@NG in magnetic targeted drug delivery applications.
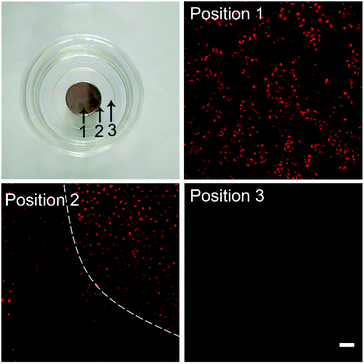 |
| | Fig. 6 Photo of the cell culture plate in the presence of a magnet and the corresponding fluorescence images of HeLa cells treated by DOX-Fe3O4@NG. Three positions were observed. Position 1: HeLa cells above the magnet, Position 2: HeLa cells at the edge of the magnet, Position 3: HeLa cells outside the magnet (scale bar: 100 μm). | |
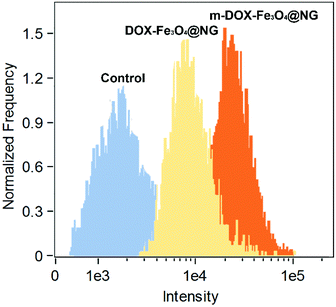 |
| | Fig. 7 Flow cytometric histograms of HeLa cells treated by DOX-Fe3O4@NG and DOX-Fe3O4@NG under magnetic field (m-DOX-Fe3O4@NG) for 2 h. | |
3.6
In vitro cytotoxicity of the DOX-Fe3O4@NG
Firstly, the cytocompatibilities of blank poly(AA-co-DMA) NG and Fe3O4@NG for 3T3-L1 normal cells were evaluated by MTT assays for a preliminary assessment purpose (Fig. 8a and b). The poly(AA-co-DMA) NG and Fe3O4@NG showed no obvious cytotoxicity on the growth of 3T3-L1 normal cells over a wide range of concentrations (0–50 μg mL−1) for both 24 and 48 h. This result indicated that both poly(AA-co-DMA) NG and different DOX concentrations (0–20 μg mL−1) were investigated (Fig. 8c and d). The cell viabilities decreased as drug concentrations Fe3O4@NG were suitable to be employed as potential candidates for drug delivery systems. Subsequently, the anti-cancer cell performances of the DOX-Fe3O4@NG, DOX-Fe3O4@NG under magnetic field (m-DOX-Fe3O4@NG), and free DOX system increased after incubation for both DOX-treated groups, indicating that the antiproliferation efficacies were drug concentration dependent. Furthermore, the cell viabilities for the DOX-Fe3O4@NG were similar to that of free DOX. After 24 h of incubation, the cell viabilities decreased to about 37.8% for the DOX-Fe3O4@NG treated cells. The value reduced to 15.6%, while the incubation time extended to 48 h. In contrast with the DOX-Fe3O4@NG group, the cell viabilities for the m-DOX-Fe3O4@NG group were slightly lower, with the values of 30.9% for 24 h and 10.4% for 48 h of incubation. The results confirmed the high anti-cancer efficiencies of the DOX-Fe3O4@NG system. Hence, the poly(AA-co-DMA) NG exhibited great potential as intelligent drug nanocarriers based on their cytocompatibility, high drug loading and controlled drug releasing abilities.
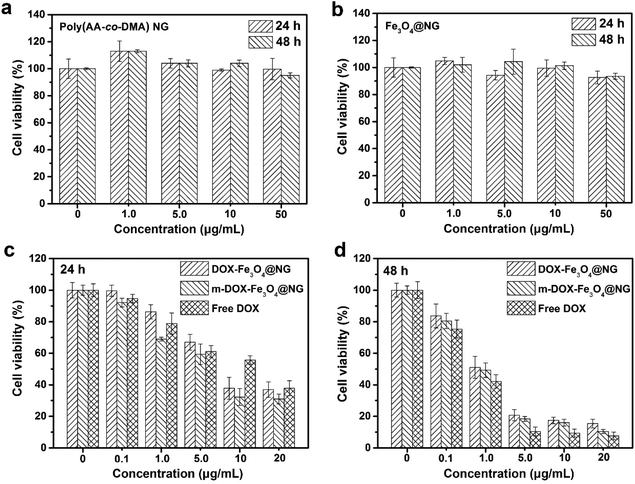 |
| | Fig. 8 Cell viabilities of 3T3-L1 cells incubated with (a) poly(AA-co-DMA) NG and (b) Fe3O4@NG for 24 h and 48 h. Cell viabilities of HeLa cells incubated with Free DOX, DOX-Fe3O4@NG and DOX-Fe3O4@NG under magnetic field (m-DOX-Fe3O4@NG) for (c) 24 h and (d) 48 h. | |
3.7 Preparation and characterization of the DOX-BTZ@NG
As a boronic acid analogue, the proteasome-inhibiting drug BTZ can be facilely conjugated to catechol functional groups in the poly(AA-co-DMA) nanohydrogels via the reversible boronate ester linkages.21,24 In a neutral or alkaline pH environment, this boronate ester conjugation is stable, which deactivates the cytotoxicity of BTZ.38,39 At low pH, the pH sensitive boronate ester bonds can be dissociated,39 leading to the BTZ release from the nanohydrogels and subsequent synergistic therapy with DOX. The loading process of the two drugs onto NG was investigated by UV-vis spectroscopy (Fig. S1†). The absorption band located at 270 nm was attributed to BTZ, while the absorption band at 480 nm was assigned to DOX.12 Both bands were observed only in the DOX-BTZ@NG, confirming the successful loading of dual drugs in poly(AA-co-DMA) NG. Moreover, the drug encapsulation efficiency was found to be dependent on the feed ratio of drugs to polymer (Table S2†). The DOX-BTZ@NG showed the highest DLC 93.7% for DOX and 26.2% for BTZ, respectively, at NG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOX
DOX![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BTZ mass ratios of 1
BTZ mass ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. In addition, the hydrodynamic diameter of the DOX-BTZ@NG system was 295 ± 38 nm, which was larger than that of NG and single drug loaded NG (BTZ@NG and DOX@NG) (Fig. 9a). The zeta potential of blank poly(AA-co-DMA) NG was −23.9 ± 0.4 mV. It increased to −15.6 ± 0.7 mV after successful loading of DOX and decreased to −30.8 ± 0.6 mV after BTZ conjugation (Fig. 9b). Accordingly, the zeta potential of DOX-BTZ@NG slightly decreased to −26.2 ± 0.7 mV due to the loading of both drugs. All these results proved the successful loading of DOX and BTZ for subsequent dual drug combination therapy.
2. In addition, the hydrodynamic diameter of the DOX-BTZ@NG system was 295 ± 38 nm, which was larger than that of NG and single drug loaded NG (BTZ@NG and DOX@NG) (Fig. 9a). The zeta potential of blank poly(AA-co-DMA) NG was −23.9 ± 0.4 mV. It increased to −15.6 ± 0.7 mV after successful loading of DOX and decreased to −30.8 ± 0.6 mV after BTZ conjugation (Fig. 9b). Accordingly, the zeta potential of DOX-BTZ@NG slightly decreased to −26.2 ± 0.7 mV due to the loading of both drugs. All these results proved the successful loading of DOX and BTZ for subsequent dual drug combination therapy.
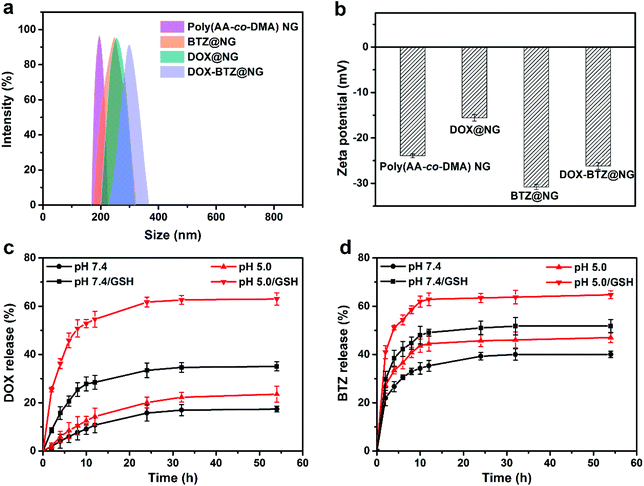 |
| | Fig. 9 (a) Size distribution determined by DLS and (b) zeta potential of poly(AA-co-DMA) NG, DOX@NG, BTZ@NG and DOX-BTZ@NG. (c) DOX and (d) BTZ release profiles of DOX-BTZ@NG in PBS at pH 5.0 and pH 7.4 at 37 °C. | |
3.8 Drug release profiles of DOX and BTZ of the DOX-BTZ@NG
In vitro DOX and BTZ release profiles of the DOX-BTZ@NG were studied at pH 7.4 and pH 5.0 at 37 °C with/without GSH, which was mimicking the physiological pH in normal tissue and cancer cells (Fig. 9c and d). Similar to the DOX release behaviors of DOX-Fe3O4@NG, the DOX release rates for DOX-BTZ@NG with GSH presence were obviously higher than that without GSH at both pH 7.4 and 5.0, while the rate also significantly increased as the pH decreased from 7.4 to 5.0 no matter whether GSH was present (Fig. 9c). The DOX release reached a plateau after 54 h with nearly 63.0% of the drug released at pH 5.0 with 10 mM GSH. As for the BTZ release profiles, a burst release of BTZ from DOX-BTZ@NG was observed in the first 10 h, and much slower release rates were observed in the following period (Fig. 9d). Similarly, the BTZ released faster at low pH with GSH presence in comparison with that of high pH and absence of GSH. Apart from the GSH triggered release caused by the disulfide bond in the poly(AA-co-DMA) NG, both drugs exhibited controlled release behaviors in response to pH variation. Specifically speaking, the pH triggered release for DOX arises from the reversible ionization of DOX and acrylic acid in the poly(AA-co-DMA) NG, while the BTZ release is attributed to the dissociation of boronate ester bonds between boric acid in BTZ and catechol in the poly(AA-co-DMA) NG. As glucose containing dihydroxyl groups could be conjugated with boric acid in BTZ,26 the existence of glucose in human blood may affect the release profiles of the BTZ and DOX. As shown in Fig. S2b,† the BTZ release with glucose of 10 mM was slightly higher than that without glucose in PBS at pH 7.4. However, this release difference induced by the glucose existence was much smaller than that caused by the GSH and pH stimuli. As for DOX, the release profiles with and without glucose presence were comparable. These results indicated the superior stability of DOX-BTZ@NG in the neutral media with the presence of glucose. Therefore, the dual stimuli-responsive poly(AA-co-DMA) NG rendered controlled drug release profiles for both DOX and BTZ in the dual drug combination therapy.
3.9 Cellular uptake studies and cell apoptosis/necrosis
The cellular uptake behaviors of the DOX-BTZ@NG were observed by CLSM. As depicted in Fig. 10, a weak red fluorescence was observed for the cells incubated with DOX-BTZ@NG in 2 h. After 5 h and 8 h, the fluorescence intensity increased progressively, indicating the internalization of DOX-BTZ@NG into HeLa cells. The apoptosis/necrosis of HeLa cells incubated with poly(AA-co-DMA) NG, BTZ@NG, DOX@NG, free DOX and BTZ and DOX-BTZ@NG were investigated by the flow cytometry (Fig. 11). The cells were double stained for viability (with PI) and apoptosis (with Annexin V-FITC). The PI− Annexin V-FITC+ cells were defined as early apoptotic cells and the PI+ Annexin V-FITC+ cells were late apoptotic cells, while PI+ Annexin V-FITC− cells were necrotic cells.24,40 The amounts of apoptotic and necrotic cells incubated with poly(AA-co-DMA) NG were very small, which were comparable to that of the untreated cells. This result also suggested the non-cytotoxic nature of the poly(AA-co-DMA) NG. As for the DOX@NG, free DOX and BTZ and DOX-BTZ@NG, a large number of apoptotic and necrotic HeLa cells were present, indicative of effectiveness of the anticancer drugs. These results confirmed that the poly(AA-co-DMA) NG could be employed as promising drug nanocarriers for co-delivery of DOX and BTZ.
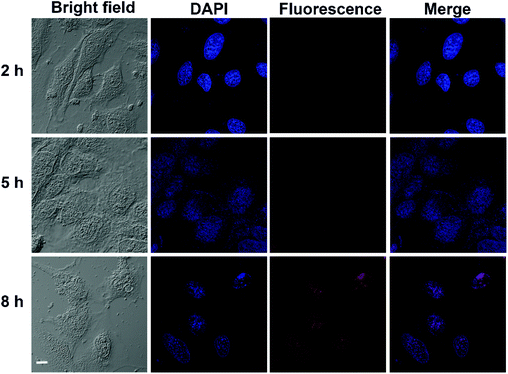 |
| | Fig. 10 CLSM images of HeLa cells incubated with DOX-BTZ@NG after 2 h, 5 h, and 8 h (scale bar: 10 μm). | |
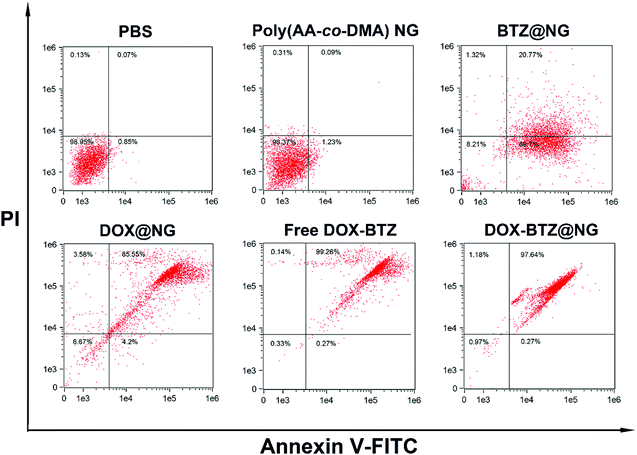 |
| | Fig. 11 Flow cytometric histograms of HeLa cells apoptosis/necrosis after incubation with PBS, poly(AA-co-DMA) NG, BTZ@NG, DOX@NG, free DOX-BTZ, and DOX-BTZ@NG using V-FITC/PI staining. | |
3.10
In vitro cytotoxicity the DOX-BTZ@NG
The anti-cancer cell performances of the DOX-BTZ@NG were investigated quantitatively (Fig. 12). Initially, the cytotoxicity of blank poly(AA-co-DMA) NG to HeLa cells was evaluated (Fig. 12a and c). After incubation with the HeLa cells for 24 and 48 h, the poly(AA-co-DMA) NG exhibited no obvious cytotoxicity in a wide concentration range from 0 to 50 μg mL−1. Subsequently, the HeLa cells were incubated with free drugs and drug loaded nanohydrogels with drug concentration ranging from 0 to 10 μg mL−1. After incubation, all free drugs and drug-loaded NG exhibited antiproliferative activities. In particular, the cancer cells decreased as the drug amounts increased, proving that the anticancer performances were concentration dependent. Moreover, the cell viabilities for the drug loaded NG treated groups were comparable to that of free drugs, as evidenced by the similar cell viabilities of around 30.9% and 6.4% after 24 and 48 h of incubation, respectively, for the cells treated by DOX-BTZ@NG and free DOX and BTZ. As expected, the DOX-BTZ@NG significantly reduced the cell viabilities in comparison with the single drug loaded nanohydrogels (BTZ@NG and DOX@NG). In order to confirm the synergistic therapeutic effect, the combination index (CI) plots calculated by the Chou–Talalay method were provided. The CI values lower than, equal to, or higher than 1.0 denote synergism, additivity or antagonism.31,40 As shown in Fig. 12e, DOX-BTZ@NG incubated with HeLa cells for 48 h showed significant synergism at lower concentrations. The half-maximal inhibitory concentration (IC50) values of DOX@NG and BTZ@NG for 48 h were 1.281 μg and 3.301 μg, respectively. In contrast, the IC50 values in the DOX-BTZ@NG system were much lower, with the value of 0.376 μg for DOX and 0.086 μg for BTZ. Accordingly, the value of CI50 was calculated as 0.32, indicating an obvious synergistic effect of two drugs (Table 1). Hence, the surface-tailorable polymer nanohydrogels are potentially ideal candidates for dual-drug delivery, equipped with good biocompatibility and synergistic therapeutic efficacy.
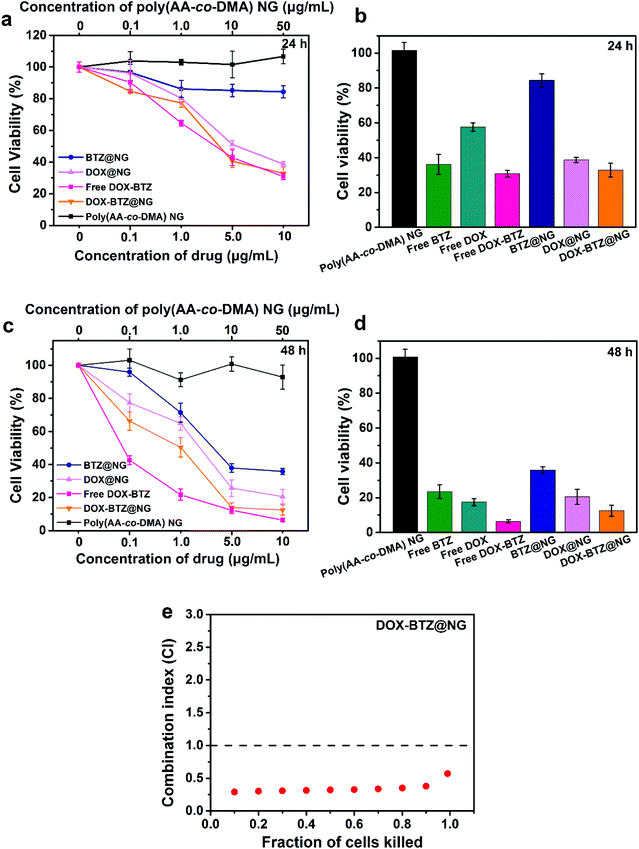 |
| | Fig. 12 Cell viabilities of HeLa cells incubated with poly(AA-co-DMA) NG, free DOX and free BTZ (Free DOX-BTZ), BTZ@NG, DOX@NG, and DOX-BTZ@NG for (a) 24 h and (c) 48 h. Cell viabilities of HeLa cells incubated with free drugs and drug-loaded nanohydrogels for (b) 24 h and (d) 48 h. (e) CI plot for DOX-BTZ@NG against HeLa cells for 48 h. | |
Table 1 IC50 (half maximal inhibitory concentration) values and combination index (CI) of drug-loaded nanohydrogels against HeLa cells for 48 h
| |
IC50 DOX (μg mL−1) |
IC50 BTZ(μg mL−1) |
CI50 |
| DOX@NG |
1.281 |
— |
— |
| BTZ@NG |
— |
3.301 |
— |
| DOX-BTZ@NG |
0.376 |
0.086 |
0.32 |
3.11 Degradation of the poly(AA-co-DMA) NG nanohydrogels
Due to the presence of disulfide and ester bonds, the poly(AA-co-DMA) NG were expected to be biodegradable in the physiological environment. Fig. 13 shows TEM images of the nanohydrogels in different external environments (pH 7.4 and pH 5.0 with 10 mM GSH). Without GSH presence, the poly(AA-co-DMA) NG maintained their spherical shape after 12 hours and became slightly deformed after 24 hours in PBS at pH 7.4. Furthermore, the nanohydrogels also exhibited relatively good stability in buffer solution with the presence of Fe3+, glucose and albumin human at pH 7.4 (Fig. S3†). These results suggested the high stability of the nanohydrogels in the neutral environment, which is preferable for the drug circulation. In contrast, the nanohydrogels were gradually decomposed in PBS at pH 5.0 with 10 mM GSH and completed degraded after 24 h. Thus, the excellent stimuli-responsive properties and biodegradable nature of thepoly(AA-co-DMA) NG were confirmed.
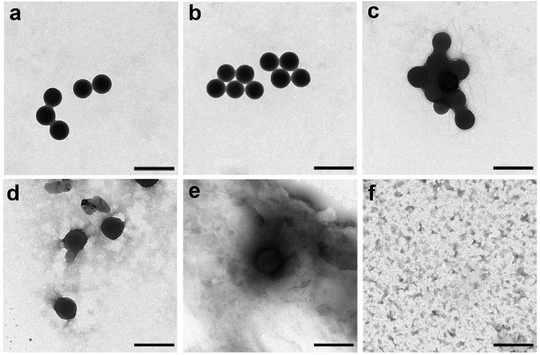 |
| | Fig. 13 Biodegradation behaviors of the nanohydrogels observed by TEM. (a) Original poly(AA-co-DMA) NG. (b and c) degraded poly(AA-co-DMA) NG in PBS at pH 7.4 for 12 h and 24 h. (d–f) degraded poly(AA-co-DMA) NG in PBS at pH 5.0 with 10 mM GSH for 1 h, 12 h and 24 h (scale bar: 500 nm). | |
4. Conclusions
The poly(AA-co-DMA) nanohydrogels containing the versatile catechol groups were prepared by one-pot reflux-precipitation polymerization. The resultant poly(AA-co-DMA) NG were not only capable of delivering DOX, but also conjugating with the magnetic Fe3O4 nanoparticles and anticancer drug BTZ. For the DOX-Fe3O4@NG system, the nanohydrogels exhibited a high drug loading capability of 98.3% for DOX and triggered drug release behaviors in the acidic and redox environment. Furthermore, the DOX-Fe3O4@NG achieved improved cellular uptake with the presence of external magnetic field due to the active magnetic targeting properties. As for the dual drug delivery system (DOX-BTZ@NG), DOX was loaded to the poly(AA-co-DMA) NG by electrostatic interaction, while BTZ was attached to the poly(AA-co-DMA) NG through chemical complexation of boric acid in BTZ and catechol groups in NG. The DOX-BTZ@NG also released the drugs in response to the external stimuli including low pH and GSH presence, indicating the intelligent drug delivery properties. In particular, the DOX-BTZ@NG showed higher antiproliferation efficacy to cancer cells in comparison with the single drug loaded nanohydrogels with a combination index of 0.32, suggesting a synergistic effect of the dual drug combination therapy. The degradable poly(AA-co-DMA) nanohydrogels with surface-tailorable functionalities are thus a promising versatile platform for conjugation of both nanomaterials and drug molecules.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
The authors would like to acknowledge the financial support of this study from the National Key Research and Development Program of China (2017YFA0205302), the Natural Science Foundation of Jiangsu Province (BK20180089, BK20160919), Program for Changjiang Scholars and Innovative Research Team in University (IRT_15R37), and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), NUPTSF (Grant No. NY218029).
References
- R. T. Chacko, J. Ventura, J. Zhuang and S. Thayumanavan, Adv. Drug Delivery Rev., 2012, 64, 836–851 CrossRef CAS PubMed.
- Y. Li, D. Maciel, J. Rodrigues, X. Shi and H. Tomas, Chem. Rev., 2015, 115, 8564–8608 CrossRef CAS PubMed.
- Y. J. Pan, Y. Y. Chen, D. R. Wang, C. Wei, J. Guo, D. R. Lu, C. C. Chu and C. C. Wang, Biomaterials, 2012, 33, 6570–6579 CrossRef CAS PubMed.
- L. Nuhn, M. Hirsch, B. Krieg, K. Koynov, K. Fischer, M. Schmidt, M. Helm and R. Zentel, ACS Nano, 2012, 6, 2198–2214 CrossRef CAS PubMed.
- M. Liu, S. Y. Shen, D. Wen, M. R. Li, T. Li, X. J. Chen, Z. Gu and R. Mo, Nano Lett., 2018, 18, 2294–2303 CrossRef CAS PubMed.
- W. J. Yang, T. T. Zhao, P. Zhou, S. M. Chen, Y. Gao, L. J. Liang, X. D. Wang and L. H. Wang, Polym. Chem., 2017, 8, 3056–3065 RSC.
- S. Jin, J. Wan, L. Meng, X. Huang, J. Guo, L. Liu and C. Wang, ACS Appl. Mater. Interfaces, 2015, 7, 19843–19852 CrossRef CAS PubMed.
- J. H. Ryu, R. T. Chacko, S. Jiwpanich, S. Bickerton, R. P. Babu and S. Thayumanavan, J. Am. Chem. Soc., 2010, 132, 17227–17235 CrossRef CAS PubMed.
- S. Kaur, C. Prasad, B. Balakrishnan and R. Banerjee, Biomater. Sci., 2015, 3, 955–987 RSC.
- Q. W. Zhu, X. J. Chen, X. Xu, Y. Zhang, C. Zhang and R. Mo, Adv. Funct. Mater., 2018, 28, 1707371 CrossRef.
- L. Nuhn, E. Bolli, S. Massa, I. Vandenberghe, K. Movahedi, B. Devreese, J. A. Van Ginderachter and B. G. De Geest, Bioconjugate Chem., 2018, 29, 2394–2405 CrossRef CAS PubMed.
- M. Duran-Lobato, B. Carrillo-Conde, Y. Khairandish and N. A. Peppas, Biomacromolecules, 2014, 15, 2725–2734 CrossRef CAS PubMed.
- R. Liu, Y. L. Guo, G. Odusote, F. L. Qu and R. D. Priestley, ACS Appl. Mater. Interfaces, 2013, 5, 9167–9171 CrossRef CAS PubMed.
- R. R. Zhang, S. S. Su, K. L. Hu, L. H. Shao, X. W. Deng, W. Sheng and Y. Wu, Nanoscale, 2015, 7, 19722–19731 RSC.
- J. Park, N. R. Kadasala, S. A. Abouelmagd, M. A. Castanares, D. S. Collins, A. Wei and Y. Yeo, Biomaterials, 2016, 101, 285–295 CrossRef CAS PubMed.
- Q. H. Feng, Y. Y. Zhang, W. X. Zhang, Y. W. Hao, Y. C. Wang, H. L. Zhang, L. Hou and Z. Z. Zhang, Acta Biomater., 2017, 49, 402–413 CrossRef CAS PubMed.
-
G. T. Hermanson, in Bioconjugate Techniques, Academic Press, Boston, 3rd edn, 2013, pp. 229–258 Search PubMed.
- E. Valeur and M. Bradley, Chem. Soc. Rev., 2009, 38, 606–631 RSC.
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS PubMed.
- E. Faure, C. Falentin-Daudre, C. Jerome, J. Lyskawa, D. Fournier, P. Woisel and C. Detrembleur, Prog. Polym. Sci., 2013, 38, 236–270 CrossRef CAS.
- J. H. Ryu, P. B. Messersmith and H. Lee, ACS Appl. Mater. Interfaces, 2018, 10, 7523–7540 CrossRef CAS PubMed.
- L.-S. Lin, Z.-X. Cong, J.-B. Cao, K.-M. Ke, Q.-L. Peng, J. Gao, H.-H. Yang, G. Liu and X. Chen, ACS Nano, 2014, 8, 3876–3883 CrossRef CAS PubMed.
- M. K. Jaiswal, M. Gogoi, H. D. Sarma, R. Banerjee and D. Bahadur, Biomater. Sci., 2014, 2, 370–380 RSC.
- W. J. Yang, P. Zhou, L. J. Liang, Y. P. Cao, J. Q. Qiao, X. H. Li, Z. G. Teng and L. H. Wang, ACS Appl. Mater. Interfaces, 2018, 10, 18560–18573 CrossRef CAS PubMed.
- J. Su, F. Chen, V. L. Cryns and P. B. Messersmith, J. Am. Chem. Soc., 2011, 133, 11850–11853 CrossRef CAS PubMed.
- Y. P. Li, W. W. Xiao, K. Xiao, L. Berti, J. T. Luo, H. P. Tseng, G. Fung and K. S. Lam, Angew. Chem., Int. Ed., 2012, 51, 2864–2869 CrossRef CAS PubMed.
- P. M. Voorhees, E. C. Dees, B. O'Neil and R. Z. Orlowski, Clin. Cancer Res., 2003, 9, 6316–6325 CAS.
- F. Wang, Y. Zhang, P. Yang, S. Jin, M. Yu, J. Guo and C. Wang, J. Mater. Chem. B, 2014, 2, 2575–2582 RSC.
- M. Fan, F. Wang and C. Wang, Macromol. Biosci., 2018, 18, 1800077 CrossRef PubMed.
- E. M. White, J. E. Seppala, P. M. Rushworth, B. W. Ritchie, S. Sharma and J. Locklin, Macromolecules, 2013, 46, 8882–8887 CrossRef CAS.
- T. C. Chou, Cancer Res., 2010, 70, 440–446 CrossRef CAS PubMed.
- S. M. Lee, T. V. O'Halloran and S. T. Nguyen, J. Am. Chem. Soc., 2010, 132, 17130–17138 CrossRef CAS PubMed.
- S. Chen, Q. Bian, P. J. Wang, X. W. Zheng, L. Lv, Z. M. Dang and G. J. Wang, Polym. Chem., 2017, 8, 6150–6157 RSC.
- W. H. Chiang, V. T. Ho, W. C. Huang, Y. F. Huang, C. S. Chern and H. C. Chiu, Langmuir, 2012, 28, 15056–15064 CrossRef CAS PubMed.
- Y. B. Cai, H. S. Shen, J. Zhan, M. L. Lin, L. H. Dai, C. H. Ren, Y. Shi, J. F. Liu, J. Gao and Z. M. Yang, J. Am. Chem. Soc., 2017, 139, 2876–2879 CrossRef CAS PubMed.
- W. B. Y. Dai, X. Wang, G. Song, T. Z. Liu, B. He, H. Zhang, X. Q. Wang and Q. Zhang, Adv. Drug Delivery Rev., 2017, 115, 23–45 CrossRef CAS PubMed.
- R. Zheng, S. Wang, Y. Tian, X. G. Jiang, D. L. Fu, S. Shen and W. L. Yang, ACS Appl. Mater. Interfaces, 2015, 7, 15876–15884 CrossRef CAS PubMed.
- A. GhavamiNejad, M. SamariKhalaj, L. E. Aguilar, C. H. Park and C. S. Kim, Sci. Rep., 2016, 6, 33594 CrossRef CAS PubMed.
- L. L. Wang, C. Y. Shi, F. A. Wright, D. D. Guo, X. Wang, D. L. Wang, R. J. H. Wojcikiewicz and J. T. Luo, Cancer Res., 2017, 77, 3293–3305 CrossRef CAS PubMed.
- X. Wu, C. He, Y. Wu and X. Chen, Biomaterials, 2016, 75, 148–162 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The characterization of poly(AA-co-DMA) nanohydrogels with different monomer ratios; drug loading efficiencies and drug loading capacities of DOX and BTZ in poly(AA-co-DMA) nanohydrogels; the UV-vis spectra of poly(AA-co-DMA) NG, DOX@NG, BTZ@NG, and DOX-BTZ@NG; the drug release profiles and the stability of the nanohydrogels in PBS solution containing glucose, Fe3+ and human albumin. See DOI: 10.1039/c8bm01093e |
|
| This journal is © The Royal Society of Chemistry 2019 |
Click here to see how this site uses Cookies. View our privacy policy here.  ,
Lijun
Liang
,
Xiaodong
Wang
,
Yanpeng
Cao
,
Wenya
Xu
,
Dongqing
Chang
,
Yu
Gao
,
Lijun
Liang
,
Xiaodong
Wang
,
Yanpeng
Cao
,
Wenya
Xu
,
Dongqing
Chang
,
Yu
Gao
 and
Lianhui
Wang
and
Lianhui
Wang
 *
*
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 3 min and washed in acetonitrile under ultrasonic bathing three times. Finally, the purified poly(AA-co-DMA) nanohydrogels were uniformly dispersed in deionized water and freeze-dried with a lyophilizer.
000 rpm for 3 min and washed in acetonitrile under ultrasonic bathing three times. Finally, the purified poly(AA-co-DMA) nanohydrogels were uniformly dispersed in deionized water and freeze-dried with a lyophilizer.
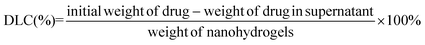
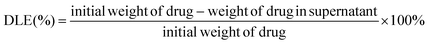
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10)) were added to PBS solution containing 1 mg poly(AA-co-DMA) nanohydrogels. The mixture was kept shaking in an incubator at 37 °C. Afterwards, the DOX-BTZ@NG were collected by centrifugation (10
10)) were added to PBS solution containing 1 mg poly(AA-co-DMA) nanohydrogels. The mixture was kept shaking in an incubator at 37 °C. Afterwards, the DOX-BTZ@NG were collected by centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 5 min) and washed with deionized water three times to remove the unbound BTZ and DOX. The loading content of BTZ was determined by using a UV-vis spectrometer at 270 nm. The DLC and DLE for BTZ were also calculated according to eqn (1) and (2).
000 rpm, 5 min) and washed with deionized water three times to remove the unbound BTZ and DOX. The loading content of BTZ was determined by using a UV-vis spectrometer at 270 nm. The DLC and DLE for BTZ were also calculated according to eqn (1) and (2).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMA) to investigated the effect of the monomer composition on the morphology of nanohydrogels. The typical reaction parameters are listed in Table S1† and the corresponding transmission electron microscopy (TEM) images are shown in Fig. 1. All the nanohydrogels exhibited a spherical shape with good monodispersity. With a decrease of the molar ratio of AA
DMA) to investigated the effect of the monomer composition on the morphology of nanohydrogels. The typical reaction parameters are listed in Table S1† and the corresponding transmission electron microscopy (TEM) images are shown in Fig. 1. All the nanohydrogels exhibited a spherical shape with good monodispersity. With a decrease of the molar ratio of AA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMA from 8
DMA from 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 4
2 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, the diameter of the poly(AA-co-DMA) NG increased from 183 nm to 401 nm, indicating that the size of the nanohydrogels increased as the content of DMA increased. The poly(AA-co-DMA) NG, prepared by the molar ratio of AA and DMA monomers of 7
6, the diameter of the poly(AA-co-DMA) NG increased from 183 nm to 401 nm, indicating that the size of the nanohydrogels increased as the content of DMA increased. The poly(AA-co-DMA) NG, prepared by the molar ratio of AA and DMA monomers of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, were used in all the following experiments. The TEM, scanning electronic microscopy (SEM) and dynamic light scattering (DLS) characterization results are shown in Fig. 2. Obviously, the poly(AA-co-DMA) NG were monodisperse with an average diameter of about 205 nm determined by TEM and around 260 nm determined by DLS (Fig. 2a–c). Due to the high swelling in the aqueous solution, the DLS data of the nanohydrogels were much larger than those of TEM. The FT-IR spectra of the DMA, poly(acrylic acid) (poly(AA) NG) and poly(AA-co-DMA) nanohydrogels are shown in Fig. 2d. The absorption band at a wavenumber of 1715 cm−1, present in the FT-IR spectra of both poly(AA) and poly(AA-co-DMA) NG, was associated with the stretching vibration of the carboxylic acid group in AA.3,6 The absorption bands at a wavenumber of 1651 cm−1 and 1516 cm−1, associated with the amide I and II absorption bands, were present in both DMA and poly(AA-co-DMA) spectra.24 These results indicated the successful preparation of poly(AA-co-DMA) NG for subsequent functionalization.
3, were used in all the following experiments. The TEM, scanning electronic microscopy (SEM) and dynamic light scattering (DLS) characterization results are shown in Fig. 2. Obviously, the poly(AA-co-DMA) NG were monodisperse with an average diameter of about 205 nm determined by TEM and around 260 nm determined by DLS (Fig. 2a–c). Due to the high swelling in the aqueous solution, the DLS data of the nanohydrogels were much larger than those of TEM. The FT-IR spectra of the DMA, poly(acrylic acid) (poly(AA) NG) and poly(AA-co-DMA) nanohydrogels are shown in Fig. 2d. The absorption band at a wavenumber of 1715 cm−1, present in the FT-IR spectra of both poly(AA) and poly(AA-co-DMA) NG, was associated with the stretching vibration of the carboxylic acid group in AA.3,6 The absorption bands at a wavenumber of 1651 cm−1 and 1516 cm−1, associated with the amide I and II absorption bands, were present in both DMA and poly(AA-co-DMA) spectra.24 These results indicated the successful preparation of poly(AA-co-DMA) NG for subsequent functionalization.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, (b) 7
2, (b) 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, (c) 6
3, (c) 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, and (d) 4
4, and (d) 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (scale bar: 200 nm).
6 (scale bar: 200 nm).

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DOX
DOX![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) BTZ mass ratios of 1
BTZ mass ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. In addition, the hydrodynamic diameter of the DOX-BTZ@NG system was 295 ± 38 nm, which was larger than that of NG and single drug loaded NG (BTZ@NG and DOX@NG) (Fig. 9a). The zeta potential of blank poly(AA-co-DMA) NG was −23.9 ± 0.4 mV. It increased to −15.6 ± 0.7 mV after successful loading of DOX and decreased to −30.8 ± 0.6 mV after BTZ conjugation (Fig. 9b). Accordingly, the zeta potential of DOX-BTZ@NG slightly decreased to −26.2 ± 0.7 mV due to the loading of both drugs. All these results proved the successful loading of DOX and BTZ for subsequent dual drug combination therapy.
2. In addition, the hydrodynamic diameter of the DOX-BTZ@NG system was 295 ± 38 nm, which was larger than that of NG and single drug loaded NG (BTZ@NG and DOX@NG) (Fig. 9a). The zeta potential of blank poly(AA-co-DMA) NG was −23.9 ± 0.4 mV. It increased to −15.6 ± 0.7 mV after successful loading of DOX and decreased to −30.8 ± 0.6 mV after BTZ conjugation (Fig. 9b). Accordingly, the zeta potential of DOX-BTZ@NG slightly decreased to −26.2 ± 0.7 mV due to the loading of both drugs. All these results proved the successful loading of DOX and BTZ for subsequent dual drug combination therapy.










