Selective growth of palladium nanocrystals on the (100) facets of truncated octahedral Cu2O for UV plasmonic photocatalysis†
Wei
Zhou
a,
Denghui
Jiang
 b,
Jianbin
Xue
a and
Xinheng
Li
b,
Jianbin
Xue
a and
Xinheng
Li
 *a
*a
aThe State Key Laboratory for Oxo Synthesis and Selective Oxidation and Suzhou Research Institute of LICP, Lanzhou Institute of Chemical Physics (LICP), Chinese Academy of Sciences, Suzhou, 215123, China. E-mail: xinhengli@licp.cas.cn
bCentre for Mineral Materials, School of Minerals Processing and Bioengineering, Central South University, Changsha 410083, China
First published on 29th November 2018
Abstract
We report Pd-based UV plasmon-enhanced photocatalysis and its hot electron effect. The selective crystalline growth of Pd nanocrystals on the (100) facets of truncated octahedral Cu2O was conducted and proved. The photocatalytic activities of Pd/Cu2O at λ = 254 nm and at λ = 365 nm respectively had ca. 18- and 10-fold enhancements due to the hot electron effect.
Plasmonic photocatalysts have emerged as promising photocatalysts because they have a variety of advantages, such as a full solar spectrum of adjustable light absorption, enhanced charge separation, and hot electron effects.1,2 Plasmonic photocatalysts generally comprise metal nanocrystals (NCs) as a cocatalyst and semiconductors as a light harvester.3 In conventional photocatalysts, metal NCs enhance photocatalytic activity by a Schottky junction with their counterpart semiconductors at the interface,4,5 which speeds up charge separation via a built-in electric field. However, metal NCs in plasmonic photocatalysts often have many more effects beyond the Schottky junction effect, such as the electric field effect, the hot electron effect, and so on.6–10 So far, the most investigated metallic nanoparticles are gold and silver due to their ease of preparation, visible light absorption and good stability.11–13 Moreover, the crystalline growth location and morphology of metallic NCs like Au have been found to influence photocatalytic performance and optoelectronic properties.14,15 For example, Au NCs were preferentially grown on the (111) planes of truncated octahedral Cu2O.14c Another example is the selective growth of metals and metal oxides on BiVO4 photocatalysts.16,17 It is known that Pd has a plasmonic absorption peak in the UV light region.18,19 However, Pd NCs have rarely been investigated for plasmonic photocatalysts, to the best of our knowledge. There is no doubt that Pd is of great importance in terms of its catalytic function in catalytic reactions and the UV plasmon effect for enhancement.
Cuprous oxide was chosen as a semiconductor due to its non-toxicity, earth-abundance, and well-established synthetic methods.20,21 Moreover, Li et al. reported spatial charge separation between adjacent facets and the selective growth of metals/metal oxides on a given facet of a semiconductor under light irradiation.16,17,22 But, the selective growth of Pd NCs on truncated octahedral Cu2O for the UV-plasmon enhancement of photocatalytic activity is still elusive. To this end, we report the selective deposition of Pd NCs on the (100) facets of truncated octahedral Cu2O, prepared using a Pd precursor ethanol solution under visible light irradiation. The selective deposition of Pd NCs was determined by SEM, TEM/HRTEM, XRD and UV-vis absorption spectra. The UV plasmon-enhanced photocatalytic activities were proved by testing under single wavelength UV light irradiation. The photocatalytic mechanism of the hot electron effect was then proposed after comparing with control experiments.
Fig. 1 shows the selective growth of Pd NCs on truncated octahedral Cu2O. Fig. 1A shows that the truncated octahedral Cu2O microcrystals had an edge length of about 550 nm. It was indeed seen that only the (100) facets of Cu2O were covered with Pd NCs with a diameter of ca. 17 nm, as shown in Fig. S1.† Note that 0.06 mL of Na2PdCl4 ethanol solution was used therein. The inset image in Fig. 1A clearly shows that Pd NCs were predominantly formed on the (100) facets of Cu2O with high selectivity. It seems that Pd nanoparticles were polyhedral and well dispersed on the surface. Note that the Pd NCs were assumed to be spherical in order to estimate their size. Pd nanoparticles were seen on the outer edges of the (100) facet of Cu2O and were well dispersed. A lattice spacing of 2.25 Å was ascribed to the (111) facet of the Pd NCs. The XRD patterns of the products shown in Fig. 1D present strong Cu2O diffraction peaks and a Pd peak as indicated with an asterisk. The inset image in Fig. 1D is a magnification of the Pd peak, clearly indicating the formation of Pd NCs. In addition, as shown in Fig. 1B, the Pd NCs had intimate contact with the Cu2O octahedra at the interface. In conjunction with the SEM image, Pd NCs preferentially deposited on the (100) facet of truncated octahedral Cu2O were proved. The very weak XRD peak of Pd suggests that the tiny spots obscurely seen were not Pd.
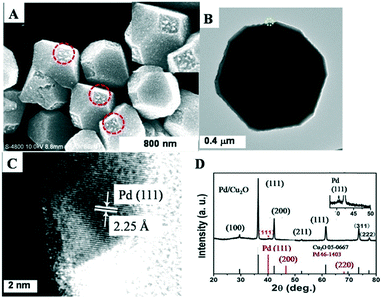 | ||
| Fig. 1 SEM image (A), TEM image (B), HRTEM image (C), and XRD spectra (D) of Pd/truncated octahedral Cu2O. Inset in (A) and (D) are the corresponding magnifications. | ||
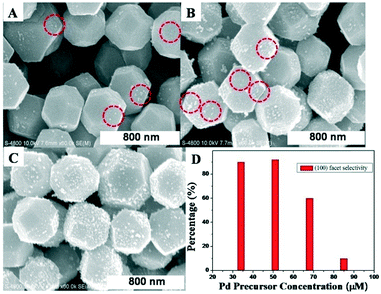
Fig. 2 demonstrates the effect of Pd precursor concentration on the growth selectivity of the Pd NCs. Roughly 90% selective deposition of the Pd NCs on the (100) facet was observed for the 0.04 mL and 0.06 mL cases, as shown in Fig. 1A and Fig. 2A and D. This showed preferential deposition of the (100) facet deposition. As the volume of Na2PdCl4 ethanol solution was further increased to 0.08 mL and 0.1 mL, respectively, Pd NCs started to grow on the (111) facets and lost high selectivity gradually, as shown in Fig. 2B and C. Nevertheless, as for the 0.08 mL case, more Pd NCs were seen on the (100) facets than on the (111) facets, revealing that (100) facet deposition was still dominant. The Pd precursor concentration-dependence of selective deposition indicates that this method was feasible and controllable. In addition, the size of the Pd NCs increased as the volume of Na2PdCl4 ethanol solution increased due to Ostwald ripening, as shown in Table S1.†
In order to explore the selective growth mechanism of the Pd NCs, we performed control experiments where the samples were prepared under no visible light irradiation, as shown in Fig. S2.† It was seen that Pd NCs were preferentially grown on the (111) facet rather than on the (100) facet, similar to Au deposition on the (111) facet reported in an earlier study.14c Au NC selective growth was attributed to the higher surface energy of the (111) facet compared to the (100) facet. However, under light irradiation, the growth mechanism is obviously different from that under no light irradiation. So, it is plausible to assume that the selective growth of Pd NCs on the (100) facets was mainly the result of photo-generated electrons transferring to the (100) facet and holes transferring to the (111) facet. In order to further prove our hypothesis, we studied the selective growth of NiO under similar conditions. It was indeed observed that NiO NCs were preferentially grown on the (111) facets of truncated octahedral Cu2O, as shown in Fig. S3.† The formation of NiO NCs was proved by XRD data. These results proved that photo-generated holes moved to the (111) facets to oxidize Ni precursors, resulting in the formation of NiO NCs therein, which was in good agreement with Pd NC deposition on the (100) facets. The above observations fully complied with the spatial charge separation between the adjacent facets.16,17
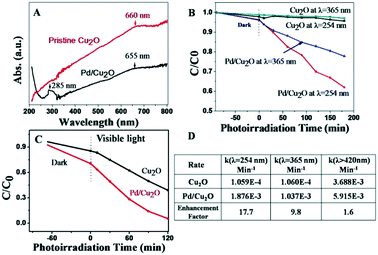
Fig. 3 shows that the as-obtained Pd/Cu2O had an absorption peak at ca. λ = 285 nm. The peak at ca. λ = 285 nm was attributed to the plasmonic absorption of Pd NCs according to the literature and our control experiment, as shown in Fig. S4.† Cu2O did not absorb UV light but it had a broad scattering peak at ca. λ = 660 nm covering its other visible light absorption peaks. Pristine Cu2O did not show noticeable photocatalytic activity at both λ = 365 nm and λ = 254 nm, as shown in Fig. 3B. By contrast, Pd/Cu2O showed excellent photocatalytic activities at both λ = 365 nm and λ = 254 nm, respectively. Moreover, the photocatalytic activity at λ = 254 nm was better than that at λ = 365 nm. The corresponding enhancement factors by the Pd nanoparticles were ca. 18- and 10- fold, respectively, as shown in Fig. 3D. We conducted further photocatalytic activity tests under visible light irradiation, as shown in Fig. 3C, and its enhancement factor was ca. 1.6. Under visible light irradiation, Pd did not produce a plasmon peak so the enhancement was attributed to the Schottky junction. Moreover, the photocatalytic activity of Pd on the (111) facet was obviously lower than that of Pd on the (100) facet, as shown in Fig. S5.†
Regarding UV-plasmon enhancement, it has been reported that Al nanoparticles caused more than a 10-fold enhancement of the photocatalytic activity of TiO2 in the range of 260–340 nm.23 But, their enhancement mechanism is complicated because TiO2 itself has excellent UV light photocatalytic activity. TiO2-tipped Au nanorods showed excellent photocatalytic activity under visible light irradiation but TiO2 had no visible light absorption, so they proposed the hot electron effect.8b In our case, since Cu2O did not absorb UV light, the Pd-based UV plasmon-enhancement mechanism should be predominated by the hot electron effect.
In order to explore the role of O2 on photocatalytic reactions, MO aqueous solution was purged under N2 with 99.99% purity. We found that Pd/Cu2O almost lost photocatalytic activity at both single UV wavelengths with no O2 involvement, as shown in Fig. S6.† This control experiment clearly indicates O2 participation in MO photocatalytic degradation, although the exact reaction paths are unclear. Fig. S6B† shows that pristine Pd NCs almost had no considerable photocatalytic activity, excluding the effect of the Pd NCs. Note that the surface ligand PVP of the Pd NCs resulted in a small amount of MO adsorption.
So, we propose that the Pd-based UV plasmon photocatalytic mechanism is the hot electron effect, as shown in Fig. 4. In detail, Pd NCs generate hot electrons upon absorbing UV light. The hot electrons transfer to the (100) facet, as shown in Fig. 4A. Meanwhile, the hot electrons have a higher energy level than the conduction band of Cu2O and therefore transfer to its conduction band, as shown in Fig. 4B. Afterwards, with the involvement of the transferred electrons and O2 in aqueous solution, MO becomes photo-degraded on the (100) facets. Since the plasmon peak of the Pd NCs had more overlap at λ = 254 nm than at λ = 365 nm, it is reasonable to assume that Pd/Cu2O had a bigger enhancement factor at λ = 254 nm than at λ = 365 nm. It is noteworthy that the wavelength range of the UV lamp used was tens of nm.
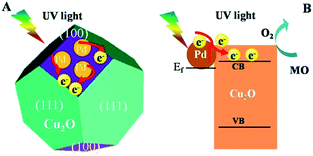 | ||
| Fig. 4 Proposed photocatalytic mechanism of the hot electron effect with respect to facets (A) and band alignment (B). | ||
Conclusions
In conclusion, the selective crystalline growth of Pd nanocrystals on the (100) facets of truncated octahedral Cu2O prepared using Na2PdCl4 ethanol solution under visible light irradiation was conducted. The preparation method made use of the spatial charge separation between the adjacent facets under light irradiation. SEM, TEM/HRTEM and XRD characterization showed that the Pd nanocrystals were preferentially grown on the (100) facets of Cu2O with high selectivity. The Na2PdCl4 concentration-dependence of the growth selectivity and control experiments proved that the selective growth of the Pd nanocrystals was caused by the (100) facets' electron-rich feature which resulted from spatial charge separation. Pd/Cu2O had a relatively broad plasmonic absorption peak at ca. λ = 285 nm. The photocatalytic activities of Pd/Cu2O at λ = 254 nm and at λ = 365 nm respectively had ca. 18- and 10- fold enhancements compared to pristine Cu2O. The photocatalytic results and control experiments proved that the photocatalytic activity enhancements were predominated by the hot electron effect.Conflicts of interest
There are no conflicts to declare.Acknowledgements
X.-H. Li acknowledges financial support from the National Natural Science Foundation of China (21573263 and 21872157), the National Key Research and Development Program of China from the Ministry of Science and Technology of China (2016YFE0105700), the Henan Provincial Open and Cooperation Foundation of China (60), and the Suzhou Science and Technology program (SYG201743). D.-H. Jiang is grateful for the financial support from the National Natural Science Foundation of China (51402346).Notes and references
- M. Xiao, R. Jiang, F. Wang, C. Fang, J. Wang and J. C. Yu, J. Mater. Chem. A, 2013, 1, 5790 RSC.
- X. Zhang, Y. L. Chen, R.-S. Liu and D. P. Tsai, Rep. Prog. Phys., 2013, 76, 046401 CrossRef.
- K. Awazu, M. Fujimaki, C. Rockstuhl, J. Tominaga, H. Murakami, Y. Ohki, N. Yoshida and T. Watanabe, J. Am. Chem. Soc., 2008, 130, 1676 CrossRef CAS.
- H. Wang, L. Zhang, Z. Chen, J. Hu, S. Z. Wang, J. Liu and X. C. Wang, Chem. Soc. Rev., 2014, 43, 5234 RSC.
- G. Zhang, Z. Lan and X. C. Wang, Chem. Sci., 2017, 8, 5261 RSC.
- D. Jiang, W. Zhou, X. Zhong, Y. Zhang and X. Li, ACS Appl. Mater. Interfaces, 2014, 6, 10958 CrossRef CAS PubMed.
- M. A. Mahmoud, W. Qian and M. A. El-Sayed, Nano Lett., 2011, 11, 3285 CrossRef CAS PubMed.
- (a) S. K. Cushing, J. Li, F. Meng, T. R. Senty, S. Suri, M. Zhi, M. Li, A. D. Bristow and N. Wu, J. Am. Chem. Soc., 2012, 134, 15033 CrossRef CAS PubMed; (b) B. Wu, D. Liu, S. Mubeen, T. T. Chuong, M. Moskovits and G. D. Stucky, J. Am. Chem. Soc., 2016, 1114 CrossRef CAS.
- S. Mubeen, J. Lee, N. Singh, S. Kramer, G. D. Stucky and M. Moskovits, Nat. Nanotechnol., 2013, 8, 247 CrossRef CAS.
- M. K. Kumar, S. Krishnamoorthy, L. K. Tan, S. Y. Chiam, S. Tripathy and H. Gao, ACS Catal., 2011, 1, 300 CrossRef CAS.
- (a) M. Moskovits, Nat. Nanotechnol., 2015, 10, 6 CrossRef CAS; (b) S. Mubeen, J. Lee, D. Liu, G. D. Stucky and M. Moskovits, Nano Lett., 2015, 15, 2132 CrossRef CAS PubMed.
- (a) P. Christopher, H. L. Xin and S. Linic, Nat. Chem., 2011, 3, 467 CrossRef CAS PubMed; (b) D. B. Ingram and S. Linic, J. Am. Chem. Soc., 2011, 133, 5202 CrossRef CAS PubMed.
- C. Gomes Silva, R. Juárez, T. Marino, R. Molinari and H. García, J. Am. Chem. Soc., 2011, 133, 595 CrossRef CAS PubMed.
- (a) Y. Shemesh, J. Macdonald, G. Menagen and U. Banin, Angew. Chem., Int. Ed., 2011, 50, 1185 CrossRef CAS PubMed; (b) P. Li, Z. Wei, T. Wu, Q. Peng and Y. Li, J. Am. Chem. Soc., 2011, 133, 5660 CrossRef CAS PubMed; (c) X. Liu, Langmuir, 2011, 27, 9100 CrossRef CAS PubMed.
- R. Costi, A. Saunders and U. Banin, Angew. Chem., Int. Ed., 2010, 49, 4878 CrossRef CAS PubMed.
- R. G. Li, F. X. Zhang, D. Wang, J. Yang, M. Li, J. Zhu, X. Zhou, H. Han and C. Li, Nat. Comm., 2013, 4, 1432 CrossRef PubMed.
- J. Zhu, F. Fan, G. Chen, H. An, Z. Feng and C. Li, Angew. Chem., Int. Ed., 2015, 54, 9111 CrossRef CAS PubMed.
- Y. Xiong, J. Chen, B. Wiley, Y. Xia, Y. Yin and Z.-Y. Li, Nano Lett., 2005, 5, 1237 CrossRef CAS PubMed.
- (a) R. Long, K. Mao, M. Gong, S. Zhou, J. Hu, M. Zhi, Y. You, S. Bai, J. Jiang, Q. Zhang, X. Wu and Y. Xiong, Angew. Chem., Int. Ed., 2014, 53, 3205 CrossRef CAS PubMed; (b) R. Long, Z. Rao, K. Mao, Y. Li, C. Zhang, Q. Liu, C. Wang, Z.-Y. Li, X. Wu and Y. Xiong, Angew. Chem., Int. Ed., 2015, 54, 2425 CrossRef CAS PubMed.
- W.-C. Huang, L.-M. Lyu, Y.-C. Yang and M. H. Huang, J. Am. Chem. Soc., 2012, 134, 1261 CrossRef CAS PubMed.
- D. Jiang, J. Xue, L. Wu, W. Zhou, Y. Zhang and X. Li, Appl. Catal., A, 2017, 211, 199 CrossRef CAS.
- R. Chen, S. Pang, H. An, J. Zhu, S. Ye, Y. Gao, F. Fan and C. Li, Nat. Energy, 2018, 3, 655 CrossRef CAS.
- M. Honda, Y. Kumamoto, A. Taguchi, Y. Saito and S. Kawata, Appl. Phys. Lett., 2014, 104, 061108 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8ce01697f |
| This journal is © The Royal Society of Chemistry 2019 |