Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of calcium chloride on the uniformity of colouring in sushi red ginger slices by modulating the properties of starch
Liping Jiaoa,
Shuqin Xia *a,
Xiaoming Zhang
*a,
Xiaoming Zhang a,
Jianzeng Liub,
Jingyang Yua,
Man Zhanga,
Xuejiao Wanga and
Xiangzhen Qib
a,
Jianzeng Liub,
Jingyang Yua,
Man Zhanga,
Xuejiao Wanga and
Xiangzhen Qib
aState Key Laboratory of Food Science and Technology, School of Food Science and Technology, Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province, Jiangnan University, 1800 Lihu Boulevard, Wuxi, Jiangsu 214122, China. E-mail: sqxia2013@hotmail.com; sqxia@jiangnan.edu.cn; Tel: +86 510 85884496
bLaiwu Manhing Foods Corporation, 1 Wanxing Road, Yangzhuang Town, Nonggao District, Laiwu, Shandong 271100, China
First published on 14th January 2019
Abstract
The colour of sushi red ginger slices without blanching is not uniform, which seriously affects their sensory quality. The effect of calcium chloride (CaCl2) pretreatment on the uniformity of colouring and the properties of ginger starch have been studied. The crystalline region of the starch in blanched ginger slices was broken, which might be beneficial for uniform colouring. The effect of CaCl2 pretreatment on starch properties depended on the concentration. The influence of CaCl2 at a concentration higher than 3.5 mol L−1 was more pronounced than that at a lower concentration. The uniformity of colouring was close to the effect of blanching treatment. Furthermore, the starch crystallization was destroyed, the granules were broken, and the polarized cross disappeared, which was consistent with that observed for the starch in blanched ginger slices. Therefore, it is possible to achieve a uniform colour in red ginger slices at room temperature through CaCl2 pretreatment.
1. Introduction
Ginger belongs to the family Zingiberaceae, and it is a perennial grass root plant with abundant nutritional and medicinal value, such as exerting anti-oxidative, anti-tumour and hypoglycaemic effects.1,2 Ginger has a long history and a wide planting area in China, Vietnam and other Southeast Asian countries, and it occupies an important position in agriculture development.3 In many countries, fresh ginger is used to prepare dishes and as a flavouring agent in beverages.4 With the development of the economy and the progress of society, deep-processed products of ginger are attracting more attention, and they have broad market prospects. Among them, sushi red ginger slices is popular with consumers for its bright colour and refreshing taste and is widely exported to Europe, America and Africa.Sushi red ginger slices is an industrial product prepared by adding colour seasoning liquid containing Allura red (E129) to desalted ginger slices after blanching at 95 °C. Allura red (2-hydroxy-1-(2-methoxy-5-methyl-4-sulphonatophe-nylazo)-naphthalene-6-sulphonate) is one of the most widely used synthetic diazo colorants.5 It is usually added to food products in order to achieve the desired colour and has been used in soft drinks, sweets,6 kimchi,7 a traditional Korean fermented food and others. Blanching is a traditional thermal treatment that applied to various fruits and vegetables. The main purpose of blanching is to inactivate enzymes and destroy microbial cells. However, heat treatment also causes loss of nutritional and sensorial quality attributes.8,9 Blanching leads to the leaching out of phytochemicals in ginger and decreases the antioxidant potential.10 In the processing of sushi red ginger slices, blanching ensures uniform colouring quality. However, this process consumes large quantities of energy and is a costly commercial operation.11 Considering energy conservation and business benefit, the industry hopes to directly add colour seasoning liquid to non-blanched ginger slices. However, we found that the problem of uneven colouring will occur during the production if the seasoning liquid contains Allura red, especially for old ginger slices. The phenomenon of uneven colouring is mainly due to the difficulty in colouring the middle part of the ginger slices and the easiness of the edge part, which seriously affects the quality of appearance of the product and, thus, affects the economic benefits.
The reason for difficulty in colouring may be related to the component of the ginger, which include water, starch, cellulose, pectin and others. The content of starch in old ginger is high, accounting for nearly 56% of the dry mass, and starch content in young ginger accounts for only about 19%. Several approaches have been made to modify starch as a potential adsorbent for dyes.12–14 In sewage treatment, crosslinked amphoteric starch flocculants have a strong adsorption effect and can effectively enhance flocculation and sedimentation.15 Starch/polyaniline nanocomposite might effectively adsorb dyes from textile effluents.16 Heat treatment during the blanching process is a common method of gelatinizing starch and destroying crystallization.17 The gelatinization onset temperature (To) of ginger starch is 69.7 °C, peak temperature (Tp) is 77.3 °C and conclusion temperature (Tc) is 90.5 °C.18 Therefore, blanching pretreatment at 95 °C can gelatinize starch, which might facilitates the adsorption of pigment and renders the ginger colouring more even. During heat treatment, the gelatinization process mainly consists of water swelling in the non-crystalline region, gradual disappearance of the crystalline region with water entry, and cracking of the starch granules. In addition, factors such as pH, sugar, salt, lipids, and emulsifiers also have different effects on the gelatinization properties of starch.19–22
Salts have an obvious effect on the gelatinization of starch.23–26 Research has shown that different types of salt exhibited different effects on the energy required for starch gelatinization and the gelatinization temperature curve.27 Salting ions increased the gelatinization temperature and enthalpy of maize and waxy maize starch, and the effect of salt-soluble ions was the opposite.28 Furthermore, the degree of influence on the gelatinization temperature was also different. Some salts, such as NaCl and KCl, have little effect on starch gelatinization temperature.29 Ahmad showed that alkali metal salts (LiCl, MaCl2 and CaCl2) changed the gelatinization temperature of sago starch. When the alkali metal salt reaches a certain concentration, the starch can gelatinize at room temperature.29 Among the alkali metal salts, calcium chloride (CaCl2) exhibits the potential to be used as a food-processing aid for ginger slices considering food safety. In addition, calcium ions are often used as a firming agents in the processing of fruits and vegetables, which may have a good effect on the texture of the ginger.30 Because desalination is performed before the use of ginger slices, we performed the pretreatment of ginger slices with CaCl2 before the desalination process and removed the residual CaCl2 during desalination. Although many studies have been conducted to disclose the relationship between ions with starch gelatinization, clear descriptions are limited regarding the effect of ions on the property of ginger slices and starch. It is challenging to address the influence of ion concentration and environmental conditions on the property of ginger slices as a coloured matrix. These investigations further inspire us to explore a non-blanching method for ginger slices with good pigment absorption property.
To achieve energy conservation, a creative non-blanching method to evenly colour ginger slices was developed in this study. The effects of blanching on ginger starch properties were compared. The effects of CaCl2 concentration, processing time and temperature on the uniformity of colouring were investigated with a* value as an index. To obtain further insights on the influence of CaCl2 treatment on ginger slices, the dependence of starch structure on the CaCl2 concentration was elucidated via X-ray diffraction and polarization microscopy. These studies could provide guidance for improving the uniformity of colouring and quality of appearance of non-blanched red ginger slices.
2. Materials and methods
2.1 Materials
Salted ginger (Kaempferia galanga) slices (10% NaCl, thickness 2–3 mm) and Allura red (food grade, 98% purity) were provided by Laiwu Manhing Foods Corporation (Shandong, China). Analytical grade reagents, including sodium hydroxide, CaCl2 and anhydrous ethanol, were purchased from Shanghai Chemical Reagent Corporation (Shanghai, China). All aqueous solutions were prepared with deionized water (Milli-Q water).2.2 Blanching treatment of ginger slices
Salted ginger slices (thickness 2–3 mm, 200 g) were immersed in a thermostatic water bath (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/v) at 95 °C for 5 min. After blanching, the sample was placed in deionized water for 20 min (1
2 w/v) at 95 °C for 5 min. After blanching, the sample was placed in deionized water for 20 min (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/v) to remove salts, and this process was repeated 4 times. The sushi ginger slices were prepared via vacuum sealing after adding the liquid material to it (2
2 w/v) to remove salts, and this process was repeated 4 times. The sushi ginger slices were prepared via vacuum sealing after adding the liquid material to it (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/v). The liquid material contains salt, edible acetic acid, citric acid, aspartame, sodium saccharin, potassium sorbate, monosodium glutamate, and 0.03% Allura red. A non-blanched sample was used as the control.
1 w/v). The liquid material contains salt, edible acetic acid, citric acid, aspartame, sodium saccharin, potassium sorbate, monosodium glutamate, and 0.03% Allura red. A non-blanched sample was used as the control.
2.3 CaCl2 treatment of ginger slices
The CaCl2 solution was prepared with different concentration (2, 2.5, 3, 3.5, 4, 4.5 and 5 mol L−1). Salted ginger slices (200 g) were weighed and added to CaCl2 solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/v) respectively. Then the system were mixed for 10, 20, 30, 60, 90 and 120 min with a stirring paddle at 50 rpm. The processing temperature was set at 20, 30, 40 and 50 °C respectively. The ginger slices were then separated from the CaCl2 solution and placed in deionized water for 20 min (1
2 w/v) respectively. Then the system were mixed for 10, 20, 30, 60, 90 and 120 min with a stirring paddle at 50 rpm. The processing temperature was set at 20, 30, 40 and 50 °C respectively. The ginger slices were then separated from the CaCl2 solution and placed in deionized water for 20 min (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 w/v) to remove salts and CaCl2. The desalting process was repeated 4 times. After desalting, the sushi ginger slices were prepared by vacuum sealing after adding the liquid material to it (2
2 w/v) to remove salts and CaCl2. The desalting process was repeated 4 times. After desalting, the sushi ginger slices were prepared by vacuum sealing after adding the liquid material to it (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/v). The liquid material used was the same as that described above.
1 w/v). The liquid material used was the same as that described above.
2.4 Measurement of ginger slices colouring
The colour of ginger samples were assessed using a high-precision chromometer (UltraScan Pro1166, Hunterlab Corporation, Reston, VA, USA). Before measurement, the blackboard and the whiteboard were used for zero correction. Five ginger slices were randomly chosen from each pack, and 4 points were selected on each ginger slice to determine the colour value of the ginger slices. The L* (lightness), a* (redness) and b* (yellowness) values were recorded. The average a* value and the coefficient of variation were calculated to indicate the adsorption degree of pigment on the ginger and the uniformity of ginger colouring, respectively.To reflect the relationship between the concentration of Allura red and a* value, the a* value of the pigment solution (2, 4, 6, 8, 10 and 12 μg mL−1) was measured using the high-precision chromometer. The regression equation is Y = 2.115X + 8.4467 (R2 = 0.94), in which Y represents a* value, and X represents the concentration of Allura red.
2.5 Isolation of starch
The desalted ginger slices were macerated at a low speed in a Jiuyang blender (200 g of ginger slices![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 g of deionized water) for 1 min. The homogenate was poured through four layers of muslin cloth, and the yellow residue that settled was washed with deionized water twice. Next, the mixture was sieved through a 100-mesh sieve to remove the residue. Ethanol was added to the filtrate to make its concentration more than 30%, followed by centrifugation at 8000 rpm for 10 min. The colourless precipitate was deproteinated using 0.01 mol L−1 NaOH and was washed repeatedly until the washing water was rendered neutral. The starch was collected and freeze dried (Scientz-18N, Ningbo Scientz Biotechnology Corporation, China) for 48 h. Then, the dried starch was ground with a mortar and pestle, and passed through a 200-mesh sieve to obtain starch powder which was stored in a sealed container at room temperature.31
200 g of deionized water) for 1 min. The homogenate was poured through four layers of muslin cloth, and the yellow residue that settled was washed with deionized water twice. Next, the mixture was sieved through a 100-mesh sieve to remove the residue. Ethanol was added to the filtrate to make its concentration more than 30%, followed by centrifugation at 8000 rpm for 10 min. The colourless precipitate was deproteinated using 0.01 mol L−1 NaOH and was washed repeatedly until the washing water was rendered neutral. The starch was collected and freeze dried (Scientz-18N, Ningbo Scientz Biotechnology Corporation, China) for 48 h. Then, the dried starch was ground with a mortar and pestle, and passed through a 200-mesh sieve to obtain starch powder which was stored in a sealed container at room temperature.31
2.6 Microstructure of starch granules
The starch and water were used to prepare starch milk at a proportion of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 w/v. A drop of starch milk was placed onto the glass slide, and was gently shaken to evenly distribute the starch, and was covered with a glass lid. The starch samples were observed under a microscope (BX41, Olympus Corporation, Tokyo, Japan) at a 500× magnification.
100 w/v. A drop of starch milk was placed onto the glass slide, and was gently shaken to evenly distribute the starch, and was covered with a glass lid. The starch samples were observed under a microscope (BX41, Olympus Corporation, Tokyo, Japan) at a 500× magnification.
2.7 X-ray diffraction
The X-ray diffraction patterns of the starch from ginger were analysed using a D2 PHASER X-ray powder diffractometer (Phaser, Bruker AXS Corporation, Karlsruhe, German) with Cu Kα radiation at a voltage of 300 W and a power of 2.2 kW. The scattered radiation was detected in the angular range of 3–45° (2θ), with a scanning speed of 2° (2θ) min−1 and a step of 0.05° (2θ). Starch crystallinity was analysed using the Jade5 software.2.8 Data analysis
Each experiment was conducted in triplicate under the same conditions. The results were reported as the mean values ± standard deviation. Statistical analysis was performed using Origin 8.5. Analysis of variance (ANOVA) and bilateral Tukey's test (p < 0.05) were conducted to determine significant differences.3. Results and discussion
3.1 Colouring difference between blanched and non-blanched ginger slices
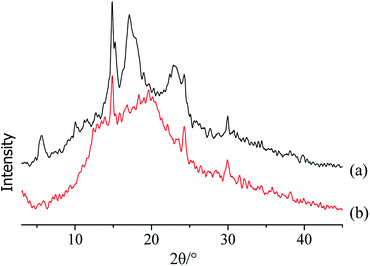
To directly reflect the colouring of ginger slices, the a* value and coefficient of variation were measured. The difference between the colouring effect in the blanched and non-blanched ginger slices was obvious. The results are shown in Fig. 1.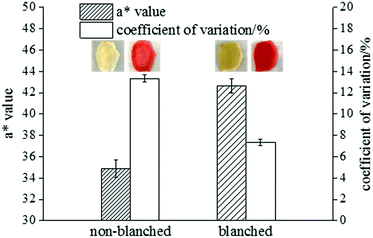 | ||
| Fig. 1 The colouring result for blanched and non-blanched ginger slices. The inserted images represent the appearances of blanched and non-blanched ginger slices before and after colouring. | ||
The a* value of the blanched ginger slices increased by 22.19%, and the coefficient of variation significantly decreased by 44.87%, compared to those for the non-blanched ginger slices; this indicated that heat treatment was beneficial for pigment adsorption, and it exhibited a better colouring effect. The inserted pictures in Fig. 1 presents the appearance of blanched and non-blanched old ginger slice. The middle part of the uncoloured and untreated ginger slice was difficult to colour, and it was observed as opaque white. The content of starch in old ginger was higher than that in fresh ginger. Therefore, the whitish colour in old ginger slices might be attributed to the aggregation of more starch. When the ginger slices were coloured with Allura red, the non-blanched ginger slices exhibited poor colour uniformity because the middle part of these slices were hard to colour. The blanched ginger slices exhibited a better uniformity of colouring than the untreated ginger slices. Gelatinized starch was beneficial for the absorption of pigment by the ginger slices, and it enhanced the uniformity of colouring. The reason for this effect might be that the structure of starch was broken and more hydroxyl groups were available. The highly polar hydroxyl groups tended to form intermolecular and intramolecular hydrogen bonds.32 Therefore, Allura red, a hydroxyl-containing compound, could form hydrogen bonds with starch.
The starch crystal characteristics of these two types of ginger slices were analysed via X-ray diffractometry (Fig. 2). X-ray diffraction has been used to reveal the presence and characteristics of the starch crystalline structure.33 The X-ray diffractogram of starch in non-blanched ginger slices showed five peaks (Fig. 2a), and the strongest diffraction peak was observed at approximately 15° at 2θ. A doublet between 17° and 18° and small peaks approximately 5.7° and 23° indicated that native ginger had a characteristic C-type starch.34,35 The crystallinity of the starch from non-blanched ginger was 19.05%; however, the crystallinity of the starch in blanched ginger slices was 0.51%. The latter no longer possessed the characteristic absorption of the C-type, which indicated that the high temperature broke the hydrogen bond and destroyed the crystalline region of starch in the ginger slices. This further proved that gelatinization of starch occurred in ginger slices after blanching.
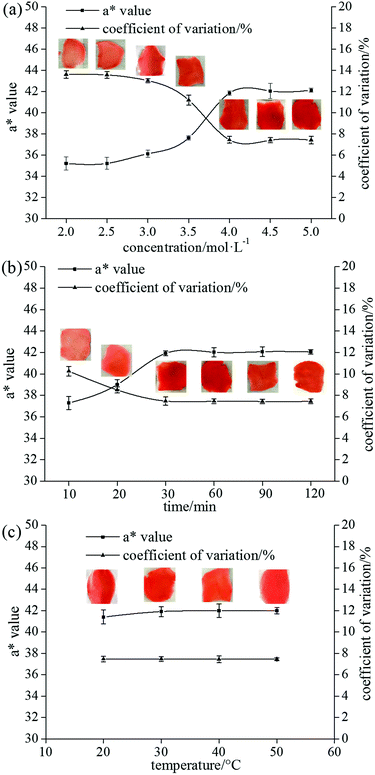
3.2 Colouring difference in ginger slices treated by CaCl2
The blanching method was conducive to the uniform colouring of ginger slices mainly because of the gelatinization of starch and breaking of the crystalline region according to the above analysis. Existing research showed that alkali metal salts with appropriate concentration could reduce the gelatinization temperature of sago starch.29 Considering food safety, CaCl2 was selected as a food-processing aid to pretreat the ginger slices. The influence of CaCl2 concentration, protreatment time and temperature on the uniformity of colouring were investigated with the a* value and coefficient of variation as indicators.Fig. 3a shows the dependence of the ginger-slice colouring on the concentration of CaCl2. The concentration of CaCl2 exhibited an important influence on the colouring of the ginger. When the concentration of CaCl2 was less than 3.5 mol L−1, the a* value and its coefficient of variation were similar to those of the untreated ginger slices, and the colour uniformity of the ginger slices was not improved. When the concentration of CaCl2 was 3.5 mol L−1, the a* value increased by 7.83% compared to non-blanched ginger slices, and the coefficient of variation decreased by 16.18%. The uniformity of colouring of the ginger slices improved slightly, but the effect was not obvious. The a* value increased by approximately 20%, and the coefficient of variation reduced by nearly 45% when the concentration was high (≥4 mol L−1), which was similar to that of the blanched ginger slices. Moreover, the colouring effect of the ginger slices improved significantly. Therefore, 4 mol L−1 was determined to be the best concentration. However, studies had shown that 3.5 mol L−1 CaCl2 could gelatinize starch at room temperature.29 It is possible that the concentration was slightly lower because the study was conducted in a pure starch system and the contact area was wider.
The influence of pretreatment time on the colouring of the ginger slices is shown in Fig. 3b. Pretreatment time slightly affected the colour. The a* value increased, and the coefficient of variation decreased with the extension of the processing time (10–30 min). The colouring of the ginger slices following 30 min of treatment was similar to that of the blanched ginger slices, and the colouring tended to be stable with longer processing times. Therefore, 30 min was determined to be the best processing time.
The influence of pretreatment temperature on the colouring of the ginger slices is shown in Fig. 3c. Pretreatment temperature had little effect on the uniformity of ginger colouring (Fig. 3c). The a* value and its coefficient of variation showed a steady trend in the range of 20–50 °C, indicating that the effect of temperature was not obvious and that the treatment could be performed at room temperature.
The experimental results showed that the pretreatment time and temperature had little effect on the colouring of ginger slices. When the pretreatment time was less than 30 min, the colouring improved with increasing time. However, the effect of time was not obvious when the time was more than 30 min. This might be because CaCl2 could not be in full contact with starch in a short period of time. A certain concentration of salt solution could change the starch gelatinization temperature.28,36 CaCl2 at 4 mol L−1 had already lowered the starch gelatinization temperature to room temperature; therefore, changing the temperature had no effect on the result.
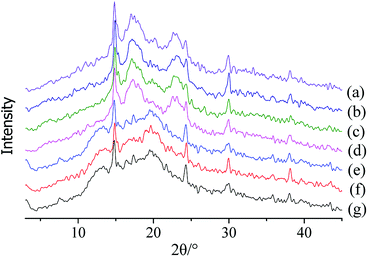
3.3 Effect of CaCl2 treatment on starch properties of ginger slices
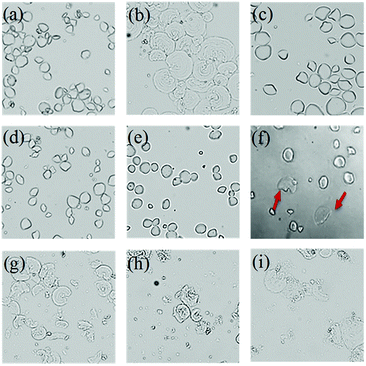
Blanched sushi ginger slices were more likely to exhibit even colouring because the high temperature of 95 °C changed the properties of the starch in the ginger slices. The effect of different concentrations of CaCl2 on the colouring of sushi ginger was different. A high concentration of CaCl2 (≥4 mol L−1) produced a colouring effect in the ginger close to that obtained with the blanching treatment; therefore, this concentration might also transform starch properties. The crystallization characteristics, starch particle shape and other properties were compared by separating the starch from ginger slices that were treated with different concentrations of CaCl2.The starch structure is different for different sources.40 The starch granule shape for non-blanched ginger slices appeared to be oval or irregular polygonal (Fig. 5a). The starch granule shape for blanched ginger slices changed and exhibited an increase in size (Fig. 5b). In this process, the granules swelled and broke, and the structure was destroyed. Different concentrations of CaCl2 had different effects on starch morphology. When the concentration of CaCl2 was less than 3.5 mol L−1, there was no obvious change in the morphology of the starch granules, which remained oval or polygonal (Fig. 5c–e). When CaCl2 was 3.5 mol L−1, there was no effect on most of the starch granules (Fig. 5f). However, it could be seen that edges became blurred for a small amount of starch granules, and the shape of the granules changed. When the concentration of CaCl2 was more than 4 mol L−1 (Fig. 5g–i), almost all starch granules were destroyed, which was consistent with the result obtained for the starch from blanched ginger.
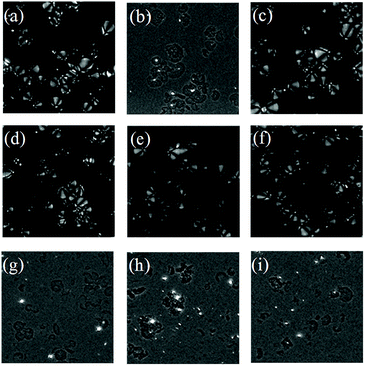
The starch granules were spherical crystals that were birefringent under polarized light microscopy. There were different structural regions within the starch, divided into crystalline regions and amorphous regions. The arrangement of starch chains in the crystalline regions was regular and that in the amorphous regions was disorganized. The starch chains were anisotropic, rendering the starch grains polarized.41 Once the crystalline region of the starch granules was destroyed, the anisotropy vanished, and the polarization disappeared; therefore, the starch structure and crystallographic information can be identified through change in the polarization of the starch granules. The polarization of starch was observed using a polarized microscope (Fig. 6).
The position and degree of visibility of the polarizing cross were related to the type of native starch granules. The starch granules from non-blanched ginger slices exhibited an obvious polarized cross, and the umbilical point was located at one end of the granules (Fig. 6a). After the blanching treatment of ginger slices, the polarized cross of the starch particles disappeared, and only broken starch granules could be seen (Fig. 6b). Different concentrations of CaCl2 induces different effects on the polarization of starch. When the concentration of CaCl2 was lower than 3.5 mol L−1 (Fig. 6c–e), there was still a clear polarization. Treatment with 3.5 mol L−1 CaCl2 influenced a small amount of starch granules, according to a previous analysis; however, it did not affect the polarization (Fig. 6f). Starch granules without crystalline regions lost their polarized cross under the polarized microscope.42 3.5 mol L−1 CaCl2 did less damage to the crystalline region and the starch maintained a relatively high degree of crystallinity; therefore, it still retained the polarized cross. When the concentration of CaCl2 was higher than 4 mol L−1, the polarization cross completely disappeared (Fig. 6g–i), indicating that the crystalline region of the starch was destroyed at this time, which was consistent with the result for the starch from blanched ginger.
Starch granules from blanched ginger slices were broken under light microscope. Research had shown that microwave-treated starch granules that were heated to a final temperature of 95 °C appeared completely ruptured.41 Furthermore, through microscopic observation, Jangchud found that starch granules from blanched flours were broken and concluded that the starch was gelatinized.17 Therefore, the gelatinization of the starch from blanched ginger slices might be due to the blanching treatment. Additionally, the polarized cross of the starch particles disappeared under polarized microscope. This could also explain that the blanching treatment of ginger slices destroyed the starch crystal region, broke the hydrogen bonds and caused the starch to gelatinize. A high concentration of CaCl2 (more than 4 mol L−1) could also break the starch granules and induce the polarized cross to disappear, which was consistent with the result for blanched ginger starch. This result showed that at room temperature, a high concentration of CaCl2 (more than 4 mol L−1) could lead to hydrogen-bond breaking and the gelatinization of ginger starch.
4. Conclusions
The uneven colour of ginger slices without blanching pretreatment is related to the characteristics of starch. The crystalline region of the starch is broken in blanched ginger slices. The properties of starch were evaluated in ginger slices treated with CaCl2. The experiments showed that different concentrations of CaCl2 had different effects on the starch crystallization structure, morphology, and polarization cross in ginger slices. Pretreatment with 4 mol L−1 CaCl2 almost completely destroyed the crystallization structure of the starch in ginger slices, broke the starch granules and led to the disappearance of the polarized light cross; this effect was close to that observed with blanching treatment. A high concentration of CaCl2 might be used in the pretreatment of ginger slices instead of the traditional blanching treatment. It could also cause the starch to gelatinize at room temperature, and the sushi red ginger slices exhibited even colouring and good sensory qualities.Conflicts of interest
The authors have declared no conflict of interest.Acknowledgements
This research was financially supported by projects of the National Key R&D Program (2016YFD0400801), National First-class Discipline of Food Science and Technology (JUFSTR20180204), and the program of the “Collaborative Innovation Center of Food Safety and Quality Control in Jiangsu Province”.References
- A. Ghasemzadeh, H. Z. Jaafar and A. Rahmat, Molecules, 2010, 15, 4324–4333 CrossRef CAS PubMed.
- B. Tepe, M. Sokmen, H. A. Akpulat and A. Sokmen, Food Chem., 2006, 95, 200–204 CrossRef CAS.
- E. J. Park and J. M. Pezzuto, Cancer Metastasis Rev., 2002, 21, 231–255 CrossRef CAS PubMed.
- Y. Shukla and M. Singh, Food Chem. Toxicol., 2007, 45, 683–690 CrossRef CAS PubMed.
- B. Fallico, E. Chiappara, E. Arena and G. Ballistreri, Food Addit. Contam., 2011, 28, 1501–1515 CrossRef CAS PubMed.
- S. Chanlon, L. Joly-Pottuz, M. Chatelut, O. Vittori and J. L. Cretier, J. Food Compos. Anal., 2005, 18, 503–515 CrossRef CAS.
- H. J. Kim, J. L. Mi, H. J. Park, H. J. Kim, S. K. Cho and H. J. Min, Food Sci. Biotechnol., 2018, 1–6 Search PubMed.
- S. M. Castro, J. A. Saraiva, J. A. Lopes-da-Silva, I. Delgadillo, A. V. Loey, C. Smout and M. Hendrickx, Food Chem., 2008, 107, 1436–1449 CrossRef CAS.
- L. T. Nguyen, A. Tay, V. M. Balasubramaniam, J. D. Legan, E. J. Turek and R. Gupta, LWT--Food Sci. Technol., 2010, 43, 525–534 CrossRef CAS.
- V. Kumar, R. Kushwaha, A. Goyal, B. Tanwar and J. Kaur, Food Chem., 2018, 245, 168–177 CrossRef CAS PubMed.
- S. R. Drake and B. G. Swanson, J. Food Sci., 2010, 51, 1081–1082 CrossRef.
- D. K. Kweon, J. K. Choi, E. K. Kim and S. T. Lim, Carbohydr. Polym., 2001, 46, 171–177 CrossRef CAS.
- R. Cheng, S. Ou, M. Li, Y. Li and X. Bo, J. Hazard. Mater., 2009, 172, 1665–1670 CrossRef CAS PubMed.
- R. Cheng, B. Xiang, Y. Li and M. Zhang, J. Hazard. Mater., 2011, 188, 254–260 CrossRef CAS PubMed.
- S. Xu, J. Wang, R. Wu, J. Wang and H. Li, Chem. Eng. J., 2006, 117, 161–167 CrossRef CAS.
- V. Janaki, K. Vijayaraghavan, B. T. Oh, K. J. Lee, K. Muthuchelian, A. K. Ramasamy and S. Kamala-Kannan, Carbohydr. Polym., 2012, 90, 1437–1444 CrossRef CAS PubMed.
- K. Jangchud, Y. Phimolsiripol and V. Haruthaithanasan, Starch/Staerke, 2003, 55, 258–264 CrossRef CAS.
- R. S. Policegoudra and S. M. Aradhya, Food Hydrocolloids, 2008, 22, 513–519 CrossRef CAS.
- D. Lund, CRC Crit. Rev. Food Sci. Nutr., 1984, 20, 249–273 CrossRef CAS PubMed.
- U. Uthumporn, I. S. M. Zaidul and A. A. Karim, Food Bioprod. Process., 2010, 88, 47–54 CrossRef CAS.
- S. Puncha-arnon, W. Pathipanawat, C. Puttanlek, V. Rungsardthong and D. Uttapap, Food Res. Int., 2008, 41, 552–561 CrossRef CAS.
- C. S. Cheow and S. Y. Yu, J. Food Process. Preserv., 1997, 21, 161–177 CrossRef CAS.
- M. B. A. Ghani, Y. B. Che Man, A. B. Ali and D. B. Mat Hashim, J. Sci. Food Agric., 1999, 79, 2001–2009 CrossRef CAS.
- B. A. Rumpold and D. Knorr, Starch/Staerke, 2005, 57, 370–377 CrossRef CAS.
- A. G. Maaurf, Y. B. Che Man, B. A. Asbi, A. H. Junainah and J. K. Kemmedy, Carbohydr. Polym., 2001, 45, 335–345 CrossRef CAS.
- Q. Li, L. Zhang, Y. Ye and Q. Y. Gao, Food Hydrocolloids, 2015, 51, 468–475 CrossRef CAS.
- H. Zhou, C. Wang, L. Shi, T. Chang, H. Yang and M. Cui, Food Chem., 2014, 156, 137–143 CrossRef CAS PubMed.
- W. Wang, H. Zhou, H. Yang, S. Zhao, Y. Liu and R. Liu, Food Chem., 2017, 214, 319–327 CrossRef CAS PubMed.
- F. B. Ahmad and P. A. Williams, J. Agric. Food Chem., 1999, 47, 3359–3366 CrossRef CAS PubMed.
- H. C. L. Tang and R. F. Mcfeeters, J. Food Sci., 2010, 48, 66–70 CrossRef.
- L. Zhang, W. Xie, X. Zhao, Y. Liu and W. Gao, Thermochim. Acta, 2009, 495, 57–62 CrossRef CAS.
- L. T. Sin, W. A. W. A. Rahman, A. R. Rahmat and A. A. Samad, Polymer, 2010, 51, 1206–1211 CrossRef CAS.
- N. Singh, J. Singh, L. Kaur, N. Singh Sodhi and B. Singh Gill, Food Chem., 2003, 81, 219–231 CrossRef CAS.
- L. A. Bello-Pérez, A. Aparicio-Saguilán, G. MÉNdez-Montealvo, J. Solorza-Feria and E. Flores-Huicochea, Plant Foods Hum. Nutr., 2005, 60, 7–12 CrossRef.
- M. Braga, S. Moreschi and M. Meireles, Carbohydr. Polym., 2006, 63, 340–346 CrossRef CAS.
- J. O. Brumovsky, L. A. Brumovsky and D. B. Thompson, Int. J. Food Prop., 2006, 9, 889–896 CrossRef CAS.
- S. Narpinder, S. Jaspreet, K. Lovedeep, S. S. Navdeep and S. G. Balmeet, Food Chem., 2003, 81, 219–231 CrossRef.
- Q. Sun, Y. Xu and L. Xiong, Int. J. Biol. Macromol., 2014, 68, 86–91 CrossRef CAS PubMed.
- J. Singh, L. Kaur and O. J. McCarthy, Food Hydrocolloids, 2007, 21, 1–22 CrossRef CAS.
- K. Svegmark and A. M. Hermansson, Food Struct., 1993, 12, 181–193 CAS.
- T. Palav and K. Seetharaman, Carbohydr. Polym., 2006, 65, 364–370 CrossRef CAS.
- P. L. Liu, B. S. Zhang, Q. Shen, X. S. Hu and W. H. Li, Int. J. Food Eng., 2010, 6, 61–64 Search PubMed.
| This journal is © The Royal Society of Chemistry 2019 |