A novel MnO2/MXene composite prepared by electrostatic self-assembly and its use as an electrode for enhanced supercapacitive performance†
Shu
Chen
a,
Yuanfang
Xiang
a,
Weijian
Xu
 *a and
Chang
Peng
*ab
*a and
Chang
Peng
*ab
aCollege of Chemistry and Chemical Engineering, Hunan University, Hunan 410082, P.R. China. E-mail: weijianxu_59@sina.com; pch1026@126.com
bCollege of Science, Hunan Agricultural University, Hunan 410128, P.R. China
First published on 19th November 2018
Abstract
MXene is a new 2D transition metal carbide possessing metallic conductivity and hydrophilic surfaces. It has drawn widespread attention as a potential material for electrode use. However, the applications of MXene are limited by its property of low electrical capacity. In this paper, a novel MnO2/MXene composite is prepared by electrostatic self-assembly. Firstly, delaminated MnO2 nanosheets are obtained through the intercalation delamination of multilayered H-MnO2 in a cationic Gemini surfactant (Gem) solution, leading to a positively charged surface. Then, the positive MnO2 nanosheets are assembled on negative MXene nanosheets through electrostatic self-assembly to form a MnO2/MXene composite. The characterization results show that the MnO2 nanosheets are intimately assembled on the MXene nanosheets. As an electrode material, the MnO2/MXene composite displays a specific capacitance of 340 F g−1 at 1 A g−1, which is three times the performance of an MXene electrode. In addition, the MnO2/MXene electrode shows a high retention rate (90.3% retention at 10 A g−1) and good cycling life (87.6% of the initial specific capacitance is retained after 2000 cycles at 4 A g−1). The properties of the proposed composite are attributed to the excellent conductivity of MXene and the high specific capacitance of MnO2.
1. Introduction
With the rapidly growing energy demand, the development of energy storage systems with high-efficiency and low-cost has become a critical issue.1–4 The exploration of new electrode materials is an essential topic to improve power storage. An ideal electrode material is expected to deliver the electrical charges promptly and store the charges at high density. However, it is not easy to find a material with the above two requirements at the same. In recent years, supercapacitors have been attracting more and more attention due to their good electrochemical properties, such as excellent cycling stability, ultrafast charge-discharge, and long-term cycling.5–7Therefore, researchers have investigated new electrode materials, such as transition metal oxides and carbon materials, with outstanding electrochemical properties for energy storage applications. Some of the most widely used electrode materials are carbon materials, with active sites for redox reactions and high conductivity.8,9 However, carbon materials exhibit a low specific capacitance and limited voltage window. Thus, transition metal oxides have attracted more attention and become the most important electrode materials because of their high-performance in pseudocapacitors.10–16 Recently, a two-dimensional titanium carbide material labeled MXene was reported, which shows both metallic conductivity and hydrophilic features.17 This novel material is prepared by etching aluminium from the corresponding ‘MAX’ phase carbides in hydrofluoric acid. MXene is classified as an inorganic graphene oxide, since it is highly conductive, like the ‘MAX’ phase before etching.18–20 Thus, MXene has the potential to be used as an effective material for electrochemical supercapacitors, to manufacture powerful energy storage devices.21,22 During the HF etching process, the surfaces of MXene are terminated by –F, –O, and –OH groups, and then the MXene can be named as Mn+1XnTx, where M is the transition metal, X is the carbon, and T is the terminating group.23,24 Thus, MXene can be made to express different properties by modifying these terminating groups with various addends.
MXenes exhibit high volumetric capacitance, stable cycling performance, and long-term cycling.17 However, compared to graphene, the MXenes still have lower gravimetric capacitance, which could be improved in future studies. In general, the pseudocapacitive transition metal oxides provide a large capacity of charge storage, and then enhance the electrochemical performance of carbon materials. Meanwhile, the high theoretical pseudocapacitance of the transition metal oxides can be effectively utilized by carbon materials with good mechanical properties and conductivity.25,26 For example, MnO2 nanowhiskers have been used to fabricate MnO2 nanowhisker/MXene composites by direct chemical synthesis at 60 °C, and the specific capacitance of the MXene can be improved due to the additional contribution of the pseudocapacitance provided by the MnO2 nanowhiskers.26 Thus, MnO2 is an ideal candidate for enhancing the supercapacitive performance of MXene owing to its low cost, high theoretical value, and electron transfer/storage ability.27,28
Recently, birnessite-type MnO2 nanosheets29–33 have been reported as a new class of electrode materials due to their long cycling life and high specific capacitance. Thus, surface decoration using birnessite-type MnO2 nanosheets may also improve the electrochemical properties of MXene. However, the simple physical and mechanical mixing of MnO2 nanosheets and MXene nanosheets may not efficiently prevent the self-stacking of these two types of nanosheets due to van der Waals interactions. A suitable integration strategy using the MnO2 nanosheets for the preparation of MnO2/MXene composites remains a scientific problem. Electrostatic self-assembly is one of the simplest and most effective ways to fabricate advanced composites for power source applications.34–36 MXene is a negatively charged material due to its surface functional groups.24 When the two types of nanosheets have opposite surface charges to each other, the electrostatic interactions between the MnO2 nanosheets and MXene nanosheets induce a self-assembly process that results in the formation of a MnO2/MXene composite. The contact between the two types of nanosheets via this self-assembly synthesis can greatly promote the conductivity of the composite. However, to the best of our knowledge, the use of electrostatic self-assembly to fabricate a layered MnO2/MXene composite has not been reported before.
Gemini surfactants (Gem) are a new type of surfactant with two cationic head groups, and two hydrophobic tail chains, linked by a spacer at the heads, which can alter the electrostatic charges to hybridize the 2D materials and provide many attractive properties such as good aqueous dispersion and modifiable ability.37 In our work, MnO2 nanosheets were obtained through Gem intercalation and exfoliation of multilayered H-MnO2 to change the charges on the surface of the MnO2 nanosheets to positive and form a stable dispersion. Since the surface charges of the MnO2 nanosheets and MXene nanosheets are opposite, the two types of nanosheets can attract each other via electrostatic interactions. As a result, we have fabricated a novel layered MnO2/MXene composite for the first time through electrostatic self-assembly. The architecture of the composite enabled excellent contact, thereby enhancing the interfacial electron transfer. Furthermore, we carefully studied the electrochemical performance of the MnO2/MXene electrode using a three-electrode system, which led to excellent results of high specific capacitance and good stability.
2. Materials and characterization
Synthesis of multilayered H-birnessite MnO2 (H-MnO2)
The birnessite MnO2 was synthesized according to the literature.38 The obtained birnessite MnO2 (1 g) was added to 500 mL of HCl (0.1 M) under stirring for 48 h. After the mixture was filtered and dried, multilayered H-MnO2 was obtained.Synthesis of the delaminated MnO2 nanosheets
Gem (C18N22+Br2−) was synthesized according to the literature.39 The 1H nuclear magnetic resonance (1H NMR) of Gem is shown in Fig. S1 (ESI†). The synthesis of the MnO2 nanosheets was carried out in Gem solution. First, 0.5 g of H-MnO2 was added to 500 mL of Gem solution (0.5 M) under stirring for 6 h and bath-sonicated for 2 h. The obtained dispersion solution was centrifuged for 30 min at 8000 rpm. The brown supernatant was collected to obtain the delaminated MnO2 suspension. Finally, after washing the unbound Gem in the colloidal suspension and filtering the mixture, MnO2 nanosheets were obtained.Synthesis of multilayered Ti3C2Tx
Multilayered Ti3C2Tx was prepared by etching Ti3AlC2. 1 g of LiF (98.5%) was added to 10 mL of HCl solution (9 M), and 1 g of Ti3AlC2 was added to the above solution under stirring for 24 h at 35 °C. Then, the mixture was washed with water using centrifugation until the pH of the supernatant was above 5. Multilayered Ti3C2Tx was then obtained by freeze drying.Synthesis of delaminated Ti3C2Tx (MXene)
Multilayered Ti3C2Tx (1 g) was added to 250 mL of deionized water and sonicated for 1 h under Ar flow. Then, the dispersion solution was centrifuged for 1 h at 3500 rpm. The dark green supernatant was collected to obtain the delaminated MXene suspension. This MXene suspension was filtered and freeze-dried to measure the concentration of the delaminated MXene.Synthesis of MnO2/MXene composite
The MnO2/MXene composite was prepared by very slowly mixing two dispersion solutions of positive MnO2 nanosheets (50 mL, 0.2 mg mL−1) and negative MXene nanosheets (50 mL, 0.2 mg mL−1). The composite quickly transforms into a precipitate and settles to the bottom due to electrostatic self-assembly. Finally, the MnO2/MXene composite was obtained by filtering the mixture.Characterization
A Varian Inova-400 spectrometer was used to record the 1H NMR of the Gem. A Malvern Zetasizer Nano ZS90 (Malvern Instruments, UK) was used to measure all zeta potentials of the samples. Scanning electron microscopy (SEM) (JSM-6700F, Hitachi Ltd) and transmission electron microscopy (TEM) (JEM-2100F, JEOL) were used to inspect the morphology of the materials. The element mapping analysis and energy dispersive spectroscopy (EDS) results were acquired by using TEM. An X-ray diffraction (XRD) instrument (Rigaku, Ltd, JP) was used to measure the crystallinity of the materials. A PHI 5000c ESCA photoelectron spectrometer was used to record the X-ray photoelectron spectroscopy (XPS) spectra of the materials.A CHI 760C workstation (CH Instruments Inc.) with a three-electrode system was used to measure the cycle voltammetry (CV) and galvanostatic charge-discharge (GCD) curves. The working electrode was prepared by mixing the active electrode material, acetylene black, and polyvinylidene fluoride with a mass ratio of 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 to form a slurry, and then the slurry was coated onto stainless steel foil. The active material loading on the stainless steel foil, which served as the working electrode, was 5 mg cm−2. The stainless steel foil was ultrasonicated in Na2SO4 (1 M) for 10 minutes before use, and rinsed with acetone and deionized water, followed by vacuum drying. All electrochemical experiments were carried out in 1 M Na2SO4. Platinum wire and a calomel electrode were used as the counter and reference electrodes, respectively. The gravimetric capacitances can be calculated using eqn (1) and (2):
5 to form a slurry, and then the slurry was coated onto stainless steel foil. The active material loading on the stainless steel foil, which served as the working electrode, was 5 mg cm−2. The stainless steel foil was ultrasonicated in Na2SO4 (1 M) for 10 minutes before use, and rinsed with acetone and deionized water, followed by vacuum drying. All electrochemical experiments were carried out in 1 M Na2SO4. Platinum wire and a calomel electrode were used as the counter and reference electrodes, respectively. The gravimetric capacitances can be calculated using eqn (1) and (2):
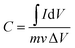 | (1) |
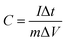 | (2) |
3. Results and discussion
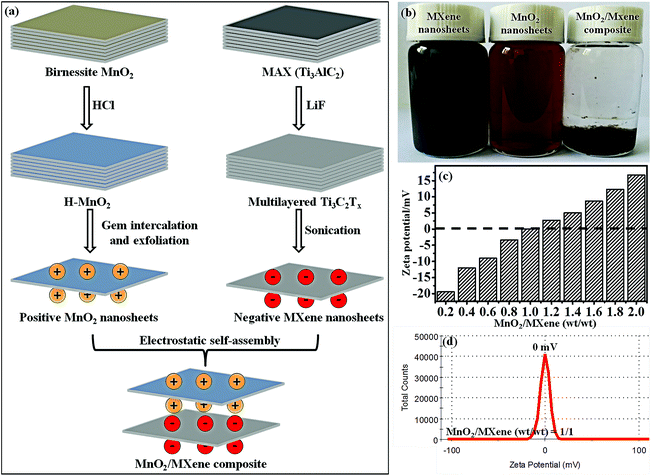
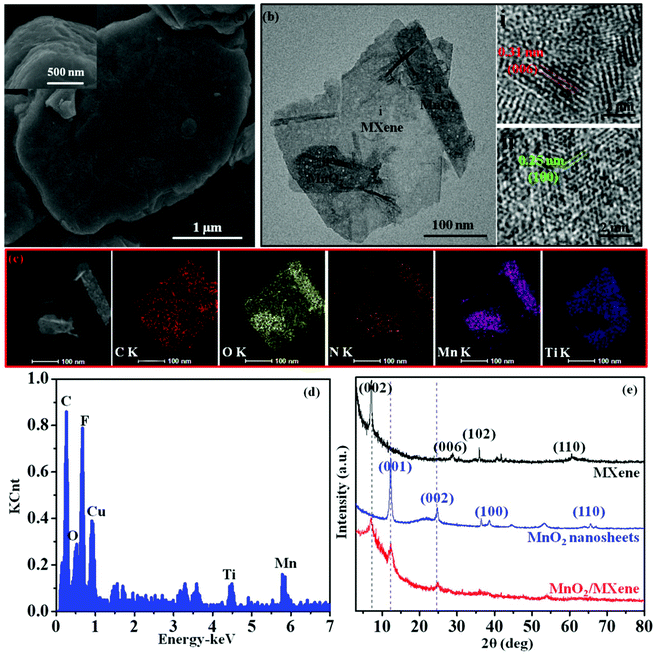
The fabrication of the MnO2/MXene composite, which was obtained by electrostatic self-assembly between positive the MnO2 nanosheets and negative MXene nanosheets, is schematically illustrated in Fig. 1a. In our work, the Gem (1) makes the MnO2 surface positively charged and forms a stable aqueous dispersion due to electrostatic repulsion between the different positive MnO2 nanosheets, and (2) improves the surface reaction between the MnO2 nanosheets and the MXene nanosheets. The delaminated MnO2 nanosheets were obtained through the Gem intercalation and exfoliation of H-MnO2 in Gem solution. For the purpose of confirming this modification, the zeta potentials of the H-MnO2 and MnO2 nanosheets were measured to be −23.5 and +26.1 mV, respectively (Fig. S2a and b in the ESI†). Here, the zeta potential of the MnO2 turned from a negative value to a positive value. Since the Gem has two hydrophilic cation heads, the charge density and surface activity of the MnO2 nanosheets can be increased by Gem. Then, the zeta potential result of MnO2 verifies that the Gem is firmly attached. Besides, the thin layered structure of the MnO2 nanosheets is observed (Fig. S2c and d in the ESI†). The formation of thin layers is a result of ion-intercalated functionalization by the Gem with cationic groups. Owing to the electrostatic repulsion, the positive MnO2 nanosheets disperse well in water (Fig. 1b). On the other hand, MXene was prepared by etching Ti3AlC2 and exfoliating the multilayered Ti3C2Tx, yielding a well dispersible suspension (Fig. 1b). The zeta potential of MXene is −26.6 mV, and the thin sheets of MXene clearly layered on top of each other (Fig. S3a–c in the ESI†). When the positive MnO2 nanosheets were added into the negative MXene nanosheet solution, the MnO2 nanosheets were adsorbed on the MXene nanosheets through electrostatic self-assembly. An MnO2/MXene composite is formed and transforms into a precipitate at the bottom of the container (Fig. 1b). Fig. 1c shows the zeta potentials of the composites with different weight (wt) ratios between the MnO2 nanosheets and MXene nanosheets. The zeta potential value increases with increasing wt ratio of MnO2/MXene. Notably, when the wt ratio equals 1/1, the zeta potential is measured to be about 0 mV (Fig. 1d). Furthermore, when the wt ratios are more than 1/1, the zeta potentials pass from negative to positive due to the unbound MnO2 nanosheets with positive charges, resulting in the zeta potential changing to a positive value in the composite solution. These results indicate that the positive MnO2 nanosheets and negative MXene nanosheets fully self-assemble when the wt ratio between the two types of nanosheets equals 1/1. From the analyses above, we can conclude that electrostatic self-assembly is a mild and efficient approach for MnO2/MXene composite preparation.The morphology of the MnO2/MXene composite (wt ratio = 1/1) was studied using SEM and TEM. The SEM of the composite presents a well-aligned multilayer microstructure (Fig. 2a). As shown in Fig. 2b, the TEM shows two characteristic morphologies including the large plate-like MXene and small plate-like MnO2, in which the first type of nanosheet exhibits lattice fringes with a d-spacing of 0.31 nm reflecting the (006) plane of MXene (i),26 and the other type of nanosheet exhibits lattice fringes with a d-spacing of 0.25 nm indexed to the (100) plane of the MnO2 nanosheets (ii).40 This result indicates that the MnO2 nanosheets are intimately reassembled on the MXene nanosheets. The formation of the as-prepared MnO2/MXene composite is also verified by the elemental mapping analysis (Fig. 2c). The signals of Mn and Ti are well distributed according to the sites of the MnO2 nanosheets and MXene nanosheets on the surface of the MnO2/MXene composite, respectively. Besides, the EDS result verifies the elementary composition of the composite (Fig. 2d). It is believed that the self-assembly can prevent the MXene sheets from restacking, and the effective contact between the MnO2 and MXene could promote the conductivity of the composite. Furthermore, XRD was used to analyze the chemical structure of the MnO2/MXene composite (Fig. 2e). The typical diffraction peak of MXene (002) at 7.1°,41 with an interlayer spacing of 1.24 nm, represents the pure MXene. The MnO2 nanosheets have a layered structure, and strong diffraction peaks are observed at about 12.5° (001) and 25.0° (002), suggesting the formation of birnessite MnO2.34,40 The MnO2/MXene shows almost all of the characteristic peaks of both the MnO2 and MXene. However, the (002) peak of MXene and the (001) peak of MnO2 are both broadened, suggesting that the MnO2 nanosheets have been deposited on the surface of MXene. From this analysis, we can conclude that the structure of the MnO2/MXene composite is analogous to a layered framework, which consists of layered MnO2 and layered MXene.
The surface chemical composition and chemical valence states of the MnO2/MXene composite were analyzed by XPS. As shown in Fig. 3a, N-atoms are found in the survey spectrum of the MnO2 nanosheets and MnO2/MXene, while no N-atoms are found in the MXene. Furthermore, after grafting the MnO2 nanosheets onto the MXene, Mn 3s (84.5 eV) and Mn 2p (641.9 eV)42 are detected in the MnO2/MXene, which verified that the N-atoms in MnO2/MXene come from Gem and the Mn-atoms in MnO2/MXene come from MnO2. Table S1 in the ESI† provides the element atomic ratio data from the XPS analysis results. Fig. 3b–e are the high-resolution XPS spectra of MnO2/MXene. In Fig. 3b, the Ti 2p peak of MnO2/MXene can be fitted to five constituent peaks: Ti–C at 455.4 eV, Ti–OH at 457.1 eV, Ti–O (2p3/2) at 459.2 eV, Ti–F at 461.7 eV, and Ti–O (2p1/2) at 464.7 eV.43 Compared with the C 1s of MXene (Fig. S4 in the ESI†), a new C–N group can be observed at 285.5 eV44 in the C 1s of MnO2/MXene, which originates from the nitrogen in Gem (Fig. 3c). Fig. 3d and e show the Mn 3s and Mn 2p in the high-resolution XPS of MnO2/MXene, respectively. These XPS results indicate that a large number of MnO2 nanosheets are present in the MnO2/MXene composite due to electrostatic self-assembly.
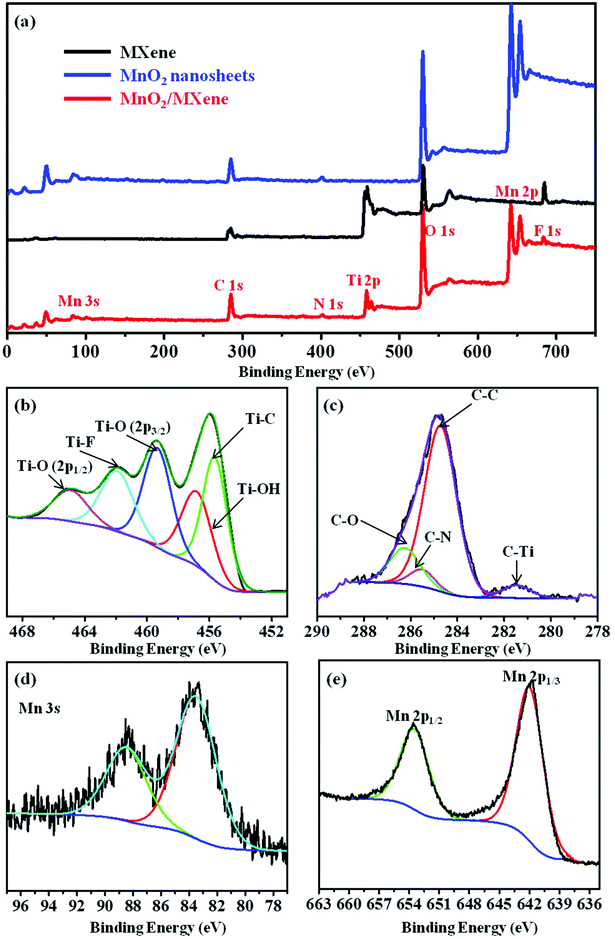 | ||
| Fig. 3 (a) Survey scan XPS of the MXene, MnO2 nanosheets, and MnO2/MXene; high-resolution XPS of (b) Ti 2p, (c) C 1s, (d) Mn 3s, and (e) Mn 2p of MnO2/MXene. | ||
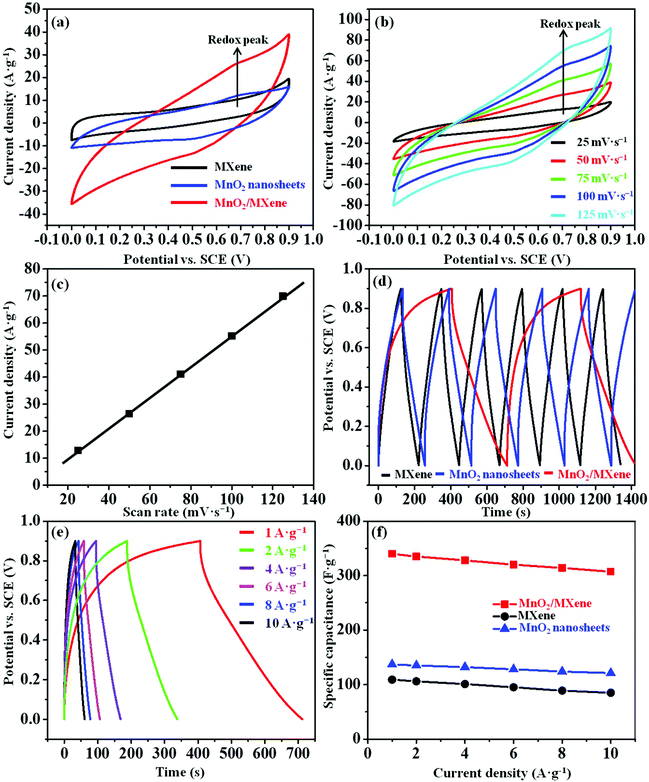
The electrochemical properties of the MnO2/MXene composite were examined using a three-electrode system in 1 M Na2SO4. The MXene and MnO2 nanosheet samples were also tested in the same system as control samples. In Fig. 4a, the MnO2 nanosheets and MnO2/MXene (wt ratio = 1/1) electrodes exhibit an oxidation peak appearing at about 0.7 V due to the pseudocapacitive effect of MnO2, whereas no obvious redox peak is observed for the pure MXene. Compared to the MXene and MnO2 nanosheets, the MnO2/MXene sample has a larger area in the closed-loop CV curve, which indicates that the MnO2/MXene electrode has better capacitive performance than the others. This demonstrates that electrostatic self-assembly improves the surface reaction between the MnO2 nanosheets and MXene nanosheets. In Fig. 4b, symmetric oxidation and reduction peaks can be observed at scan rates as high as 125 mV s−1 for the MnO2/MXene, indicating the excellent reversibility of this electrode. In Fig. 4c, the oxidation peak currents and scan rate are linearly correlated, which suggests that the MnO2/MXene electrode has good kinetic performance and rate capability. The MnO2 nanosheets that are immobilized on the MXene surface can prompt ion dispersion at the electrode, and thus improve these electrochemical characteristics. Moreover, the wt ratio between the MnO2 nanosheets and MXene nanosheets could influence the capacitive behavior of the MnO2/MXene electrode. Thus, we also evaluated the specific capacitances of the MnO2/MXene composites with different wt ratios of the two types of nanosheets using GCD (Fig. S5a and b in the ESI†). From this data, the optimized wt ratio in the MnO2/MXene composite was chosen to be 1/1. The specific capacitance of the MnO2/MXene (wt ratio = 1/1) electrode (340 F g−1) calculated from GCD is about two and a half times, and three times higher than that of the MnO2 nanosheets (137 F g−1) and MXene (109 F g−1) at 1 A g−1, respectively (Fig. 4d), which basically corresponds to the results calculated from CV (Table S2 in the ESI†). Besides, the specific capacitance values of the MnO2/MXene, MXene, and MnO2 nanosheets remain at 90.3%, 78.0%, and 88.3% of the initial capacitance, respectively, when the constant current density of GCD is increased to 10 A g−1 (Fig. 4e and f). These results show that the MnO2/MXene composite has a good capacitance retention rate and higher specific capacitance. Compared with the previously reported MnO2 nanowhisker/MXene composite prepared by direct chemical synthesis,26 our layered MnO2/MXene composite prepared by electrostatic self-assembly shows higher specific capacitance and better stability. The attractive electrochemical performance of the composite is mainly attributable to the following important reasons:
(1) The moderate immobilized MnO2 nanosheets in the MXene material effectively improve the electron conductivity and enhance the specific capacitance.
(2) The electrostatic self-assembly is mild and efficient and effectively inhibits the self-stacking of the MnO2 layers and MXene layers, which could significantly facilitate ion diffusion of the active material and enhance the surface accessibility to the electrolyte.
(3) The Gem can increase the stability of the MnO2/MXene composite. Hence, the enhanced high capability of the MnO2/MXene electrode can be understood by the layered framework of this composite.
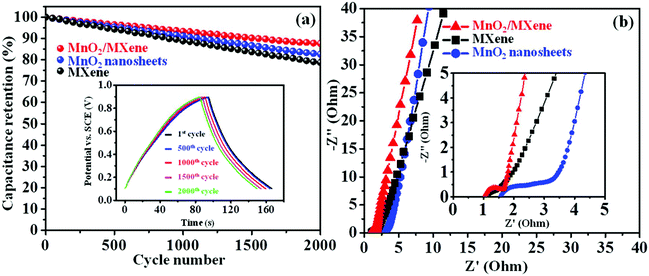
The cycling stability is an essential parameter to evaluate the performance of electrode materials. The GCD was tested with 2000 galvanostatic cycles at 4 A g−1 to examine the MnO2/MXene electrode cycling life. The specific capacitance values of the MnO2/MXene, MXene, and MnO2 nanosheets remain at 87.6%, 78.7%, and 82.6% of the initial value, respectively (Fig. 5a). In addition, the inset in Fig. 5a shows that the MnO2/MXene electrode has excellent cycling reversibility during the 2000 GCD cycle test, indicating its good cycling performance. To further testify the cycling stability of the MnO2/MXene composite in the GCD process, XRD was employed before and after the 2000 GCD cycles (Fig. S6 in the ESI†). The main diffraction peaks of the composite could still be indexed to the MnO2 nanosheets and MXene after 2000 GCD cycles, indicating the outstanding reversible behavior during the GCD process. Therefore, both a high capacitance value and good cycling stability can be achieved due to electrostatic self-assembly to form the MnO2/MXene composite. EIS analysis was conducted to further investigate the electrochemical performance of the MnO2/MXene composite. Fig. 5b shows the Nyquist plots of the MXene, MnO2 nanosheet, and MnO2/MXene electrodes, which consist of two regions including a low frequency region and a high frequency region. The curve at the juncture of the axis at high frequency represents the resistance of active material-based electrodes. The resistance of MnO2/MXene is lower than that of the MnO2 nanosheets, demonstrating that MnO2/MXene has better conductivity. Moreover, in the low frequency region, the curve of the MnO2/MXene electrode is more vertical than that of the MnO2 nanosheets and MXene, which is a sign of faster diffusion of the ions in the active material, and also a more ideal capacitive behavior for the electrode. From this analysis, we can conclude that the MnO2/MXene composite shows significant advantages compared to the pure MXene and MnO2 nanosheets, and it could be a promising electrode material.
4. Conclusion
We have developed a suitable strategy for the preparation of a novel MnO2/MXene composite by electrostatic self-assembly between MnO2 nanosheets and MXene nanosheets. The structure and properties of the MXene material can be effectively modified by this approach at the nanoscale. An electrode made from the MnO2/MXene composite shows high specific capacitance, excellent retention rate, and good cycling life. The improved performance can be explained by the function of the MnO2 nanosheets, which mildly doped the MXene material. We believe that this composite could be a promising electrode material for application in supercapacitors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the research funds for the National Natural Science Foundation of China (21606081).References
- S. Chu and A. Majumdar, Opportunities and challenges for a sustainable energy future, Nature, 2012, 488, 294–303 CrossRef CAS PubMed.
- Y. Z. Zhang, Y. Wang, T. Cheng, W. Y. Lai, H. Pang and W. Huang, Flexible supercapacitors based on paper substrates: a new paradigm for low-cost energy storage, Chem. Soc. Rev., 2015, 44, 5181–5199 RSC.
- V. Palomares, P. Serras, I. Villaluenga, K. B. Hueso, J. Carretero-González and T. Rojo, Na-ion batteries, recent advances and present challenges to become low cost energy storage systems, Energy Environ. Sci., 2012, 5, 5884–5901 RSC.
- W. Kang, N. Deng, J. Ju, Q. Li, D. Wu and X. Ma, et al., A review of recent developments in rechargeable lithium-sulfur batteries, Nanoscale, 2016, 8, 16541–16588 RSC.
- Z. Yu, L. Tetard, L. Zhai and J. Thomas, Supercapacitor electrode materials: nanostructures from 0 to 3 dimensions, Energy Environ. Sci., 2015, 8, 702–730 RSC.
- P. Simon and Y. Gogotsi, Materials for electrochemical capacitors, Nat. Mater., 2008, 7, 845–854 CrossRef CAS PubMed.
- M. Zhi, C. Xiang, J. Li, M. Li and N. Wu, Nanostructured carbon-metal oxide composite electrodes for supercapacitors: a review, Nanoscale, 2012, 5, 72–88 RSC.
- E. Frackowiak, Carbon materials for supercapacitor application, Phys. Chem. Chem. Phys., 2007, 9, 1774–1785 RSC.
- A. G. Pandolfo and A. F. Hollenkamp, Carbon properties and their role in supercapacitors, J. Power Sources, 2006, 157, 11–27 CrossRef CAS.
- S. Faraji and F. N. Ani, Microwave-assisted synthesis of metal oxide/hydroxide composite electrodes for high power supercapacitors-a review, J. Power Sources, 2014, 263, 338–360 CrossRef CAS.
- Y. Zhang, L. Li, H. Su, W. Huang and X. Dong, Binary metal oxide: advanced energy storage materials in supercapacitors, J. Mater. Chem. A, 2014, 3, 43–59 RSC.
- R. S. Kate, S. A. Khalate and R. J. Deokate, Overview of nanostructured metal oxides and pure nickel oxide (NiO) electrodes for supercapacitors: a review, J. Alloys Compd., 2018, 734, 89–111 CrossRef CAS.
- D. Chen, Q. Wang, R. Wang and G. Shen, Ternary oxide nanostructured materials for supercapacitors: a review, J. Mater. Chem. A, 2015, 3, 10158–10173 RSC.
- P. C. Chen, G. Shen, Y. Shi, H. Chen and C. Zhou, Preparation and characterization of flexible asymmetric supercapacitors based on transition-metal-oxide nanowire/single-walled carbon nanotube hybrid thin-film electrodes, ACS Nano, 2010, 4, 4403 CrossRef CAS PubMed.
- Q. J. Le, T. Wang, D. N. H. Tran, F. Dong, Y. X. Zhang and D. Losic, Morphology-controlled MnO2 modified silicon diatoms for high-performance asymmetric supercapacitors, J. Mater. Chem. A, 2017, 5, 10856–10865 RSC.
- W. Xu, J. Wan, W. Huo, Q. Yang, Y. Li, C. Zhang, X. Gu and C. Hu, Sodium ions pre-intercalation stabilized tunnel structure of Na2Mn8O16 nanorods for supercapacitors with long cycle life, Chem. Eng. J., 2018, 354, 1050–1057 CrossRef CAS.
- M. Ghidiu, M. R. Lukatskaya, M. Q. Zhao, Y. Gogotsi and M. W. Barsoum, Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance, Nature, 2014, 516, 78–81 CAS.
- M. Naguib, V. N. Mochalin, M. W. Barsoum and Y. Gogotsi, 25th anniversary article: mxenes: a new family of two-dimensional materials, Adv. Mater., 2014, 26, 992–1005 CrossRef CAS PubMed.
- O. Mashtalir, M. Naguib, V. N. Mochalin, Y. D. Agnese, M. Heon, M. W. Barsoum and Y. Gogotsi, Intercalation and delamination of layered carbides and carbonitrides, Nat. Commun., 2013, 4, 1716 CrossRef PubMed.
- M. R. Lukatskaya, O. Mashtalir, C. E. Ren, Y. D. Agnese, P. Rozier, P. L. Taberna, M. Naguib, P. Simon, M. W. Barsoum and Y. Gogotsi, Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide, Science, 2013, 341, 1502 CrossRef CAS PubMed.
- X. Liang, A. Garsuch and L. F. Nazar, Sulfur cathodes based on conductive mxene nanosheets for high-performance lithium-sulfur batteries, Angew. Chem., Int. Ed., 2015, 54, 3907–3911 CrossRef CAS PubMed.
- C. Lin, W. Zhang, L. Wang, Z. Wang, W. Zhao, W. Duan, Z. Zhao, B. Liu and J. Jin, Few-layered Ti3C2 nanosheet/glass fiber composite separator as lithium polysulphide reservoir for high-performance lithium-sulfur battery, J. Mater. Chem. A, 2016, 4, 5993–5998 RSC.
- T. Zhao, J. Zhang, Z. Du, Y. Liu, G. Zhou and J. Wang, Dopamine-derived n-doped carbon decorated titanium carbide composite for enhanced supercapacitive performance, Electrochim. Acta, 2017, 254, 308–319 CrossRef CAS.
- Z. Ling, C. E. Ren, M. Q. Zhao, J. Yang, J. M. Giammarco, J. Qiu, M. W. Barsoum and Y. Gogotsi, Flexible and conductive mxene films and nanocomposites with high capacitance, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 16676 CrossRef CAS PubMed.
- S. K. Kim, Y. K. Kim, H. Lee, S. B. Lee and H. S. Park, Superior pseudocapacitive behavior of confined lignin nanocrystals for renewable energy-storage materials, ChemSusChem, 2014, 7, 1094–1101 CrossRef CAS PubMed.
- R. B. Rakhi, B. Ahmed, D. Anjum and H. N. Alshareef, Direct chemical synthesis of MnO2 nanowhiskers on transition-metal carbide surfaces for supercapacitor applications, ACS Appl. Mater. Interfaces, 2016, 8, 18806–18814 CrossRef CAS PubMed.
- S. Zhu, L. Li, J. Liu, H. Wang, T. Wang, Y. Zhang, L. Zhang, R. Ruoff and F. Dong, Structural Directed Growth of Ultrathin Parallel Birnessite on β-MnO2 for High-Performance Asymmetric Supercapacitors, ACS Nano, 2018, 12, 1033–1042 CrossRef CAS PubMed.
- W. Xu, Z. Jiang, Q. Yang, W. Huo, M. S. Javed, Y. Li, L. Huang, X. Gu and C. Hu, Approaching the lithium-manganese oxides’ energy storage limit with Li2MnO3 nanorods for high-performance supercapacitor, Nano Energy, 2018, 43, 168–176 CrossRef CAS.
- Z. Sun, D. Shu, C. Lv, Q. Zhang, C. He and S. Tian, Fabrication and supercapacitive behavior of tetramethylammonium ion-intercalated MnO2, prepared by an exfoliation and self-assembly process, J. Alloys Compd., 2013, 569, 136–143 CrossRef CAS.
- M. Huang, Y. Zhang, F. Li, L. Zhang, R. S. Ruoff and Z. Wen, et al., Self-assembly of mesoporous nanotubes assembled from interwoven ultrathin birnessite-type MnO2 nanosheets for asymmetric supercapacitors, Sci. Rep., 2014, 4, 3878 CrossRef PubMed.
- Y. Liu, D. Yan, R. Zhuo, S. Li, Z. Wu and J. Wang, et al., Design, hydrothermal synthesis and electrochemical properties of porous birnessite-type manganese dioxide nanosheets on graphene as a hybrid material for supercapacitors, J. Power Sources, 2013, 242, 78–85 CrossRef CAS.
- T. Wang, F. Dong and Y. X. Zhang, Diverse birnessite MnO2 nanosheets-based nanocomposites for supercapacitors, Mater. Lett., 2016, 171, 319–322 CrossRef CAS.
- Z. Li, J. Zhang and X. W. Lou, Frontispiece: hollow carbon nanofibers filled with MnO2 nanosheets as efficient sulfur hosts for lithium-sulfur batteries, Angew. Chem., Int. Ed., 2015, 54, 12886 CrossRef CAS PubMed.
- H. Cheng, L. Long, D. Shu, J. Wu, Y. Gong, C. He, Z. Kang and X. Zou, The supercapacitive behavior and excellent cycle stability of graphene/MnO2 composite prepared by an electrostatic self-assembly process, Int. J. Hydrogen Energy, 2014, 39, 16151–16161 CrossRef CAS.
- J. Hao, Y. Zhong, Y. Liao, D. Shu, Z. Kang, X. Zou, C. He and S. Guo, Face-to-face self-assembly graphene/MnO2 nanocomposites for supercapacitor applications using electrochemically exfoliated graphene, Electrochim. Acta, 2015, 167, 412–420 CrossRef CAS.
- Y. Zhong, Y. Liao, A. Gao, J. Hao, D. Shu, Y. Huang, J. Zhong, C. He and R. Zeng, Supercapacitive behavior of electrostatic self-assembly reduced graphene oxide/CoAl-layered double hydroxides nanocomposites, J. Alloys Compd., 2016, 669, 146–155 CrossRef CAS.
- C. Song, D. Wu, F. Zhang, P. Liu, Q. Lu and X. Feng, Gemini surfactant assisted synthesis of two-dimensional metal nanoparticles/graphene composites, Chem. Commun., 2012, 48, 2119–2121 RSC.
- X. Zhang, P. Yu, H. Zhang, D. Zhang, X. Sun and Y. Ma, Rapid hydrothermal synthesis of hierarchical nanostructures assembled from ultrathin birnessite-type MnO2 nanosheets for supercapacitor applications, Electrochim. Acta, 2013, 89, 523–529 CrossRef CAS.
- R. Zana, M. Benrraou and R. Rueff, Alkanediyl-α, ω-bis(dimethylalkylammonium bromide) surfactants. 1. Effect of the spacer chain length on the critical micelle concentration and micelle ionization degree, Langmuir, 1991, 7, 1072–1075 CrossRef CAS.
- M. S. Song, K. M. Lee, R. L. Yu, I. Y. Kim, T. W. Kim and J. L. Gunjakar, Porously assembled 2D nanosheets of alkali metal manganese oxides with highly reversible pseudocapacitance behaviors, J. Phys. Chem. C, 2010, 114, 22134–22140 CrossRef CAS.
- M. Q. Zhao, C. E. Ren, Z. Ling, M. R. Lukatskaya, C. Zhang and K. L. Van Aken, et al., Flexible mxene/carbon nanotube composite paper with high volumetric capacitance, Adv. Mater., 2015, 27, 339–345 CrossRef CAS PubMed.
- G. Du, X. Liu, Y. Zong, T. S. Hor, A. Yu and Z. Liu, Co3O4 nanoparticle-modified MnO2 nanotube bifunctional oxygen cathode catalysts for rechargeable zinc-air batteries, Nanoscale, 2013, 5, 4657–4661 RSC.
- J. Halim, K. M. Cook, M. Naguib, P. Eklund, Y. Gogotsi, J. Rosen and M. W. Barsoum, X-ray photoelectron spectroscopy of select multi-layered transition metal carbides (MXenes), Appl. Surf. Sci., 2016, 362, 406–417 CrossRef CAS.
- T. Y. Kim, H. W. Lee, J. E. Kim and K. S. Suh, Synthesis of phase transferable graphene sheets using ionic liquid polymers, ACS Nano, 2010, 4, 1612–1618 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8qi00957k |
| This journal is © the Partner Organisations 2019 |