Clean synthesis of ZnCo2O4@ZnCo-LDHs yolk–shell nanospheres composed of ultra-thin nanosheets with enhanced electrocatalytic properties†
Jing
Pan
ab,
Fan
Wang
a,
Lingling
Zhang
ab,
Shuyan
Song
 *a and
Hongjie
Zhang
*a and
Hongjie
Zhang
 a
a
aState Key Laboratory of Rare Earth Resource Utilization, Changchun Institute of Applied Chemistry, Changchun 130022, P. R. China. E-mail: songsy@ciac.ac.cn
bCollege of Chemistry, Jilin University, Changchun 130012, P. R. China
First published on 19th November 2018
Abstract
A clean and facile template-engaged approach was developed for the synthesis of ZnCo2O4@ZnCo-layered double hydroxide (ZnCo-LDH) yolk–shell nanospheres via controlled hydrolysis of mixed metal glycolates. The ZnCo2O4@ZnCo-LDHs have novel structures and exhibit good oxygen evolution reaction (OER) activity, with an overpotential (η) of 375 mV at 10 mA cm−2vs. RHE and a Tafel slope of 73 mV dec−1 in 1.0 M KOH.
The global energy crisis has encouraged the search for sustainable chemical processes that create and store energy. Among the most promising approaches to obtain renewable fuels is the electrocatalytic water splitting into hydrogen and oxygen, which has received increasing attention in recent years.1–4 Previous reports have confirmed that noble metal-based oxides, such as IrO2 and RuO2, exhibit high catalytic performance. However, the high cost and poor earth abundance of Ir and Ru has limited further applications. Thanks to the recent developments in nanotechnology, crystalline transition-metal-based materials have been successfully fabricated and used as novel “noble metal-free” catalysts in energy conversion processes. Co-Based nanostructures have been extensively studied for electrochemical catalysis because of their rich composition, multiple oxidation states and high electrical activity and stability. Moreover, the introduction of additional ions can greatly increase the active sites and improve the electronic conductivity.5,6 However, some recent achievements have shown that Co-based materials could also exhibit lower overpotential caused by sluggish OER at the anodic electrode.7–12 Research on transition metal-based layered double hydroxides (LDHs) has gained attention owing to their great potential in energy-related applications.13–19 LDHs are a class of layered materials with positively charged layers and charge-balancing anions located in the interlayer regions. As successful examples, strongly coupled Ni–Fe LDHs nanomaterials have shown superior oxygen evolution reaction (OER) activity in alkaline conditions.20 Guo et al. established a relationship between the OER activity and the components of hollow Ni–Co-LDH nanospheres.21 Zhang et al. designed and facilely developed a novel composite based on Ni–Fe LDHs and graphene in the quest for high-performance oxygen evolution electrocatalysis.22 Notably, water oxidation catalysts reported thus far have some substantial disadvantages, such as low stability over long-term electrochemical reactions.
Thus, efforts were made to optimize the catalyst nanostructures. The designed hollow structures were confirmed to be beneficial for the energy conversion efficiency. One of the unrivaled advantages of these structures is the high stability provided by the inner hollow spaces and the porous structure.23 Many functional materials have been successfully composited with diverse hollow structures including hollow nanospheres,24,25 hollow nanorods,26 and hollow nano-polyhedrons.27 In addition, increasing the designability of hollow structures may open up new range of possibilities in regulating the properties of functional nanostructures to suit many applications. For example, Wang et al. reported a series of multi-shelled hollow spheres that exhibit excellent properties in lithium ion batteries (LIBs) and solar battery applications.28 Additionally, Lou et al. found that Co3O4@NiCo2O4 double-shelled hollow cages exhibited much higher LIBs and OER performance than Co3O4 nanocages,29 and Zhang et al. showed that hierarchical Fe2O3 hollow spheres composed of ultrathin nanosheets had ultra-high Li storage properties and excellent rate capabilities.30 The reported hollow nanostructures are consistently synthesized by a hard-template strategy. Further post-treatments such as sintering at high temperature or etching by strong acid (or alkali) is probably inevitable.31,32 However, certain properties of LDHs, such as poor stability at high temperatures and in acid solutions, makes the general fabrication of LDH-based hollow nanostructures much difficult. It still remains an enormous challenge to develop facile, widely applicable fabrication methods of hollow LDH nanomaterials with high structural complexity and to study the structure–property relationship.
Herein, we present a controllable hydrolysis process of mixed metal glycolates for the synthesis of yolk–shell nanospheres with ultrathin LDH shells and a metal oxide nucleus. This strategy involves the following two separate steps: the facile synthesis of transition metal glycolate nanospheres33,34 and a subsequent controllable hydrolysis treatment (Scheme 1). According to the second law of thermodynamics, force drives a reaction proceeds toward states of greater stability. The dissociation equilibrium constant (pKa) of ethylene glycol (EG) is smaller than water, which makes the metal glycolate unstable in water. Therefore, water is adopted for etching the metal glycolates to induce the metal oxide@metal hydroxides yolk@shell structure. The detailed synthesis procedures are described in the Experimental Section.
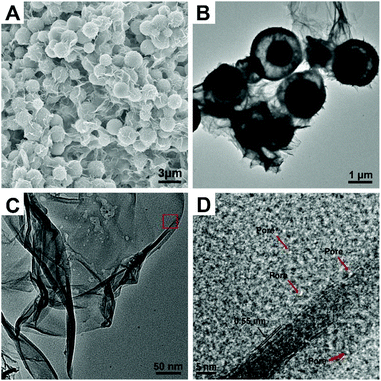
ZnCo ethylene glycol salt nanospheres (ZnCo-EG) were obtained by a previously reported method.33 The scanning electron microscope (SEM) image of ZnCo-EG in Fig. S1† indicates that the nanospheres have an average diameter of 2.4 μm. Water is then adopted for etching the metal glycolate to induce the formation of hollow spheres composed of metal oxides and metal hydroxides. Interestingly, the hollow nanostructures can be easily tuned by directly changing the amount of water in the reaction. Fig. 1 shows the typical SEM and transmission electron microscopy (TEM) images of the ZnCo2O4@ZnCo-layered double hydroxide yolk–shell nanospheres with addition of 1 mL water (ZnCo2O4@ZnCo-LDH-1000). As shown in the figure, many ultrathin wrinkled nanosheets have grown on the nanospheres and no distinct changes in the size of the nanospheres are observed in the SEM image. The TEM image also indicates the yolk–shell characteristic structure of the nanospheres. A core of about 1 μm is preserved inside the nanosphere, and a large gap is observed between the core and the shell. High resolution transmission electron microscope (HRTEM) tests were performed to examine the morphology of the as-grown ultrathin nanosheets; Fig. 1D represents the high resolution image of ZnCo2O4@ZnCo-LDH-1000 recorded from the area marked in red in the Fig. 1C. The nanosheets have a layered structure, and the spacing between neighboring layers is measured to be 0.55 nm. A pore-structure is observed above the nanosheets, which may be due to the generation of hydrogen during the reaction. In addition, a widely present but not distinctly crystalline component can be observed in the HRTEM image. A SEM image elemental mapping of ZnCo2O4@ZnCo-LDH-1000 on the conductive adhesive reveals that both Co (red) and Zn (green) elements are homogenously distributed throughout the sample (Fig. S2†). ZnCo2O4@ZnCo-LDH-500 and ZnCo-LDH-2000 were prepared by adding 0.5 mL and 2 mL H2O, respectively, for etching the ZnCo-EG. These materials also exhibit a hollow nanosphere structure composed of ultrathin inter-connected wrinkled nanosheets, as revealed by the SEM and TEM images in Fig. S3 and S4.† Because the amount of water added was too low, the reaction for preparation of ZnCo2O4@ZnCo-LDH-500 was incomplete, resulting in insufficient amount of nanosheets and formation of narrow gaps in the nanospheres. In contrast, ZnCo-LDH-2000 nanoparticles clearly aggregated because excess water was added during the reaction, and the structure of the nanospheres could not remain stable. There were no residual nuclei in the nanospheres.
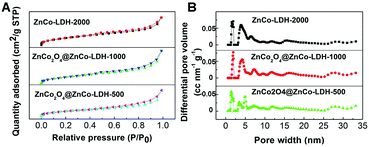
We also performed nitrogen adsorption–desorption isotherm analysis of the obtained ZnCo2O4@ZnCo-LDHs. As expected, ZnCo2O4@ZnCo-LDH-1000 possesses a high specific surface area of 244.0 m2 g−1 because of the ultrathin nanosheets in the shell and hollow structure resulting from surface roughing during hydrolysis (Fig. 2A). The estimated surface areas of ZnCo-LDH-2000 and ZnCo2O4@ZnCo-LDH-500 are 276.7 and 215.3 m2 g−1, respectively. The specific surface area increases with the increase in water content. According to the data presented in Fig. 2B, which is based on the DFT method, micropores and mesopores should both exist in the samples. The micropore size of ZnCo2O4@ZnCo-LDH-1000 is about 1.915 nm, which corresponds to the HRTEM results. Such a characteristic structure could have countless reactive sites and facilitate the elimination of the produced gaseous O2. In addition, the mesopores can shorten the electronic transmission path to the interior surface of electro-active species. The average pore sizes of ZnCo-LDH-2000 and ZnCo2O4@ZnCo-LDH-500 are 1.915 and 1.416 nm, respectively, which are similar to that of ZnCo2O4@ZnCo-LDH-1000.
The phases and structures of the prepared samples were confirmed by X-ray diffraction (XRD) analysis. Fig. S5† shows the XRD patterns of ZnCo2O4@ZnCo-LDH-500, ZnCo2O4@ZnCo-LDH-1000 and ZnCo-LDH-2000. The XRD patterns of ZnCo2O4@ZnCo-LDH-1000 show characteristic reflections corresponding to the crystal planes (110), (006), (113), (300), (119) of the well-crystallized hexagonal phase of zinc hydroxide (JCPDS 48-1066); (002), (200), (−115), (−320) of zinc cobalt hydroxide hydrate (JCPDS 21-1477); and (311), (400), (511) and (440) of the cubic phase of zinc cobalt oxide (JCPDS 23-1390). The ZnCo2O4 comes from the nucleus of the nanospheres. No characteristic peak of ZnCo2O4 exists in ZnCo-LDH-2000; however, there are significant peaks of ZnCo2O4 in ZnCo-LDH-500. The peak at about 8° is attributed to the layered structure.
In order to obtain the proportions of oxide and hydroxide, we performed thermogravimetric measurements to judge the weightlessness of the samples. The thermogravimetric analysis (TGA) of the samples were performed using an SDT 2960 thermal analyzer at a heating rate of 5 °C min−1 under air over the temperature range 50–700 °C. The TGA curves are shown in Fig. S6.† We can observe that different samples exhibit varying degrees of water loss from 50 °C–120 °C, which is attributed to the loss of water from the interlayer and absorption. The second weight loss occurs in the range of 150 °C–240 °C and is attributed to the dehydroxylation of the layers to form an oxide.35,36 The percentage of hydroxide can thus be inferred by varying the proportion of weightlessness of the dehydroxylation. The results for the percentage content of dehydroxylation in ZnCo2O4@ZnCo-LDHs from TGA and the calculated atom percentage of ZnCo-LDHs are summarized in Table S1.† The results indicate 56.00% and 84.00% hydroxide in ZnCo2O4@ZnCo-LDH-500 and ZnCo2O4@ZnCo-LDH-1000, respectively. In addition, we measured the quality of the products and calculated the theoretical value according to the stoichiometric ratio with a 100% conversion rate. The yields for the different samples, as shown in Table S2,† are 90.78%, 89.30% and 91.33% for ZnCo2O4@ZnCo-LDH-500, ZnCo2O4@ZnCo-LDH-1000 and ZnCo-LDH-2000, respectively. Due to some unavoidable losses during the centrifugation process, these may be slightly lower than the actual values.
The elemental composition and oxidation state of the as-prepared ZnCo2O4@ZnCo-LDH-1000 were analyzed in detail using X-ray photoelectron spectroscopy, and the corresponding results are presented in Fig. S7.† All signals of Zn, Co, O and C were detected in the full spectra survey (Fig. S7a†). The C signal is from adventitious carbon. The binding energies of the peaks at 1021.7 eV and 1044.7 eV are attributed to the Zn 2p3/2 and Zn 2p1/2 signals, respectively, indicating the presence of a Zn(II) species in ZnCo2O4@ZnCo-LDH-1000 (Fig. S7b†). Otherwise, the signals with the binding energies of 780.0 eV and 795.5 eV correspond to Co 2p3/2 and Co 2p1/2, suggesting that cobalt is in the +2 oxidation state in the material. In addition, the other two unconspicuous signals located at 786.5 eV and 803.0 eV are assigned to the satellite peaks of Co 2p3/2 and Co 2p1/2, respectively. At the same time, the peak with a binding energy of 531.6 eV illustrates the presence of oxygen anions.
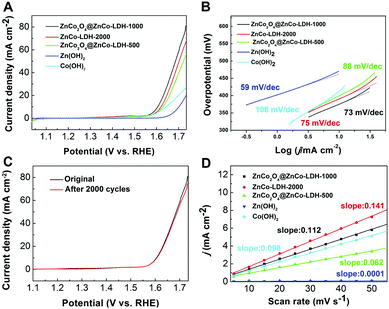
The electrocatalytic activity of ZnCo2O4@ZnCo-LDHs for OER with the Ag/AgCl electrode was investigated in a standard three electrode system using a 1 M KOH solution. The linear sweep voltammetry (LSV) of the water electrolysis of ZnCo2O4@ZnCo-LDH-1000 and the contrast at a sweep rate of 5 mV s−1 on the reversible hydrogen electrode (RHE) scale is shown in Fig. 3A. As expected, the ZnCo2O4@ZnCo-LDH-1000 electrode shows the highest activity. Lower overpotential of ZnCo2O4@ZnCo-LDH-1000 yielding a current density of 10 mA cm−2 with iR compensation was only 375 mV, which was 24 and 17 mV less than ZnCo-LDH-2000 and ZnCo2O4@ZnCo-LDH-500, respectively. In addition, the LSV exhibits a sharp onset potential of ZnCo2O4@ZnCo-LDH-1000 for OER at 365 mV, which is 19 and 25 mV less than ZnCo-LDH-2000 and ZnCo2O4@ZnCo-LDH-500, respectively. The low potential required for the current density of 10 mA cm−2 and OER onset potential reflect the excellent OER performance of ZnCo2O4@ZnCo-LDH-1000. To further confirm the synergistic role of Zn and Co, electrochemical tests on separate Zn(OH)2 and Co(OH)2 were conducted. Zn(OH)2 and Co(OH)2 were prepared by the same method, only using Zn(CH3COO)2 or Co(CH3COO)2 to synthesize the Zn-EG or Co-EG, respectively. Zn(OH)2 and Co(OH)2 show negligible OER performance, and the onset overpotentials (436 mV and 372 mV vs. RHE) are much larger than ZnCo2O4@ZnCo-LDH-1000. The current densities of Zn(OH)2 and Co(OH)2 could reach 10 mA cm−2 at overpotentials of 471 and 413 mV, respectively. After the introduction of a second metal into the Co(OH)2, it was clear that the OER performance was greatly enhanced. To probe the OER kinetics of the electrocatalysts, the Tafel slopes for ZnCo2O4@ZnCo-LDH-1000, ZnCo-LDH-2000, ZnCo2O4@ZnCo-LDH-500, Zn(OH)2 and Co(OH)2 were plotted, as shown in Fig. 3B. The results suggest that their kinetic reaction processes depend on the electrocatalysts. The Tafel slopes are obtained by fitting in the linear region of the plots according to the Tafel equation. ZnCo2O4@ZnCo-LDH-1000 has a slope as low as 73 mV dec−1, which is lower than 88 mV dec−1 for ZnCo2O4@ZnCo-LDH-500, 75 mV dec−1 for ZnCo-LDH-2000 and 106 mV dec−1 for Co(OH)2. The small Tafel slope leads to enhancement of the OER rate with increasing overpotential, which may be attributed to the barrier layer effect. The suitable quantity of water maintains the structure of the material and achieves more nanosheets inside the nanospheres, which provides sufficient active sites. However, Zn(OH)2 has the lowest Tafel slope at 59 mV dec−1. According to the classical Bulter–Volmer formalism, the Tafel slopes of the electrocatalysts imply that the first electron transfer is rate determining.37,38 The doping of Zn can change the reaction mechanism and effectively improve the reaction kinetics. To further confirm the charge-transport kinetics of the catalysts, the electrochemical impedance spectroscopy was collected. The impedance spectra of the catalysts are shown in Fig. S8.† The typical Nyquist plot consists of a depressed semicircle in the middle – high frequency region followed by a sloping line in the low frequency region. The semicircle dominating at medium frequency describes the charge transfer impedance. From the Nyquist plots, the calculated charge transfer resistances of ZnCo2O4@ZnCo-LDH-500, ZnCo2O4@ZnCo-LDH-1000 and ZnCo-LDH-2000 are 10.8 Ω, 9.3 Ω and 11.7 Ω, respectively.
Electrocatalytic durability is another important criterion for assessing the OER performance of an electrocatalyst. The stability tests of ZnCo2O4@ZnCo-LDHs were conducted in a 1 M KOH aqueous solution at room temperature. Around 2000 CV circulations were carried out between 0 and 0.7 V (vs. Ag/AgCl) at a scan rate of 100 mV s−1. As shown in Fig. 3C, the OER overpotential of the ZnCo2O4@ZnCo-LDH-1000 at 10 mA cm−2 shows no significant change after 2000 CV circulations. ZnCo-LDH-2000 and ZnCo2O4@ZnCo-LDH-500 also show no significant change and even exhibit a small improvement in potential after 2000 CV circulations of OER at the 10 mA cm−2 current density (Fig. S9†). These results confirm that ZnCo2O4@ZnCo-LDHs is a promising OER catalyst with high activity and strong durability. To gain deeper insight into the catalytic cycling performance, we have presented the SEM results in Fig. S10–S12.† By adding black carbon to the samples, we observed that the ZnCo2O4@LDHs were dispersed among small particles. After the cycles, no significant change appears in the structure. We also measured the non-faradaic region associated with double-layer capacitance (Cdl) to compare the relative active surface area (Aechem), which estimates the electrocatalyst intrinsic activity. The Cdl is extracted from the linear slope of charging current differences by utilizing cyclic voltammetry tests (CVs) (Fig. S13†) over a small potential range (0.2–0.3 vs. Ag/AgCl) at various scan rates from 5 to 50 mV s−1. The plot of Δj at 0.25 V vs. Ag/AgCl electrode against the scan rates is shown in Fig. 3D. The calculated Cdl of ZnCo-LDH-2000 is 0.141 mF cm−2, which is higher than those of ZnCo2O4@ZnCo-LDH-1000 (0.112 mF cm−2), ZnCo2O4@ZnCo-LDH-500 (0.0062 mF cm−2), Zn(OH)2 (0.0001 mF cm−2) and Co(OH)2 (0.098 mF cm−2). The larger electrochemical area reveals that the excellent electrocatalytic performance of ZnCo-LDH-2000 is related to the greater number of active sites at the solid–liquid interface, which is in accordance with the BET result.
The OER activity data for different catalysts are compared in Table S3† and show the high OER activity of ZnCo2O4@ZnCo-LDH-1000. This result may be related to the structural features of the sample. It is known that small changes in structure can lead to significant differences in chemical and physical properties. Co-Based nanomaterials are effective OER catalysts, while Zn-based nanomaterials are relatively inert electric catalysts. Yet, the two LDHs exhibit synergistic effects in this study, resulting in substantially enhanced OER activity. The contact regions between the Co(OH)2–Zn(OH)2 nanointerfaces may furnish the synergistically active sites for OER catalysis. The nanointerfaces generate the creation of open vacancy sites on the Co(OH)2 layers interacting with Zn(OH)2 layers. The advantages can cause strong electron transfer between the layers through the intermediate interfaces. The cobalt and zinc ions have alternatively arranged layers of LDH structures, which has a stabilizing effect. In ZnCo2O4, both Zn and Co have electrochemical activity, and their complementary behavior can contribute to the enhanced OER performance. Furthermore, the complex ternary compound with ZnCo2O4 and ZnCo-LDHs can possess a yolk–shell structure, which has high storage capacity, excellent cycling stability and good rate capability due to its unique hollow structure and complex synergistic effect.39,40 These results suggest that ZnCo2O4@ZnCo-LDH-1000 is a durable and robust electrocatalyst that is favourable for engineering applications and mass production of clean fuel from water electrolysis.
Conclusions
In summary, a clean and facile template-engaged approach for the synthesis of ZnCo2O4@ZnCo-LDHs yolk–shell nanospheres via controlled hydrolysis of mixed metal glycolates is reported. With this method, the generated ZnCo2O4@ZnCo-LDH-1000 exhibits excellent catalytic efficiency for OER property. The excellent property of ZnCo2O4@ZnCo-LDHs could be attributed to the following four reasons: (1) The yolk–shell material provides a large surface area that increases the number of active sites. (2) As a complex with bimetallic oxide and bimetallic hydroxide, a synergestic effect exists between the two metals. (3) The nanospheres are composed of interconnected nanosheets that reduce the transport path of electrolyte ions to the active sites. (4) The micropores and mesopores on the nanosheets effectively facilitate the transport of reactants and products. This study provides a reliable and facile route for preparing sphere-like yolk–shell structures with enhanced catalytic properties. This study may be extended to prepare other complex bi-metallic LDH hollow nanostructures.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for the financial aid from the National Natural Science Foundation of China (21590794, 21771173 and 21521092), Youth Innovation Promotion Association of Chinese Academy of Sciences (2011176), the project development plan of science and technology of Jilin Province (20180101179JC) and CAS-CSIRO project (GJHZ1730).Notes and references
- H. Jin, J. Wang, D. Su, Z. Wei, Z. Pang and Y. Wang, J. Am. Chem. Soc., 2015, 137, 2688–2694 CrossRef CAS PubMed.
- C. Qiao, Y. Zhang, Y. Zhu, C. Cao, X. Bao and J. Xu, J. Mater. Chem. A, 2015, 3, 6878 RSC.
- X. Zou, A. Goswami and T. Asefa, J. Am. Chem. Soc., 2013, 135, 17242–17245 CrossRef CAS PubMed.
- D. Chen, M. Qiao, Y. R. Lu, L. Hao, D. Liu, C. L. Dong, Y. Li and S. Wang, Angew. Chem., Int. Ed., 2018, 57, 8691–8696 CrossRef CAS PubMed.
- M. Yang, Y. Li, Y. Yu, X. Liu, Z. Shi and Y. Xing, Chem. – Eur. J., 2018, 24, 13002–13008 CrossRef CAS PubMed.
- M. Li, J. Meng, Q. Li, M. Huang, X. Liu, K. A. Owusu, Z. Liu and L. Mai, Adv. Funct. Mater., 2018, 1802016 CrossRef.
- H. Liang, F. Meng, M. Caban-Acevedo, L. Li, A. Forticaux, L. Xiu, Z. Wang and S. Jin, Nano Lett., 2015, 15, 1421–1427 CrossRef CAS PubMed.
- D. Tang, Y. Han, W. Ji, S. Qiao, X. Zhou, R. Liu, X. Han, H. Huang, Y. Liu and Z. Kang, Dalton Trans., 2014, 43, 15119 RSC.
- Y. Li, L. Zhang, X. Xiang, D. Yan and F. Li, J. Mater. Chem. A, 2014, 2, 13250 RSC.
- P. Chen, K. Xu, T. Zhou, Y. Tong, J. Wu, H. Cheng, X. Lu, H. Ding, C. Wu and Y. Xie, Angew. Chem., Int. Ed., 2016, 55, 2488–2492 CrossRef CAS PubMed.
- S. Chen, J. Duan, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed., 2013, 52, 13567–13570 CrossRef CAS PubMed.
- D. F. Yan, C. L. Dong, Y. C. Huang, Y. Q. Zou, C. Xie, Y. Y. Wang, Y. Q. Zhang, D. D. Liu, S. H. Shen and S. Y. Wang, J. Mater. Chem. A, 2018, 6, 805–810 RSC.
- L. Zhou, M. F. Shao, M. Wei and X. Duan, J. Energy Chem., 2017, 26, 1094–1106 CrossRef.
- H. Zhang, Y. Tong, J. Xu, Q. Lu and F. Gao, Chem. – Eur. J., 2018, 24, 400–408 CrossRef CAS PubMed.
- L. Zhang, R. Zhang, R. Ge, X. Ren, S. Hao, F. Xie, F. Qu, Z. Liu, G. Du and A. M. Asiri, Chem. – Eur. J., 2017, 23, 11499–11503 CrossRef CAS PubMed.
- Y. Wang, M. Qiao, Y. Li and S. Wang, Small, 2018, 14, 1800136 CrossRef PubMed.
- Y. Wang, D. Yan, S. El Hankari, Y. Zou and S. Wang, Adv. Sci., 2018, 5, 1800064 CrossRef PubMed.
- J. A. Carrasco, J. Romero, M. Varela, F. Hauke, G. Abellan, A. Hirsch and E. Coronado, Inorg. Chem. Front., 2016, 3, 478–487 RSC.
- P. Zhou, Y. Y. Wang, C. Xie, C. Chen, H. W. Liu, R. Chen, J. Huo and S. Y. Wang, Chem. Commun., 2017, 53, 11778–11781 RSC.
- L. Zhou, X. Huang, H. Chen, P. Jin, G. Li and X. Zou, Dalton Trans., 2015, 44, 11592 RSC.
- J. Nai, H. Yin, T. You, L. Zheng, J. Zhang, P. Wang, Z. Jin, Y. Tian, J. Liu, Z. Tang and L. Guo, Adv. Energy Mater., 2015, 5, 1401880 CrossRef.
- C. Tang, H. S. Wang, H. F. Wang, Q. Zhang, G. L. Tian, J. Q. Nie and F. Wei, Adv. Mater., 2015, 27, 4516–4522 CrossRef CAS PubMed.
- Z. Jiang, Z. Li, Z. Qin, H. Sun, X. Jiao and D. Chen, Nanoscale, 2013, 5, 11770 RSC.
- C. Xia and H. N. Alsharref, Chem. Mater., 2015, 27, 4661–4668 CrossRef CAS.
- T. Zhu, Z. Wang, S. Ding, J. Chen and X. Lou, RSC Adv., 2011, 1, 397–400 RSC.
- G. Zhang and X. W. D. Lou, Sci. Rep., 2013, 3, 1470 CrossRef PubMed.
- Z. Jiang, W. Lu, Z. Li, K. H. Ho, X. Li, X. Jiao and D. Chen, J. Mater. Chem. A, 2014, 2, 8603 RSC.
- S. Xu, C. M. Hessel, H. Ren, R. Yu, Q. Jin, M. Yang, H. Zhao and D. Wang, Energy Environ. Sci., 2014, 7, 632 RSC.
- H. Hu, B. Guan, B. Xia and X. Lou, J. Am. Chem. Soc., 2015, 137, 5590–5595 CrossRef CAS PubMed.
- J. Zhu, Z. Yin, D. Yang, T. Sun, H. Yu, H. E. Hoster, H. H. Hng, H. Zhang and Q. Yan, Energy Environ. Sci., 2013, 6, 987 RSC.
- Z. Jiang, Z. Li, Z. Qin, H. Sun, X. Jiao and D. Chen, Nanoscale, 2013, 5, 11770 RSC.
- C. Kuo and M. H. Huang, J. Am. Chem. Soc., 2008, 130, 12815–12820 CrossRef CAS PubMed.
- G. Zhang, H. Wu, H. E. Hoster and X. Lou, Energy Environ. Sci., 2014, 7, 302 RSC.
- L. Hu, B. Qu, C. Li, Y. Chen, L. Mei, D. Lei, L. Chen, Q. Li and T. Wang, J. Mater. Chem. A, 2013, 1, 5596–5602 RSC.
- M. Gilanizadeh and B. Zeynizadeh, New J. Chem., 2018, 42, 8553–8566 RSC.
- R. Pourfaraja, S. Y. Kazemib, S. J. Fatemia and P. Biparva, J. Solid State Chem., 2018, 265, 248–256 CrossRef.
- M. S. Burke, M. G. Kast, L. Trotochaud, A. M. Smith and S. W. Boettcher, J. Am. Chem. Soc., 2015, 137, 3638–3648 CrossRef CAS PubMed.
- X. Zhang, X. Zhang, H. Xu, Z. Wu, H. Wang and Y. Liang, Adv. Funct. Mater., 2017, 27, 1606635 CrossRef.
- Q. Wang, B. Yu, X. Li, L. Xing and X. Xue, J. Mater. Chem. A, 2016, 4, 425–433 RSC.
- S. Zhou, Z. Ye, S. Hu, C. Hao, X. Wang, C. Huang and F. Wu, Nanoscale, 2018, 10, 15771–15781 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Further details of the experiment step, additional SEM, TEM, XRD, XPS and other materials characterization. See DOI: 10.1039/c8qi00972d |
| This journal is © the Partner Organisations 2019 |