Quaternary polymer solar cells with over 13% efficiency enabled by improving film-morphologies via binary mixed fullerene additive†
Fugang
Shen
ab,
Dong
Yan
b,
Weiping
Li
b,
Huifeng
Meng
c,
Jianhua
Huang
 c,
Xuemei
Li
c,
Xuemei
Li
 *d,
Jianzhong
Xu
*a and
Chuanlang
Zhan
*d,
Jianzhong
Xu
*a and
Chuanlang
Zhan
 *ab
*ab
aCollege of Chemistry and Environmental Science, Hebei University, Baoding 071002, China. E-mail: xujz@hbu.edu.cn
bBeijing National Laboratory of Molecular Science, CAS Key Laboratory of Photochemistry, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: clzhan@iccas.ac.cn
cCollege of Materials Science and Engineering, Huaqiao University, Xiamen, 361021, China
dSchool of Chemistry & Chemical Engineering, Linyi University, Linyi 276000, China. E-mail: xuemei_li@yeah.net
First published on 11th December 2018
Abstract
In this article, we report two new cases of quaternary polymer solar cells with over 13% efficiency. By introducing bis-PC71BM:PC61BM or bis-PC71BM:IC60BA into a known nonfullerene system – PBDB-T:IT-M, the quaternary solar cells significantly outperform the nonfullerene binary and ternary (PBDB-T:IT-M:fullerene) cells with a significant increase in short-circuit current-density (18.2 vs. 16.5 and 16.8–17.4 mA cm−2, respectively) and fill-factor (0.73 vs. 0.67 and 0.705–0.726, respectively) and hence large power conversion efficiencies (13.3% for the quaternary vs. 11% for the binary and 12% for the ternary solar cells). Compared with the clear phase-separated domains in the bis-PC71BM based ternary blend, the PC61BM based film showed the finest morphologies and the IC60BA based film had the largest domains. In sequence, the bis-PC71BM:PC61BM-based quaternary blend showed finer morphology than the bis-PC71BM:IC60BA based blend, while both the quaternary blends showed clear phase-separated bright and dark domains. One-dimensional grazing incidence X-ray diffraction (1D GIXRD) data indicated that the addition of PC61BM and IC60BA helped more IT-M molecules to form compacted π–π stacks at the out-of-plane directions, which resulted in the increase in electron mobilities. Our results indicate that the use of the fourth fullerene component provides more choices and more mechanisms than the ternary systems for tuning the photon-to-electron conversion; therefore, this study sheds light on the realization of high-efficiency polymer solar cells by designing multi-acceptor component energy levels.
Introduction
Bulk-heterojunction (BHJ) polymer solar cells (PSCs) have several merits such as semi-transparency, light-weight, low-cost, and flexibility at the same time, which endow PSCs with potentials to meet the future demands of sustainable and green energy sources. The power conversion efficiencies (PCEs) in PSCs are however largely restricted by the intrinsic trade-off between the open-circuit voltage (Voc), the short-circuit current-density (Jsc), and the fill factor (FF). The ternary blended bulk-heterojunction (T-BHJ)1–5 and tandem devices6–9 are two materials that release the trade-off, and the former is of relative simplicity in both device structure and fabrication in keeping with the single junction device structure. A T-BHJ allows for the use of one more donor (D) or one more acceptor (A) to resolve the narrow absorption of organic blend films, giving rise to larger Jsc.10–13 Moreover, as the third component forms an alloy with the parent D/A component,14–17 the resulting ternary system behaves like a pseudo-binary system. The mixing becomes as easy as the fabrication of a binary system and the single-junction device structure is maintained. More importantly, the formation of the electronic alloy state allows for (1) the tuning of the interfacial charge transfer (CT) state energy level between the parent two-components' extreme values, which associates with the adjustment of the Voc between the two binary devices' extremes,15,16 and (2) the tuning of the film-morphology for the out-of-plane (OOP) charge transport and for the reduction of the recombination losses,17 leading to a higher FF. Collectively, a larger Voc·Jsc·FF product is available with the T-BHJ than with the binary blend by judiciously designing the ternary blended photo-active systems.Nevertheless, the ternary approach has several issues. First, success in the mixing of two or more polymers is still limited to rare cases because of the structure similarity requirement18 and also due to the weak intermolecular interactions and lack of entropic force between polymers.19 Second, the mixing between a polymer and a small molecule donor17,20 or between two small molecule donors21,22 is still highly challenging because achieving more efficient devices is largely restricted by the complexity in obtaining the ideal film morphologies and donor/acceptor phase crystallinity.23,24 Distinctly, the structure similarity, the small energy difference (<0.3 eV), the spherical shape, and the entropic force25 benefits the easy mixing between two fullerene acceptors, which largely relaxes the complexity in obtaining the ideal film-morphologies.26–29 A big issue in this form is the limitation of the number of fullerene derivatives.
Relative to fullerene, the frontier molecular orbitals and the optical band gap, Eoptg, of fused-ring-based nonfullerene acceptors can be tuned in a wide range with structural modifications.30–32 Thus far, reports have indicated that the highest occupied molecular orbit (HOMO) and the lowest unoccupied molecular orbit (LUMO) energy levels can be tuned between −5.3 to −6.0 eV and between −3.6 to −4.2 eV, respectively, and the Eoptg can be modulated between 2.0 and 1.2 eV through modifications on either the fused-ring electron-donating (D)/the electron-accepting (A) units and/or the π-bridges, by which over 13% PCEs have been reported.33–43 Such a wide range of tuning in both the optical and electrochemical properties provides possibilities for fabricating ternary blended organic solar cells. In the cases of two nonfullerene acceptors, the judicious selection of two structurally miscible nonfullerenes is very important to obtain improved device performance.44–51 Relatively, the mixing of one nonfullerene with one fullerene may integrate the merits from both the nonfullerene and fullerene acceptors. Particularly, the excellent film-forming ability of fullerene helps to obtain ideal film-morphologies.52–60
Recently, we reported the first case of a quaternary solar cell, in which the binary blended PCBM:ICBA was introduced into PBDB-T:ITIC, a known fullerene-free system, leading to the formation of the so-called quaternary BHJ (Q-BHJ).61 The Q-BHJ integrates the merits of both fused-ring based nonfullerene and fullerene acceptors and also those of the mixture of two fullerene acceptors for adjusting the film-morphologies, device electric properties and solar cell Voc values. In this case, we found that the minor difference in the structures of the fullerene acceptors was able to lead to a very different device performance and the structure-matched IC60BA:PC61BM combination gave rise to the best device function. In this article, we report two new cases of quaternary polymer solar cells by introducing bis-PC71BM:PC61BM or bis-PC71BM:IC60BA into another known nonfullerene system – PBDB-T:IT-M. Here, PBDB-T is poly[(2,6-(4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzo[1,2-b:4,5-b′]dithiophene)-co-(1,3-di(5-thiophene-2-yl)-5,7-bis(2-ethylhexyl)benzo[1,2-c:4,5-c′]dithiophene-4,8-dione)]62 and IT-M is 3,9-bis(2-methylene-(3-(1,1-dicyanomethylene)indanone-methyl))-5,5,11,11-tetrakis(4-n-hexylphenyl)-dithieno[2,3d:2′,3′d′]-s-indaceno[1,2b:5,6b′]dithiophene (Fig. 1).63 Compared to the bis-PC71BM-based ternary device, the PC61BM- and IC60BA-based devices had larger Jsc but lower FF. As the mixed bis-PC71BM:PC61BM or bis-PC71BM:IC60BA was used as the additive, both the quaternary devices showed improved Jsc and FF, and the IC60BA-based device exhibited slightly larger Voc than the PC61BM-based device. Finally, the quaternary solar cells showed over 13.3% PCEs, while the ternary and fullerene-free binary devices had PCEs of 12% and 11%, respectively.
 | ||
| Fig. 1 Chemical structures of polymer donor as well as nonfullerene and fullerene small-molecule acceptors. | ||
Results and discussion
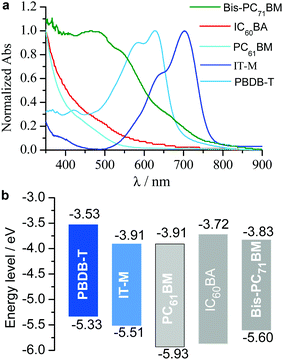
Fig. 2a shows the film absorption spectra of the polymer donor and the nonfullerene and fullerene acceptors. IC60BA and PC61BM show absorption in the visible region typically below 600 nm and bis-PC71BM has relatively stronger absorption in the visible region typically below 750 nm. Their LUMO energy levels are close to each other and match well with that of IT-M (Fig. 2b). The energy difference between IT-M and fullerene component is 0–0.19 eV, offering the possibility for the formation of alloyed energy states.64The binary, ternary and quaternary cells were fabricated with a traditional device structure of ITO/PEDOT:PSS65/active layer/ETL/Al. Here, ITO is indium tin oxide, PEDOT:PSS is poly(3,4-ethylenedioxythiophene):poly(styrene-sulfonate), and the electron transporting layer (ETL) is the binary N719:PrC60MAI (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
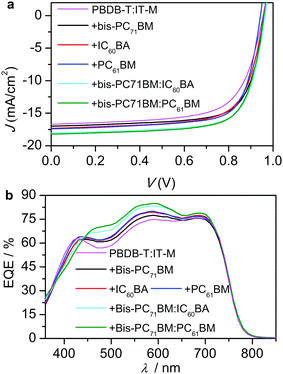
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w)-based blend, which has been recently reported by us53 with dual functions.66–71 N719 is di-tetrabutylammonium cis-bis(isothiocyanato)bis(2,2′-bipyridyl-4,4′-dicarboxylato)ruthenium(II)) and PrC60MAI is C60,N,N,N-trimethyl-1-(2,3,4-tris(2-(2-methoxyethoxy)ethoxy)phenyl)dimethanaminium monoadduct iodide salt.72 Optimizations towards the best-performing solar cells are given in Tables S1–S4 (ESI†). Table 1 lists the photovoltaic data of the optimal devices, and their current-density–voltage (J–V) curves are shown in Fig. 3a. The integrated Jsc values from their external quantum efficiencies (EQEs, Fig. 3b) agreed with the values from the J–V measurements (Table 1).
1, w/w)-based blend, which has been recently reported by us53 with dual functions.66–71 N719 is di-tetrabutylammonium cis-bis(isothiocyanato)bis(2,2′-bipyridyl-4,4′-dicarboxylato)ruthenium(II)) and PrC60MAI is C60,N,N,N-trimethyl-1-(2,3,4-tris(2-(2-methoxyethoxy)ethoxy)phenyl)dimethanaminium monoadduct iodide salt.72 Optimizations towards the best-performing solar cells are given in Tables S1–S4 (ESI†). Table 1 lists the photovoltaic data of the optimal devices, and their current-density–voltage (J–V) curves are shown in Fig. 3a. The integrated Jsc values from their external quantum efficiencies (EQEs, Fig. 3b) agreed with the values from the J–V measurements (Table 1).
| PBDB-T:IT-M:bis-PC70BM:fullerene | V oc [V] | V BI [V] | J sc [mA cm−2] | J sc [mA cm−2] | FFa | PCEavea [%] | PCEmax [%] |
|---|---|---|---|---|---|---|---|
| a Average values from 20–35 devices. b Estimated from the dark J–V curves (Fig. S1, ESI). c Integrated from the EQE spectra from 360 to 900 nm. | |||||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0.952 ± 0.007 | 1.04 | 16.59 ± 0.32 | 15.85 | 0.675 ± 0.013 | 10.66 ± 0.39 | 11.15 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0.959 ± 0.008 | 1.09 | 16.79 ± 0.30 | 16.10 | 0.726 ± 0.012 | 11.69 ± 0.32 | 12.04 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 (IC60BA) 0.2 (IC60BA) |
0.956 ± 0.006 | 1.07 | 17.21 ± 0.35 | 16.49 | 0.705 ± 0.011 | 11.60 ± 0.35 | 12.06 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 (PC61BM) 0.2 (PC61BM) |
0.948 ± 0.005 | 1.04 | 17.38 ± 0.36 | 16.54 | 0.709 ± 0.010 | 11.68 ± 0.34 | 12.09 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 (IC60BA) 0.2 (IC60BA) |
0.968 ± 0.006 | 1.11 | 18.21 ± 0.33 | 17.29 | 0.731 ± 0.011 | 12.89 ± 0.37 | 13.31 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 (PC61BM) 0.2 (PC61BM) |
0.957 ± 0.007 | 1.08 | 18.38 ± 0.31 | 17.40 | 0.731 ± 0.010 | 12.86 ± 0.35 | 13.28 |
Comparable PCEs (12%) were obtained from the bis-PC71BM, IC60BA and PC61BM-based ternary devices. The bis-PC71BM ternary cells showed higher Voc and FF, while the IC60BA- and PC61BM-based ternary devices exhibited higher Jsc. Therefore, we introduced IC60BA and PC61BM as the forth component into the bis-PC71BM-based ternary system to fabricate the quaternary devices. Interestingly, compared with the parent ternary systems – the bis-PC71BM-, IC60BA-, and PC61BM-based systems – large Voc, Jsc and FF were simultaneously obtained for the two quaternary systems, which finally gave rise to the high PCEs (13.3%). The Voc values were 0.95–0.97 V, obtained under illumination for the PBDB-T:IT-M based optimal binary, ternary and quaternary devices. In the dark, the turn-on voltage was estimated to be 1.04–1.11 V (Fig. S1, ESI†), which indicated that these devices exhibited high built-in voltage (VBI).
The electron and hole mobilities (μe/μh) were measured by the space-charge-limited current (SCLC) method (Fig. S2, ESI†). The μe/μh values were estimated to be 0.95/0.13, 1.86/0.21, and 2.07/0.21 × 10−4 cm2 V−1 s−1, respectively, for the bis-PC71BM-, IC60BA- and PC61BM-based ternary solar cell blend films. The IC60BA- and PC61BM-based blended films showed larger electron and hole mobilities, which was consistent with the observation of larger Jsc values. For the quaternary devices, even larger μe/μh values of 2.57/0.36 and 2.47/0.39 × 10−4 cm2 V−1 s−1 were obtained for the IC60BA- and PC61BM-based devices, respectively. The larger mobilities contributed to the larger Jsc values for the two quaternary solar cells.
Fig. 4 shows the transmission electron microscopy (TEM) pictures of the blended ternary and quaternary solar cell films. All the five blends showed nanoscaled film morphologies. Among the three blends, the largest bright and dark domains are observed for the IC60BA-based ternary blend, while the finest morphology is seen for the PC61BM-based blend. Very clear phase-separated bright and dark domains are seen for the bis-PC71BM-based ternary film and also in the two quaternary films, which agree with their much higher FF values compared with those of the IC60BA- and PC61BM-based ternary devices. The atomic force microscopy (AFM) height images (Fig. 4) showed similar morphologies for the three blends. The root-mean-square roughness (rms) values were 2.15, 4.06, and 1.99 nm for the IC60BA-, bis-PC71BM-, and PC61BM-based ternary blends, respectively. For the quaternary blend, larger bright and dark domains are seen for the IC60BA-based quaternary blend than the PC61BM-based quaternary blend. Again, both the IC60BA- and PC61BM-based quaternary blends showed longer fibril morphologies than the three ternary films, which might help to give rise to the larger Jsc, as observed from the J–V characterizations. The rms values were 3.16 and 3.61 nm for the IC60BA- and PC61BM-based quaternary blends, respectively.
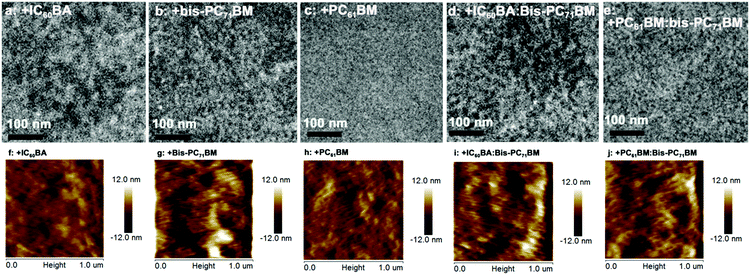 | ||
| Fig. 4 TEM (upper) and AFM height (lower) images of the ternary (a–c; f–h) and quaternary (d and e; i and j) blend films. | ||
Fig. 5 shows the (010) regions of one-dimensional graze-incidence X-ray diffraction (1D GIXRD) data of the host fullerene-free binary, the three ternary, and the two quaternary solar cell blends. The 1D GIXRD data were directly measured from the samples at their qz and qxy directions.73 First, the diffraction patterns of the six samples were quite similar, indicating that the IT-M π–π stacking was well maintained after the addition of the fullerene additives. All the six samples showed two peaks at 1.70–1.73 and 1.83–1.86 Å−1 (Fig. S3, ESI†), which corresponded to a π–π stacking distance of 3.69–3.63 and 3.43–3.39 Å, respectively. These two diffractions can be assigned as those originated from out-of-plane (OOP) π–π stacks of the acceptor IT-M. The right side diffraction tail can be contributed from the compacted π–π stacks of IT-M.74 Second, there are small differences in the intensity of the two (010) diffractions. For the host binary blend, the 1.85 Å−1 peak is much weaker than the 1.72 Å−1 diffraction peak, while for the ternary blend, we can see that the intensity of 1.83–1.86 Å−1 peak increased relatively to that of the 1.70–1.73 Å−1 peak, particularly with the use of bis-PC71BM or PC61BM as the third additive (Fig. S3, ESI†). This increase suggests that more IT-M molecules form compact π–π stacks upon the addition of bis-PC71BM, IC60BA and PC61BM. For the quaternary blend, the use of bis-PC71BM:IC60BA led to the shift in band position to a larger Q vector value, indicating the formation of more compacted π–π stacks at the OOP direction than with the use of a single fullerene component. Similar result was obtained using bis-PC71BM:PC61BM (Fig. S3, ESI†). Again, compared with the host binary blend, the larger Q-vector peak also increased, indicating that more IT-M molecules form compacted π–π stacks with the use of binary mixing fullerene, particularly with the use of bis-PC71BM:PC61BM. The compact packing of IT-M molecules along the OOP direction benefits the OOP electron transport, which is consistent with the large electron mobilities in the IC60BA- and PC61BM-based ternary and quaternary solar cell blends.
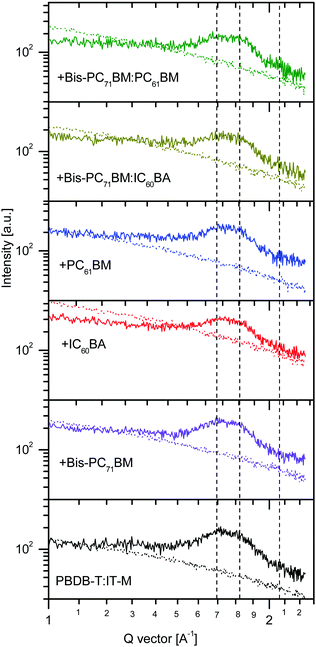 | ||
| Fig. 5 The (010) regions of the GIXRD 1D profiles of the host binary, ternary and quaternary solar cell blends at the out-of-plane (solid lines) and in-plane (dot lines), respectively. | ||
Conclusions
Two new quaternary PSCs were fabricated by introducing bis-PC71BM:PC61BM or bis-PC71BM:IC60BA into the fullerene-free PBDB-T:IT-M system, and bis-PC71BM-, PC61BM-, and IC60BA-based ternary PSCs were also fabricated for comparisons. The PC61BM- and IC60BA-based ternary devices had larger Jsc than the bis-PC71BM-based ternary device, while the latter showed higher FF and hence, all the three ternary devices showed 12% PCEs. On using mixed bis-PC71BM:PC61BM or bis-PC71BM:IC60BA as the additive, the quaternary solar cells showed improved Voc, Jsc, and FF, and finally increased PCEs, reaching 13.3%. TEM images indicated clear phase-separated bright and dark domains in the bis-PC71BM-based ternary and the two quaternary blends, while the IC60BA-based ternary and quaternary blends had larger domains and the PC61BM-based ternary and quaternary blends had finer film-morphologies. 1D GIXRD data indicated that the addition of IC60BA and PC61BM helped more IT-M molecules to form compact π–π stacks at the out-of-plane direction, which is consistent with their larger electron mobilities. These results again demonstrated the large outstanding performance of the quaternary blend devices compared with the ternary blend devices, which opens windows for the further improvement of PSCs device performance as the four-component-based active layer provides much more choices to improve the optical and electrical functions of the photoactive layer.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the financial support from the National Natural Science Foundation of China (NSFC, No. 91433202, 21773262, 21327805, 91227112, and 21521062) and Chinese Academy of Sciences (CAS, No. XDB12010200). The authors gratefully acknowledge Beijing Synchrotron Radiation Facility (BSRF) for supports of GIXRD measurements.Notes and references
- Z. Zhou, S. Xu, J. Song, Y. Jin, Q. Yue, Y. Qian, F. Liu, F. Zhang and X. Zhu, Nat. Energy, 2018, s41560 Search PubMed.
- M. Xiao, K. Zhang, S. Dong, Q. Yin, Z. Liu, L. Liu, F. Huang and Y. Cao, ACS Appl. Mater. Interfaces, 2018, 10, 25594–25603 CrossRef CAS PubMed.
- L. Yang, W. Gu, L. Hong, Y. Mi, F. Liu, M. Liu, Y. Yang, B. Sharma, X. Liu and H. Huang, ACS Appl. Mater. Interfaces, 2017, 9, 26928–26936 CrossRef CAS PubMed.
- J. Wang, J. Peng, X. Liu and Z. Liang, ACS Appl. Mater. Interfaces, 2017, 9, 20704–20710 CrossRef CAS PubMed.
- W. Su, Q. Fan, X. Guo, X. Meng, Z. Bi, W. Ma, M. Zhang and Y. Li, Nano Energy, 2017, 38, 510–517 CrossRef CAS.
- L. Meng, Y. Zhang, X. Wan, C. Li, X. Zhang, Y. Wang, X. Ke, Z. Xiao, L. Ding, R. Xia, H.-L. Yip, Y. Cao and Y. Chen, Science, 2018, 361, 1094–1098 CrossRef CAS PubMed.
- Y. Cui, H. Yao, B. Gao, Y. Qin, S. Zhang, B. Yang, C. He, B. Xu and J. Hou, J. Am. Chem. Soc., 2017, 139, 7302–7309 CrossRef CAS PubMed.
- D. Di Carlo Rasi, K. H. Hendriks, M. M. Wienk and R. A. J. Janssen, Adv. Mater., 2018, e1803836 CrossRef PubMed.
- S. Chen, H. Yao, B. Hu, G. Zhang, L. Arunagiri, L.-K. Ma, J. Huang, J. Zhang, Z. Zhu, F. Bai, W. Ma and H. Yan, Adv. Energy Mater., 2018, 8, 1800529 CrossRef.
- T. Ameri, T. Heumueller, J. Min, N. Li, G. Matt, U. Scherf and C. J. Brabec, Energy Environ. Sci., 2013, 6, 1796–1801 RSC.
- L. Lu, T. Xu, W. Chen, E. S. Landry and L. Yui, Nat. Photonics, 2014, 8, 716–722 CrossRef CAS.
- T. H. Lee, M. A. Uddin, C. Zhong, S.-J. Ko, B. Walker, T. Kim, Y. J. Yoon, S. Y. Park, A. J. Heeger, H. Y. Woo and J. Y. Kim, Adv. Energy Mater., 2016, 6, 1600637 CrossRef.
- L. Nian, K. Gao, F. Liu, Y. Kan, X. Jiang, L. Liu, Z. Xie, X. Peng, T. P. Russell and Y. Ma, Adv. Mater., 2016, 28, 8184–8190 CrossRef CAS PubMed.
- D. Angmo, M. Bjerring, N. C. Nielsen, B. C. Thompson and F. C. Krebs, J. Mater. Chem. C, 2015, 3, 5541–5548 RSC.
- R. A. Street, D. Davies, P. P. Khlyabich, B. Burkhart and B. C. Thompson, J. Am. Chem. Soc., 2013, 135, 986–989 CrossRef CAS PubMed.
- S. A. Mollinger, K. Vandewal and A. Salleo, Adv. Energy Mater., 2015, 5, 1501335 CrossRef.
- J. Zhang, Y. Zhang, J. Fang, K. Lu, Z. Wang, W. Ma and Z. Wei, J. Am. Chem. Soc., 2015, 137, 8176–8183 CrossRef CAS PubMed.
- Y. Yang, W. Chen, L. Dou, W.-H. Chang, H.-S. Duan, B. Bob, G. Li and Y. Yang, Nat. Photonics, 2015, 9, 190–198 CrossRef CAS.
- C. B. Nielsen, S. Holliday, H.-Y. Chen, S. J. Cryer and I. McCulloch, Acc. Chem. Res., 2015, 48, 2803–2812 CrossRef CAS PubMed.
- G. Zhang, K. Zhang, Q. Yin, X.-F. Jiang, Z. Wang, J. Xin, W. Ma, H. Yan, F. Huang and Y. Cao, J. Am. Chem. Soc., 2017, 139, 2387–2395 CrossRef CAS PubMed.
- Y. Zhang, D. Deng, K. Lu, J. Zhang, B. Xia, Y. Zhao, J. Fang and Z. Wei, Adv. Mater., 2015, 27, 1071–1076 CrossRef CAS PubMed.
- Q. An, F. Zhang, Q. Sun, J. Wang, L. Li, J. Zhang, W. Tang and Z. Deng, J. Mater. Chem. A, 2015, 3, 16653–16662 RSC.
- Q. An, F. Zhang, J. Zhang, W. Tang, Z. Deng and B. Hu, Energy Environ. Sci., 2016, 9, 281–322 RSC.
- H. Kang, W. Lee, J. Oh, T. Kim, C. Lee and B. J. Kim, Acc. Chem. Res., 2016, 49, 2424–2434 CrossRef CAS PubMed.
- A. D. d. Z. Mendaza, A. Melianas, S. Rossbauer, O. Backe, L. Nordstierna, P. Erhart, E. Olsson, T. D. Anthopoulos, O. Inganas and C. Muller, Adv. Mater., 2015, 27, 7325–7331 CrossRef CAS PubMed.
- H. Kang, K.-H. Kim, T. E. Kang, C.-H. Cho, S. Park, S. C. Yoon and B. J. Kim, ACS Appl. Mater. Interfaces, 2013, 5, 4401–4408 CrossRef CAS PubMed.
- S.-J. Ko, W. Lee, H. Choi, B. Walker, S. Yum, S. Kim, T. J. Shin, H. Y. Woo and J. Y. Kim, Adv. Energy Mater., 2015, 5, 1401687 CrossRef.
- H.-W. Liu, D.-Y. Chang, W.-Y. Chiu, S.-P. Rwei and L. Wang, J. Mater. Chem., 2012, 22, 15586–15591 RSC.
- B. C. Schroeder, Z. Li, M. A. Brady, G. C. Faria, R. S. Ashraf, C. J. Takacs, J. S. Cowart, D. T. Duong, K. H. Chiu, C.-H. Tan, J. T. Cabral, A. Salleo, M. L. Chabinyc, J. R. Durrant and I. McCulloch, Angew. Chem., Int. Ed., 2014, 53, 12870–12875 Search PubMed.
- F. Shen, J. Xu, X. Li and C. Zhan, J. Mater. Chem. A, 2018, 6, 15433–15455 RSC.
- J. Hou, O. Inganas, R. H. Friend and F. Gao, Nat. Mater., 2018, 17, 119–128 CrossRef CAS PubMed.
- G. Zhang, J. Zhao, P. C. Y. Chow, K. Jiang, J. Zhang, Z. Zhu, J. Zhang, F. Huang and H. Yan, Chem. Rev., 2018, 118, 3447–3507 CrossRef CAS PubMed.
- W. Zhao, S. Li, H. Yao, S. Zhang, Y. Zhang, B. Yang and J. Hou, J. Am. Chem. Soc., 2017, 139, 7148–7151 CrossRef CAS PubMed.
- W. Li, L. Ye, S. Li, H. Yao, H. Ade and J. Hou, Adv. Mater., 2018, 30, e1707170 CrossRef PubMed.
- S. Li, L. Ye, W. Zhao, H. Yan, B. Yang, D. Liu, W. Li, H. Ade and J. Hou, J. Am. Chem. Soc., 2018, 140, 7159–7167 CrossRef CAS PubMed.
- Z. Zheng, Q. Hu, S. Zhang, D. Zhang, J. Wang, S. Xie, R. Wang, Y. Qin, W. Li, L. Hong, N. Liang, F. Liu, Y. Zhang, Z. Wei, Z. Tang, T. P. Russell, J. Hou and H. Zhou, Adv. Mater., 2018, 30, e1801801 CrossRef PubMed.
- Y. Cui, S. Zhang, N. Liang, J. Kong, C. Yang, H. Yao, L. Ma and J. Hou, Adv. Mater., 2018, 30, e1802499 CrossRef PubMed.
- H. Zhang, H. Yao, J. Hou, J. Zhu, J. Zhang, W. Li, R. Yu, B. Gao, S. Zhang and J. Hou, Adv. Mater., 2018, 30, e1800613 CrossRef PubMed.
- J. Sun, X. Ma, Z. Zhang, J. Yu, J. Zhou, X. Yin, L. Yang, R. Geng, R. Zhu, F. Zhang and W. Tang, Adv. Mater., 2018, 30, e1707150 CrossRef PubMed.
- B. Kan, H. Feng, H. Yao, M. Chang, X. Wan, C. Li, J. Hou and Y. Chen, Sci. Chin. Chem, 2018, 62, s11426 Search PubMed.
- D. He, F. Zhao, J. Xin, J. J. Rech, Z. Wei, W. Ma, W. You, B. Li, L. Jiang, Y. Li and C. Wang, Adv. Energy Mater., 2018, 8, 1802050 CrossRef.
- W. Gao, T. Liu, R. Ming, Z. Luo, K. Wu, L. Zhang, J. Xin, D. Xie, G. Zhang, W. Ma, H. Yan and C. Yang, Adv. Funct. Mater., 2018, 8, 1803128 CrossRef.
- W. Wang, B. Zhao, Z. Cong, Y. Xie, H. Wu, Q. Liang, S. Liu, F. Liu, C. Gao, H. Wu and Y. Cao, ACS Energy Lett., 2018, 3, 1499 CrossRef CAS.
- T. Liu, Y. Guo, Y. Yi, L. Huo, X. Xue, X. Sun, H. Fu, W. Xiong, D. Meng, Z. Wang, F. Liu, T. P. Russell and Y. Sun, Adv. Mater., 2016, 28, 10008–10015 CrossRef CAS PubMed.
- D. Baran, R. S. Ashraf, D. A. Hanifi, M. Abdelsamie, N. Gasparini, J. A. Rohr, S. Holliday, A. Wadsworth, S. Lockett, M. Neophytou, C. J. M. Emmott, J. Nelson, C. J. Brabec, A. Amassian, A. Salleo, T. Kirchartz, J. R. Durrant and I. McCulloch, Nat. Mater., 2017, 16, 363–370 CrossRef CAS PubMed.
- X. Ma, W. Gao, J. Yu, Q. An, M. Zhang, Z. Hu, J. Wang, W. Tang, C. Yang and F. Zhang, Energy Environ. Sci., 2018, 11, 2134–2141 RSC.
- M. Zhang, W. Gao, F. Zhang, Y. Mi, W. Wang, Q. An, J. Wang, X. Ma, J. Miao, Z. Hu, X. Liu, J. Zhang and C. Yang, Energy Environ. Sci., 2018, 11, 841–849 RSC.
- L. Zhan, S. Li, H. Zhang, F. Gao, T.-K. Lau, X. Lu, D. Sun, P. Wang, M. Shi, C.-Z. Li and H. Chen, Adv. Sci., 2018, 5, 1800755 CrossRef PubMed.
- P. Cheng, J. Wang, Q. Zhang, W. Huang, J. Zhu, R. Wang, S.-Y. Chang, P. Sun, L. Meng, H. Zhao, H.-W. Cheng, T. Huang, Y. Liu, C. Wang, C. Zhu, W. You, X. Zhan and Y. Yang, Adv. Mater., 2018, 30, e1801501 CrossRef PubMed.
- W. Jiang, R. Yu, Z. Liu, R. Peng, D. Mi, L. Hong, Q. Wei, J. Hou, Y. Kuang and Z. Ge, Adv. Mater., 2018, 30, 1703005 CrossRef PubMed.
- W. Wu, G. Zhang, X. Xu, S. Wang, Y. Li and Q. Peng, Adv. Funct. Mater., 2018, 28, 1707493 CrossRef.
- H. Lu, J. Zhang, J. Chen, Q. Liu, X. Gong, S. Feng, X. Xu, W. Ma and Z. Bo, Adv. Mater., 2016, 28, 9559–9566 CrossRef CAS PubMed.
- W. Li, D. Yan, W. Liu, J. Chen, W. Xu, C. Zhan and J. Yao, Sol. RRL, 2017, 1, 1700014 CrossRef.
- W. Zhao, S. Li, S. Zhang, X. Liu and J. Hou, Adv. Mater., 2016, 29, 1604059 CrossRef.
- G. Huang, J. Zhang, N. Uranbileg, W. Chen, H. Jiang, H. Tan, W. Zhu and R. Yang, Adv. Energy Mater., 2018, 8, 1702489 CrossRef.
- Y. Chen, Y. Qin, Y. Wu, C. Li, H. Yao, N. Liang, X. Wang, W. Li, W. Ma and J. Hou, Adv. Energy Mater., 2017, 7, 1700328 CrossRef.
- M. An, F. Xie, X. Geng, J. Zhang, J. Jiang, Z. Lei, D. He, Z. Xiao and L. Ding, Adv. Energy Mater., 2017, 7, 1602509 CrossRef.
- B. Fan, W. Zhong, X.-F. Jiang, Q. Yin, L. Ying, F. Huang and Y. Cao, Adv. Energy Mater., 2017, 7, 1602127 CrossRef.
- H. Zhang, X. Wang, L. Yang, S. Zhang, Y. Zhang, C. He, W. Ma and J. Hou, Adv. Mater., 2017, 29, 1703777 CrossRef PubMed.
- N. Zhu, W. Zhang, Q. Yin, L. Liu, X. Jiang, Z. Xie and Y. Ma, ACS Appl. Mater. Interfaces, 2017, 9, 17265–17270 CrossRef CAS PubMed.
- W. Li, D. Yan, F. Liu, T. Russell, C. Zhan and J. Yao, Sci. China: Chem., 2018, 61, 1609–1618 Search PubMed.
- D. Qian, L. Ye, M. Zhang, Y. Liang, L. Li, Y. Huang, X. Guo, S. Zhang, Z. a. Tan and J. Hou, Macromolecules, 2012, 45, 9611–9617 CrossRef CAS.
- S. Li, L. Ye, W. Zhao, S. Zhang, S. Mukherjee, H. Ade and J. Hou, Adv. Mater., 2016, 28, 9423–9429 CrossRef CAS PubMed.
- Y. Chen, P. Ye, Z.-G. Zhu, X. Wang, L. Yang, X. Xu, X. Wu, T. Dong, H. Zhang, J. Hou, F. Liu and H. Huang, Adv. Mater., 2017, 29, 1603154 CrossRef PubMed.
- W. Li, X. Zhang, X. Zhang, J. Yao and C. Zhan, ACS Appl. Mater. Interfaces, 2017, 9, 1446–1452 CrossRef CAS PubMed.
- W. Liu, W. Li, J. Yao and C. Zhan, Chin. Chem. Lett., 2018, 29, 381–384 CrossRef CAS.
- M. Gupta, D. Yan, J. Xu, J. Yao and C. Zhan, ACS Appl. Mater. Interfaces, 2018, 10, 5569–5576 CrossRef CAS PubMed.
- M. Gupta, D. Yan, F. Shen, J. Xu and C. Zhan, Acta Phys. – Chim. Sin., 2019, 35, 496–502 Search PubMed.
- M. Gupta, D. Yan, J. Yao and C. Zhan, ACS Appl. Mater. Interfaces, 2018, 10, 35896–35903 CrossRef CAS PubMed.
- M. Gupta, D. Yan, J. Yao and C. Zhan, Mater. Chem. Front., 2018, 2, 1876–1883 RSC.
- T. Gatti, F. Lamberti, P. Topolovsek, M. Abdu-Aguye, R. Sorrentino, L. Perino, M. Salerno, L. Girardi, C. Marega, G. A. Rizzi, M. A. Loi, A. Petrozza and E. Menna, Sol. RRL, 2018, 2, 1800013 CrossRef.
- C.-Z. Li, C.-C. Chueh, H.-L. Yip, K. M. O'Malley, W.-C. Chen and A. K. Y. Jen, J. Mater. Chem., 2012, 22, 8574–8578 RSC.
- X. Zhang, W. Li, J. Yao and C. Zhan, ACS Appl. Mater. Interfaces, 2016, 8, 15415–15421 CrossRef CAS PubMed.
- D. Yan, W. Liu, J. Yao and C. Zhan, Adv. Energy Mater., 2018, 8, 1800204 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8qm00571k |
| This journal is © the Partner Organisations 2019 |