Open Access Article
Open Access ArticleResearch progress in the development of organic small molecule fluorescent probes for detecting H2O2
Yuanyuan Liuabcd,
Chunpeng Jiaoabcd,
Wenjuan Lubcd,
Pingping Zhangbcd and
Yanfeng Wang *bcd
*bcd
aSchool of Medicine and Life Sciences, University of Jinan-Shandong Academy of Medical Sciences, Jinan 250200, Shandong, China
bInstitute of MateriaMedica, Shandong Academy of Medical Sciences, Jinan 250062, Shandong, China. E-mail: wyfshiwoya@126.com
cKey Laboratory for Biotech-Drugs Ministry of Health, Jinan 250062, Shandong, China
dKey Laboratory for Rare & Uncommon Diseases of Shandong Province, Jinan 250062, Shandong, China
First published on 7th June 2019
Abstract
Hydrogen peroxide (H2O2), as an important signaling molecule during biological metabolism, is a key member of the reactive oxygen species (ROS) family. The excess of H2O2 will lead to oxidative stress, which is a crucial factor in the production of various ROS-related diseases. In order to study the diverse biological roles of H2O2 in cells and animal tissues, many methods have been developed to detect H2O2. Recently, fluorescence imaging has attracted more and more attention because of its high sensitivity, simple operation, experimental feasibility, and real-time online monitoring. Based on the response group, this study will review the research progress on hydrogen peroxide and summarizes the mechanisms, actualities and prospects of fluorescent probes for H2O2.
1. Introduction
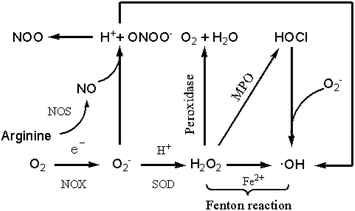
In the oxygen-containing metabolism process, the living organism produces a variety of free radicals and derivatives with redox activity,1,2 and reactive oxygen species (ROS) are one of them. Reactive oxygen species includes superoxide (O2−), hydroxyl radical (·OH), hydrogen peroxide (H2O2), hypochlorous acid (HOCl) and so on, and their initial active substance is O2−. In living organisms, O2− is the basis for the formation of other reactive oxygen species.3,4 As is shown in Fig. 1, oxygen is catalyzed by NADPH (nicotinamide adenine dinucleotide phosphate) oxidase (NOX) to form O2− via single electron reduction.5,6 Subsequently, the two O2− are capable of forming H2O2 under the action of superoxide dismutase (SOD).7–9 H2O2 is converted to ·OH in the presence of a single electron metal ion such as Fe2+ undergoing Fenton reaction, and interacts with Cl− to form HOCl in the presence of myeloperoxidase (MPO).10–12 As shown in the figure, when reacting with O2−, HOCl can convert into ·OH further.5,13 Arginine reacts with various nitric oxide synthase (NOS) enzymes to produce nitric oxide (NO), which reacts with O2− to produce peroxynitrite (ONOO−).14–17 Peroxynitrite can decompose to form hydroxyl radicals by interaction with hydrogen ions (H+).H2O2 in cells is mainly derived from mitochondria, endoplasmic reticulum (ER) and various oxidases.18–21 Among the above sources, protein-type oxidase plays a critical role in generating H2O2 for signal conversion purposes. H2O2, one of the key members of the ROS family, has a certain regulatory effect on many physiological processes of organisms.22,23 First, H2O2 is relevant to cell migration, proliferation and differentiation. Second, H2O2 has an immense influence on angiogenesis and vascular diameter regulation. Third, H2O2 can reversibly oxidize the proteins involved in the reaction, thereby activating or deactivating the pathway. In addition, it can effectively regulate the body's immune defence mechanism. For example, NADPH in neutrophils produces μM-grade H2O2 to kill pathogens.24 Finally, H2O2 is closely connected with oxidative stress-associated cancers. In the case of oxidative stress, the concentration of H2O2 will increase to 10–15 μM, which will further aggravate the condition of vasodilation cancer.25
As a vital signal molecule in the process of biological metabolism, hydrogen peroxide is attracting more and more attention for its ideal signal characteristics. First, it has adequate chemical reactivity to react with potential signal targets, and has sufficient stability to diffuse within the cell for a certain distance to react with the target selectively.26 Moreover, it can be generated rapidly, diffused and eliminated quickly, which makes it suitable as a signal generating substance. Normally, the average concentration of H2O2 in healthy mammalian cells ranges from 1 to 700 nM, and this low concentration of homeostasis is beneficial for normal cell growth. High concentrations of H2O2 can contribute to oxidative stress in the biological system and trigger apoptosis.27 Excessive accumulation of hydrogen peroxide in the human body can cause a series of inflammations and diseases, such as cancers,28 Alzheimer's disease29 and cardiovascular diseases30 and also affects early embryological development in mammals. H2O2 is mainly used as a bleaching agent and disinfectant in life and plays an irreplaceable and indispensable role in the living system.
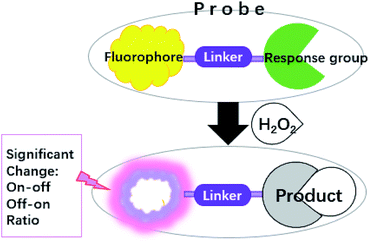
At present, studying the dynamic distribution of H2O2 in living cells and monitoring the concentration change of H2O2 in the human body are going to become a pivotal focus for biology. As an important tool, fluorescent probes have become a research hotspot in life sciences because it can greatly reduce the detection limit and has strong sensitivity and selectivity. As Scheme 1 shows, the binding of H2O2 to response group can lead to either enhancement (turn-on), quenching (turn-off) or ratio change of the fluorescence intensity, coupled with a red or blue shift of the emission band or absorption band. Here selective fluorescent probes are used for monitoring H2O2. In order to gain a better analysis on how H2O2 is generated in living systems as well as to illustrate its biological roles.
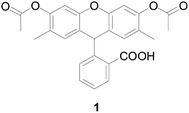
In 1965, Richard Brandt and Albert Keston designed a stable reagent probe 1 (Fig. 2) to detect H2O2, inspired by Schales.31,32 In the presence of H2O2, nonfluorescent compound 1 can rapidly oxidized to a fluorescent compound. Shortly after publication of this paper, Stephen Hempel and Duane Wessels used probe 1 as a detector of intracellular oxidants.33 Their work demonstrated that probe 1 was a better fluorescent probe for detecting intracellular oxidants such as hydroxyl radical and nitric oxide because it is more reactive toward specific oxidizing species.
With the development of technology, fluorescence analysis is becoming maturer and maturer. Thus, the number of fluorescent probes for detecting H2O2 is increasing gradually. The application of fluorescent probe to expound biological events relate to H2O2 has significantly promote the progress of H2O2-probes in the field of medicine and biology. In this review, five types of H2O2-specific fluorescent probes in probes treasury will mainly summarized and introduced based on their response group, including arylboronate-based probes, sulfonate-based probes, benzoyl-based probes, o-benzenediol based probes and enamine-based probes. These probes can not only largely improve selectivity and sensitivity to H2O2, but are also widely used as agents for bioimaging of H2O2. In addition, this review will discuss the molecular mechanism, current situation and prospects of H2O2 fluorescent probes.
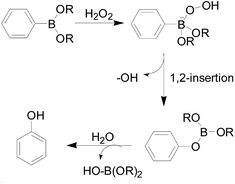
2. Arylboronate-based fluorescent H2O2 probes
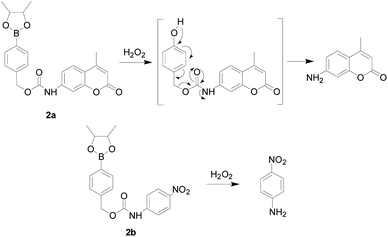
Conventional H2O2 fluorescent probes often comprise a H2O2-sensing moiety, a fluorophore and a linker. The most commonly used response moiety for H2O2 is arylboronate, which has developed in many fluorophores to monitor H2O2 in living cells and tissues. As early as 1957, Henry and Albert34 studied the response mechanism of hydrogen peroxide to arylboronic acids in aqueous solutions (Fig. 3). The nucleophilic H2O2 attacks the electrophilic aryl borate to form an unstable intermediate, which was hydrolyzed to produce phenol and boric acid quantitatively.In 2003, Lo and co-workers35 first developed probe 2a and 2b (Fig. 4), whose skeleton consisted of the same response site and the different amino group as suitable reporter groups. Both of these stable probes could be activated upon reaction with H2O2 and released the corresponding sensing group. A good linear reaction between the released p-nitroaniline or coumarin group and the hydrogen peroxide in the solution. The difference was that probe 2a provided a more sensitive detection range (0.1 to 5 mM) than probe 2b. Carried different special properties reporter groups could not affect the detecting mechanism was the major advantages in the design of the probes in this article, which could be used to meet the demands. However, the response time of both probes was much longer. Therefore, these probes have not been used in cell imaging experiments.
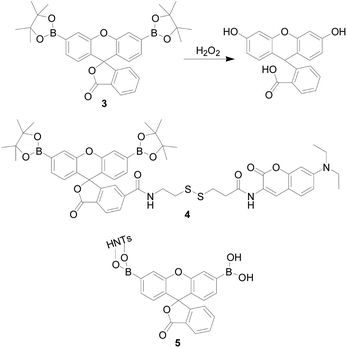
In 2004, Chang's group36 reported a H2O2 probe 3 (Fig. 5), they first introduced a pinacol borate into a fluorescent probe. It was a new water-soluble, turn-on optical probe, containing two boronic ester groups at the 3′ and 6′ positions of a xanthenone scaffold as a response moiety. It is worth mentioning that probe 3 had a high selectivity and sensitivity, exhibiting a >500-fold higher response for H2O2 over other similar ROS such as ·OH or OCl−. The authors believe this is because the reaction is a deprotection process rather than an oxidation reaction. Furthermore, the probe was applied to fluorescence imaging of exogenous H2O2 in human embryonic kidney cells (HEK293T cells) and good results were obtained. Subsequently, this research group37–40 designed a series of probes based on this mechanism. The commonality of these probes were fluorescein used as a fluorophore, an aryl boronate used as a response site, and structural modification performed there on. In the same year, Zhao and colleagues41 designed a dual-response fluorescent-opening probe 4 (Fig. 5), which connected the PF1-derivative to the coumarin unit via a disulfide bond, and simultaneously responds to H2O2 and bio-thiols. The team evaluated the selectivity and sensitivity of the probe and found that probe 4 showed a positive response to H2O2 and thiol-containing DL-dithiothreitol (DTT), glutathione (GSH), homocysteine (Hcy), and cysteine (Cys) only. Furthermore, H2O2 exhibited a different response than DTT, GSH, Hcy and Cys. When the probe was treated with H2O2, the di-borate group converted to a hydroxyl to form a highly fluorescent fluorescein derivative, and the original PET process converted to an intramolecular FRET mechanism to cause the fluorescence to turn on. Probe 4 could be activated by endogenous thiol and exogenous H2O2, and thus had the applicability to both inside and outside the cell. However, the response time and detection limit were not satisfactory. In 2017, Hailei Zhang's team42 modified the nanoparticle HNTs with fluorescein derivative PA to prepare probe 5 (PA-HNTs), which has a highly specific fluorescent “turn-on” response to H2O2. Nanocomposites exhibit regular morphology and good dispersibility in aqueous solution (Fig. 5). Experiments showed that 5 can monitor H2O2 produced by A549 cells under physiological conditions, which provided a good tool for tumor diagnosis. In order to track H2O2 at a low level to prevent and diagnose related diseases, it was necessary to develop a H2O2 sensitive fluorescent probe.
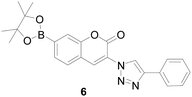
The group headed by Binghe Wang43 reported a simple fluorescent probe that directly binds the borate ester group to coumarin. This probe showed 100-fold increase in fluorescent intensity upon reaction with H2O2 and showed selectivity over other ROS, but the probe had a short emission wavelength at 454 nm. Subsequently, the research group44 improved this conjugate system by introducing a triazole into the molecule to obtain a new probe 6 (Fig. 6). Its emission wavelength was red shifted to 474 nm, and its selectivity to H2O2 was 2–4 times higher than that of other ROS, which was more suitable for cell and in vivo imaging. But the disadvantage was that the fluorescent intensity only increased 5-fold. In the above reports, borate ester group could be used as the response site of hydrogen peroxide, and both have good selectivity. However, the sensitivity were not high, and the absolute fluorescence intensity was low, which affected the test in cells imaging. In recent years, more and more fluorophore was used as the framework of the probe.
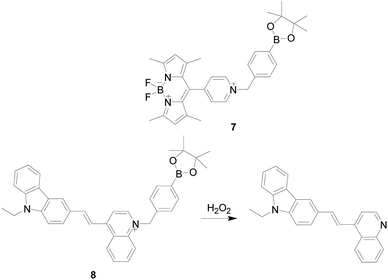
In 2014, Shijun Shao's group45 reported a case of BODIPY derivatives 7 (Fig. 7), which were synthesized by introducing 4-(bromomethyl)benzeneboronic acid pinacol eater moiety, and showed good solubility in water and “turn-on” fluorescent probe for the discrimination of H2O2. Probe 7 expresses excellent response toward H2O2 among the various ROS or RNS and biologically relevant species. Before binding with H2O2 the probe hardly had fluorescence due to the photo-induced electron transfer (PET) progress. Incubation of probe with H2O2 would release fluorophore, and the fluorescence of BODIPY was restored. Furthermore, the probe was successfully applied for both in vitro and in vivo imaging of H2O2 using cells and angelfish. In 2016, this research group46 designed and synthesized another H2O2 fluorescence probe 8 (Fig. 7), which also based on borate ester hydrolysis mechanism. Similar to the luminescence mechanism of probe 7, before binding with H2O2 probe 8 showed no fluorescence due to the PET effect, and the PET effect disappeared after the reaction with H2O2, the fluorescence of carbazole group was recovered. Moreover, probe 8 has excellent selectivity and only H2O2 shows significant fluorescence enhancement. Other ROS or RNS, such as HOCl, O2−, ·OH, NO, ONOO−, and GSH, ascorbic acid, glucose do not initiate or cause only minor changes. The probe has been successfully applied for monitoring and imaging of endogenous H2O2 in HeLa cells under physiological conditions. In addition, the structural modification of the carbazole pyridinium core provides a unique fluorescent material that opens the way for the construction of new fluorescent materials with unique optical properties.
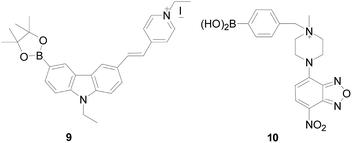
Based on the PET mechanism, Weiying Lin's group47 synthesized a fluorescent “turn-off” probe 9 (Fig. 8), which introduced carbazole-based fluorophore as a fluorescent dye and have PET process after reaction with H2O2. But unlike the above PET probe, there was no PET effect in the molecule before combined with H2O2. Probe 9 displayed strong green fluorescence before reaction with H2O2 but showed low fluorescence after reaction with H2O2. The probe has a large Stokes shift (157 nm in water) in various solutions, which can maintain the accuracy of bioimaging, minimize the self-quenching effect, and achieve highly sensitive detection of H2O2 in aqueous solution. The next year, Xiaowei Xu's group48 designed probes 10 (Fig. 8), in that they introduced 4-chloro-7-nitro-1,2,3-benzoxadiazole as the fluorophore core, and aryl boronate as a specific H2O2-reactive group. Upon exposure to H2O2 probe 10 exhibited distinct “turn-off” behaviors, and could be used in both neutral and alkaline aqueous conditions.
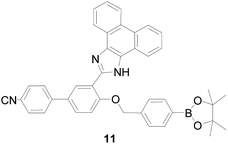
Although many probes for detection of H2O2 have been explored, rapid response probes are still expected. Based on intramolecular charge transfer (ICT), Wang and co-workers49 synthesized fluorescent probe 11 (Fig. 9), which applied phenanthroimidazol, benzonitrile, and phenyl boronate as the three-component. Through introduction of benzonitrile as an electron-withdrawing group formation of a push–pull molecular mechanism modulation ICT process of probe 11, it can be detected H2O2 with good sensitivity not only in vitro but also in vivo. Moreover, compared to H2O2, probe 11 has high selectivity and negligible interference with other substances, including peroxyacetic acid (CH3COOOH), ·OH, O2− and HOCl. The probe 11 can image H2O2 in the peritoneal cavity of mice. However, the emission is at blue, so it was not perfect to used in vivo.
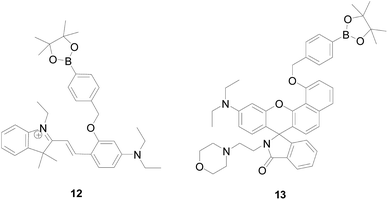
There is increasing evidence indicating that lysosomes are important site for H2O2 production under both physiological and pathological conditions. Therefore, to design and synthesis of targeted H2O2 probe molecules is more important for the application of probes in organisms. In 2016 and 2017, Yibing Zhao's group50,51 designed two lysosomal-targeted H2O2 probes 12 and 13 (Fig. 10). Probe 12 is a pH-switchable fluorescent H2O2-probe that responds sequentially to intracellular H2O2 and lysosomal pH, enabling the detection of the overall level of intracellular H2O2 by detecting the fluorescent signal intensity of lysosomes. This was because that probe 12 possesses a spirobenzopyran fluorophore emitting strongly at lower pH, showing high specificity to H2O2 and used for live cell imaging. The sensing strategy enables the visualization of the intracellular H2O2 through the lysosome windows more specifically, providing a new strategy for the spatially confined imaging in certain cell regions. Whereas, probe 13 is a novel acid activating probe with the same benzorhodol fluorophore as the probe described above. Probe 13 exhibits higher fluorescence intensity and longer emission wavelengths to H2O2, showing that 13 is more suitable for the monitoring of H2O2 under acidic conditions compared to Lyso-B-L2 and Lyso-B-L3. The fluorescence response of probe 13 to H2O2 and H+ is highly selective, while the presence of other ROS, small bio-thiols, and metal ions does not cause a change in fluorescence. It is worth noticing that probe 13 uses morpholine as a localization group to provide pKa suitable for lysosomal environment and efficient lysosomal localization ability. The probe is capable of fluorescently sensing endogenous lysosomal H2O2 in living cells, without signal interference from cytoplasm and other intracellular organelles.
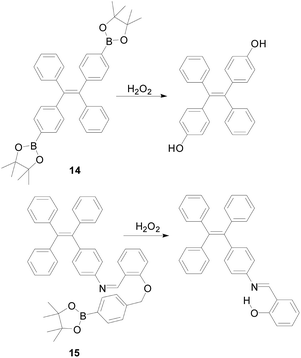
The probe 14 (Fig. 11) was designed by Bo Tang's group,52 which is a powerful tool for monitoring H2O2. The probe based on the aggregation induced luminescence (AIE) mechanism. When H2O2 was present in the system, the benzoborate groups in the probe were transformed into phenol groups, which increased the tetraphenylethylene aggregation. Upon reaction with H2O2, the fluorescence intensity of the probe 14 increased at 500 nm and reached a maximum value within 10 minutes. Probe 14 displayed a high selectivity toward H2O2 and a linear relationship with the concentration of (10–200 μM), and the detection limit was as low as 0.52 μM. Based on the above advantages, probe 14 has successfully applied to the imaging of RAW264.7 cells in mice. One of the most significant advantages of AIE-active probes is that they can applied to different conditions. For example, they can workable in solutions, aggregated and even solid states. Based on this, Benzhong Tang's research group53 synthesized probe 15 (Fig. 11), which introduces phenylboronic pinacol ester on the AIE fluorogens (AIEgens). The same as probe 14, when combined with H2O2 the probe's benzoborate groups were transformed into phenol. At the same time, the probe forms a product with bright yellow fluorescence and the hydrogen bond in the product interact to detect of hydrogen peroxide with high sensitivity and high selectivity. The probe can also detect the concentration of glucose. The detection limit of probe 15 is as low as 100 nm, which is more sensitive than probe 14.
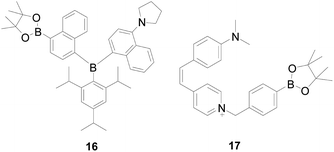
Yang and colleagues54 loaded a triarylboron-based AIE fluorophore on poly-urethane nanogel achieved probe 16 (Fig. 12). The boronate group can functionalize the triarylboron compound and give it the property of a fluorescence probe, which can provide continuous responses to H2O2. In addition, the introduced boronate group can also endow the triarylboron compound with AIE properties based on the dark TICT mechanism. By incorporating 16 into a polyurethane nanogel, they successfully utilized its photophysical properties to achieve a ratio readout for H2O2, and cells imaging can experience a color change from yellow green to blue green, which makes it suitable for potential applications in biology and medicine.
Hydrolysis of benzoborate ester groups was also used, Weiying Lin's research group55 designed probe 17 (Fig. 12). In this probe, the double bond and benzene were used as a connector to connect the electron donor (N,N-dimethylamino) and electron acceptor (pyridinium cations), which can make the molecular free rotation in the excited state. Because the ability of pyridinium cations has a significant change in the probe 17 before and after reacting with H2O2, the excited state of the reaction product could not form the twisted internal charge transfer (TICT) state, the product have a strong fluorescence emission. The probe's emission intensity and the concentration of H2O2 in range of 0–30 μM shows a linear relationship. The probe 17 showed a highly sensitivity and the detection limit was 2.1 × 10−6 M which is sensitive enough to determine the H2O2 level of the lesion. Moreover, probe 17 is more selective for H2O2 than other reference species such as ROS/RNS. In addition, pyridinium cation, as one of the mitochondrial targeting groups, possesses superior membrane permeability and can rapidly enter the mitochondria in cells in a short time. The discovery of this probe laid the foundation for future research on Alzheimer's disease. Based on similar mechanism, Xueping Feng's group56 synthesized a probe HPQB, which also can detect H2O2 and glucose in cells.
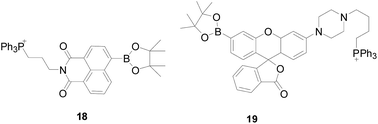
In 2018, Zhou Bo's group57 reported a probe 18 (Fig. 13), which introduced another mitochondrial targeting group alkylated triphenylphosphine in it. Probe 18 is specific for H2O2, with only H2O2 inducing significant fluorescence enhancement, while other ROS and RNS triggering no or limited changes. In addition, probe 18 demonstrated excellent water solubility and applied under physiological pH condition along with low cytotoxicity, and tracked of mitochondrial H2O2 in living cells. Therefore, the probe was expected to use as a tool to explore the biological effects of mitochondrial H2O2, and to facilitate the study of mitochondrial-targeted anticancer drugs. As early as 2008, the probe 19 (Fig. 13) was designed and synthesized by Chang's research group,39 which introduced triphenylphosphine as a mitochondrial targeting group. Probe 19 could selectivity detect H2O2 in the mitochondria of living cells over other ROS like ·OH, O2−, NO. Moreover, the level of H2O2 in mitochondria of various mammals and the increase of H2O2 caused by oxidative stress in Parkinson's disease model can be observed through imaging changes.
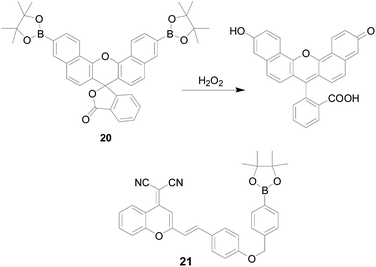
In order to reduce background interference and cell damage, enhance optical transparency of tissue and the ability to interrogate thicker specimens, it is increasingly important to design and synthesize probes that can emit spectra in the visible far-red to near infrared-region. Chang's group40 synthesized probe 20 (Fig. 14), which is a new borate-based red-emitting fluorescent indicator for H2O2. The probe was capable of responding to changes in exogenous H2O2 levels within RAW264.7 cells. However, as the absolute fluorescent brightness of naphthofluorescein was weaker than fluorescein and other fluorophore used previously, attempts to use this probe to study oxidative stress in cells have failed. Haitao Li's team58 designed a new near-infrared probe 21 (Fig. 14) with dicyanomethylene-4H-pyran moiety as fluorophore and borate ester as the recognition unit. The probe was “turn-off” response based on the ICT mechanism. After probe 21 was cultivated with H2O2, the recovery of the ICT process resulted in an obvious increase in fluorescence emission at 688 nm, accompanied by a distinct color change from yellow to purple, revealing the feasibility of “naked-eye” monitoring H2O2. Encouraged by the properties of near-infrared emission, probe 21 was successfully applied in the imaging of exogenous and endogenous H2O2 in HepG2 cells.
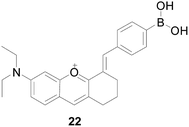
Liyi Zhou's group59 constructed a two-photon near-infrared fluorescent probe 22 (Fig. 15), which introduced the oxonium ion as the mitochondrial targeting unit and phenylboric acid as the H2O2 reaction moiety. Probe 22 could successfully applied for H2O2 imaging of liver tissue up to 170 μm depth in red fluorescence channels. After reacting with H2O2, the fluorescence intensity of probe 22 increased significantly by more than 105 times at the emission wavelength of 665 nm, which is much larger than other ROS, which meets the selectivity requirements of practical applications. Furthermore, the probe has good dyeing ability and high penetrating ability to H2O2 image tissue, and can be applied to liver tissue H2O2 images with a depth of 50–170 μm in a red fluorescent channel.
More and more borate fluorescent probes were used to detect hydrogen peroxide in cells. However, most of these probes were single-photon imaging technology. In addition to the description above, there were many probes designed using this technique.60–70 The photo-damage and photo-bleaching properties of single photon imaging technology have prevented long-term imaging during bioimaging process, and it has strong background interference and low UV transmittance, which limits their applications in deep tissue imaging. By contrast, two-photon (TP) fluorescent probes can greatly overcome these defects. By using near-infrared (NIR) lights as the excitation source, the two-photon fluorescent probes can reduce the photo-damage and photo-bleaching caused by single-photon fluorescent probes, increase tissue penetration, and promote three-dimensional imaging of living tissue. Hence, the two-photon probes are more suitable for biological detection and imaging, providing sharper tool for life science research.
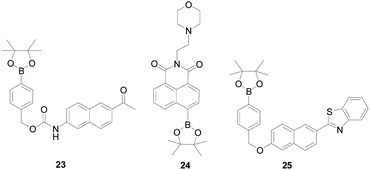
Probe 23 (ref. 71) (Fig. 16) was the first developed and reported two-photon fluorescent probe for H2O2, which introduced a boronate-based carbamate leaving group to 2-acetyl-6-aminonaphthalene (AN1), designed by Bong Rae Cho and co-workers, suitable for deep tissue imaging. After adding H2O2 to the solution, probe 23 was converted into AN1. Probe 23 can be excited by 750 nm femto-second pulses, showing a significant TP cross section, a marked blue-to-green emission color change in response to H2O2, good ROS selectivity, and insensitivity to pH in the biologically. In addition, probe 23 was capable of visualizing H2O2 levels in both RAW264.7 cells and tissue models at depths of 90–180 mm without interference from other biologically relevant species. In order to study the complex relationship between lysosomes and physiological H2O2, Weiying Lin's team72 developed a fast-response and lysosomal targeted two-photon fluorescent probe 24 (Fig. 16), which has a relatively high targeting effect on lysosomes. The probe itself has an “A–π–A” structure, and after reacting with H2O2, the borate group becomes a hydroxyl group (electron donating group), and its electronic structure becomes donor–π–acceptor “D–π–A” structure. This change shows a distinct fluorescence on signal with an 80-fold increase in fluorescence signal, which is attractive for H2O2 imaging in living tissue with deep tissue penetration. Qiujuan Ma's team73 synthesized a highly selected two-photon fluorescent probe 25 (Fig. 16) for H2O2, based on a naphthalene platform and a boric acid ester H2O2 response site. Naphthalene backbone with a D–π–A structure, which can improve fluorescence quantum yield and photochemical stability. Probe 25 has good selectivity to H2O2, and only H2O2 can cause strong fluorescence intensity enhancement at 505 nm, while other ROS substances will not, including ONOO−, O2−, ClO−, ·OH, tert-butylhydroperoxide (TBHP), 1O2 and NO. It could be applied for quantitative detection of H2O2 in live cells and tissues with good cell-permeability, and the detection limit was 0.7 μM. The ointment is that the probe's response time is long and the sensitivity was not wonderful, which requires a further improvement.
Two-photon fluorescence probes for bio-detection and imaging reported in the literature,74 the two-photon absorption cross section of most probes was not very satisfactory, and needs further development to improve the sensitivity of detection and the clarity of imaging, and broaden the biological range of imaging. Fluorescence ratiometric eliminates the effects of source intensity and instrument sensitivity. After the probe was combined with H2O2, the fluorescence intensity ratios measured at two different wavelengths can be calibrated to overcome the artifacts of the fluorescent signal due to changes in ion concentration.
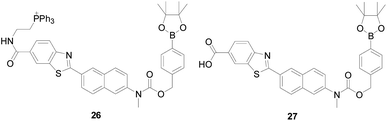
Subsequently, Bong Rae Cho research group75 designed and reported probe 26 (Fig. 17), the first two-photon probe for ratiometric detection of H2O2, which introduced triphenylphosphonium salt as the mitochondria-targeting site, could detected H2O2 level change in mitochondrial in cellular environments and intact tissues at depth >100 μM. After adding 1 mM H2O2 to a solution of 1 μM probe 26, the ratio (Fyellow/Fblue) of fluorescence intensity increased by 75-folds. Hwan Myung Kim's team76 used a carboxyl group to replace the triphenylphosphine in probe 26 and synthesized the probe 27 (Fig. 17), which also showed a change in fluorescence color from yellow to blue in response to H2O2. The ratio two-photon microscopy of 27 images displayed that H2O2 levels gradually increased from brain to kidney, skin, heart, lung, and then liver tissue. In addition, the above two probes exhibited higher selectivity for H2O2 than competitive biological species, ROS and RNS, and the ratio of Fyellow/Fblue is unperturbed after adding diverse ROS and RNS, including TBHP, O2−, HOCl, NO, ·OH, tert-butoxy radicals (·OtBu) and ONOO−. These results indicate that this type of probe will be useful for application in studies of biological and medical research.
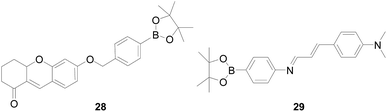
Chunhua Fan and colleagues77 reported a two-photon H2O2-specific fluorescent probe 28 (Fig. 18), which based on the ICT mechanism, with 6-hydroxy-2,3,4,4a-tetrahydro-1H-xanthen-1-one (TX) as a fluorophore. The probe 28 has a fast fluorescence response to H2O2 in 9 minutes, which with high sensitivity, high selectivity and biocompatibility. This probe has been successfully used for imaging H2O2 in mouse liver tissue. Manoj Kumar and colleagues78 also published a charge transfer assisted fluorescent probe 29 (Fig. 18), the dimethylamino group is protonated, inhibiting the twisted intramolecular charge transfer from the dimethylamino group to the imine moiety, which causes the emission at 566 nm to disappear and the higher energy emission occurring at 484 nm. In the case of the 29-H2O2 system, the fluorescence intensity ratio of I484/I566 is 9.5-times. It can be said that the probe 29 is H2O2-specific, and no significant change is observed in other reactive oxygen species. In summary, this probe can detect H2O2 in prostate cancer (PC3) cell lines in vitro, exploring the potential biological applications of probe.
The fluorescence probe designed with the change of fluorescence intensity at a single wavelength is easily interfered by external factors such as equipment efficiency, environmental conditions and probe concentration, so as to reduce the selectivity and sensitivity of fluorescence probe detection. In contrast, the ratiometric fluorescent probes can overcome these shortcomings by allowing simultaneous detection of two signals resulting from reacted and unreacted forms of the probe in the same sample.
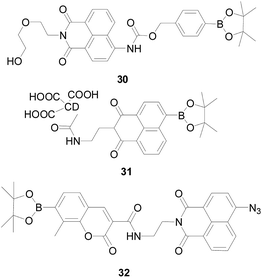
Chang' group79 designed a ratiometric fluorescent probe 30 (Fig. 19), which exhibited a blue fluorescence emission at 475 nm, after the interaction with H2O2, its emission wavelength was red shifted to 540 nm by ICT mechanism, resulting in a 12-fold change in the ratio (F540/F475). Using the ratiometric property of 30, the authors studied the changes of H2O2 in RAW264.7 cells under stimulated by PMA, found that after probe 30 induction, the fluorescence changes in phagocytic vesicles were more pronounced than in other parts of the cell, which indicated that H2O2 was produced during phagocytosis.
Shuizhu Wu's research group80 developed a nano-fluorescent probe 31 (Fig. 19) based on FRET mechanism, which was stable in spectral properties and easy to modify. Probe 31 is capable of selectively detecting the fluorescence intensity ratio (I550/I457) in the presence of H2O2 and other potentially competitive ROS/RNS. With the concentration of H2O2 increases, the fluorescence ratio (I550/I457) increased steadily. In addition, the coexistence of other species does not affect the detection of H2O2. As an energy donor and carrier for H2O2 recognition, carbon dots (CD) have good biocompatibility.81 CD-based nanoprobes are small in size and low in toxicity, and can respond quickly to H2O2, which is beneficial in vivo detection. The probe 31 is currently capable of monitoring and localizing endogenous H2O2 in zebrafish caused by oxidative damage to the drug.
Also based on FRET mechanism, Shubao Wu's group82 developed a fluorescent probe 32 (Fig. 19), which combined two different fluorophores (coumarin and naphthalimide) forming a two-channel imaging method through dual sensing strategies to detect H2S and H2O2 in living cells, which can be applied to diagnose cancer. This dual response strategy overcomes the uneven distribution of dual probes, providing accurate information about multiple analytes within the cell, and encourages the development of more fluorescent markers for detecting multiple analytes to label cancer cells.
In recent years, the development of near-infrared and multiphoton quantitative detection of ratiometric probes has become a new trend, with its unique background interference, no biological tissue damage and other unique properties, attracting a wide range of academic attention.
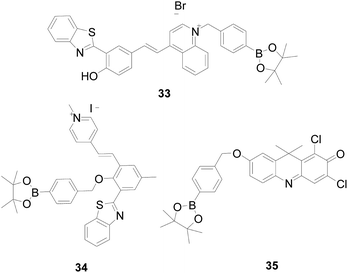
Xiangzhi Song and Wenqiang Chen83 combined the ICT and ESIPT mechanisms to design a novel mitochondria-targeted fluorescent probe 33 (Fig. 20) that showed a red fluorescence ratio of I594 nm/I666 nm at a single excitation wavelength of 440 nm. Unlike other benzothiazole-based ratio probes, probe 33 reacts with H2O2 to suppress the ICT process, exhibiting a large stoke shift (from 102 nm to 254 nm) and a significant ratio of fluorescence changes. Large Stokes shifts can minimize self-absorption and auto-fluorescence interference. Moreover, it can improve detection sensitivity, and facilitate biological cell imaging. Lijun Tang's team84 also developed a near-infrared mitochondria-targeted ratiometric probe 34 (Fig. 20) with a large stoke shift (357 nm), good sensitivity and selectivity, and has been successfully applied for A549 cell imaging.
By adjusting the intramolecular charge transfer process of dye 9H-1,3-dichloro-7-hydroxy-9,9-dimethylacridine-2-one (DDAO), Yuguang Song's team85 synthesized a ratio-type “turn-on” near-infrared probe 35 (Fig. 20) with high sensitivity and low background interference. Probe 35 produces a strong orange fluorescence emission at 620 nm, which was converted to DDAO after H2O2 response, and the wavelength is red shifted to 660 nm with red fluorescence. Except for H2O2, no palpable improvement in the fluorescence ratio (F660/F620) were observed for biological relevant ROS/RNS, GSH, Cys, VC, cations and amino acids. The probe satisfies the need for quantitative monitoring of H2O2 in the body.
Phenylborate is a classical group that recognizes H2O2 and has made outstanding contributions to the study of H2O2. However, the reaction of phenylborate to H2O2 also has certain disadvantages. Firstly, although a large number of ratiometric probes86–88 have been designed for the quantitative detection of H2O2, the concentration change of H2O2 cannot be monitored in real time. In addition, in recent years, there have been reports in the literature89–91 that phenylborate can react with ONOO− and ClO−, and the reaction rate is much faster than that with H2O2.
Therefore, developing and synthesizing high-selectivity, high-sensitivity, fast responding fluorescent probes for H2O2 based on new response groups is a significant and challenging task.
3. Sulfonate-based fluorescent H2O2 probes
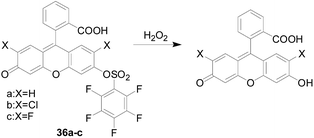
Similar to the response of the borate to H2O2, the sulfonate can also be used as a responsive group for H2O2.In 2004, Maeda and co-workers92 first discovered pentafluorobenzenesulfonyl fluorescein derivatives 36a–c (Fig. 21) as novel selective fluorescent probes for H2O2 with a sulfonate as a responsive site. The fluorescent precursor of the probe has a high fluorescence quantum yield; the sulfonate is more stable than the general ester and not easily hydrolyzed; the structure of the pentafluorobenzene ring increases the reactivity of the probe with H2O2. After H2O2 reacts with the probe 36, the sulfonate was hydrolyzed to release fluorescein, and the fluorescence is enhanced. The fluorescence response intensity of this series of probes was also positively correlated with the temperature. As the temperature increased, the detection limit decreased.
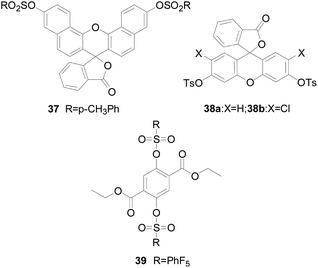
Tang Bo's group93 presented a disulfonate-responsive probe 37 (Fig. 22) as a fluorescent imaging agent to monitor intracellular H2O2. Probe 37 is an excellent naphthalene-based fluorescent reagent. Compared with fluorescein, the stent has red-shifted spectral characteristics. After treatment with various concentrations of H2O2, the probe produces a linear fluorescence response with a low detection limit, good selectivity, reported as a smart probe for H2O2 in mouse peritoneal macrophages. The same group designed probe 38a and 38b (ref. 94) (Fig. 22), which was based on the mechanism of H2O2-mediated hydrolysis of sulfonates. The probes introduced two sulfonate groups into the fluorescein chromophore as response sites. Fluorescence experiments showed that probes 38a and 38b had excellent selectivity to H2O2 over other intracellular ROS and some biological compounds and low detection limit. In addition, the probes based on sulfonate were cell permeable, which can detect the micromole change of H2O2 concentration and observe living cells with confocal microscope. In consideration of the excellent performance of the probe, the study was conducted to detect both H2O2 and superoxide in pleural lavage fluid and lung cells. The study was conducted on Wistar rats with bleomycin-induced pulmonary fibrosis. The signal transduction system for determining the content of H2O2 was developing.
Since the induction of H2O2 by the fluorescent probe acts based on the different reaction process, the sensitivity of the probe is greatly affected by the reaction rate. It is difficult to improve the reaction rate by changing the chemical structure of the single-molecule probe system. However, as has been reported,93 it can be known that real-time imaging of intracellular H2O2 can be achieved by using a switchable photoluminescent inorganic nanoprobe. Considering the reaction kinetics, Soo Young Park and Sehoon Kim95 devised a simple method to catalyze the sensing reaction by binding a fluorescent probe to a nanoreactor through a catalyst, so that the imaging of H2O2 has improved spatiotemporal resolution during cellular process. The method was proposed entirely based on the hydrolysis of the new probe 39 sulfonate group (Fig. 22), which showed a “turn-off” fluorescence response and real-time imaging of H2O2.
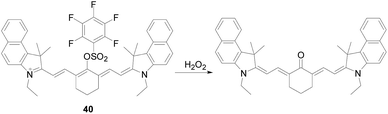
Recently, Fabiao Yu's team96 developed a near-infrared ratio fluorescent probe 40 (Fig. 23). The probe 40 itself showed the maximum absorbance centered at 830 nm, with the increase of H2O2, the fluorescence intensity decreased gradually, and a new absorption peak appeared at 635 nm, which had an excellent linear relationship. The ratiometric signals showed negligible changes for these reactive species including various ROS/RNS (HOCl, ·OH, 1O2, O2−, NO and ONOO−) and mercaptan (GSH, Cys, and Glu). The team used the probe 40 to examine the critical role of endogenous H2O2 in cell proliferation in viable hippocampal neurons and successfully demonstrated a close association between changes in endogenous H2O2 levels and brain development at different stages.
4. Benzoyl-based fluorescent probes for H2O2
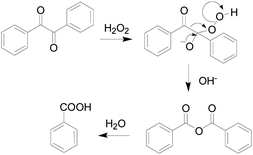
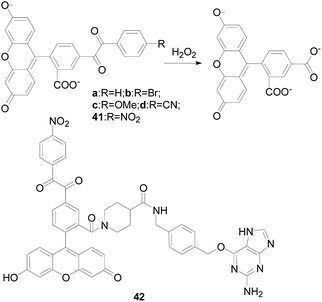
In 1979, S. Foote team97 elaborated the reaction mechanism of benzil with H2O2, as shown in the Fig. 24. At the beginning, H2O2 nucleophilic attack on the carbonyl of benzil, then the Baeyer–Villiger reaction occurs to form an unstable intermediate, and the intermediate is rapidly hydrolyzed to produce benzoic acid.Nagano's group98 described a fluorescent “turn-on” H2O2-probe 41 (Fig. 25) for the first time using benzil as a specific recognition group for H2O2. They synthesized 5-benzoylcarbonylfluorescein derivatives as candidate fluorescence probes using PET mechanism, finding that when the electron-withdrawing groups (–Br, –CN, and –NO2) were attached to the benzil, the reactivity with H2O2 was significantly enhanced. The stronger the electron absorption of the group, the higher the reactivity of the probe 41. The results demonstrated that probe 41 had the highest reactivity, better signal-to-noise ratio with H2O2, and the highest fluorescence enhancement ratio. Indeed, the enhancement of fluorescence intensity after reaction with hydrogen peroxide was as high as 150-fold. Compared with previously reported fluorescence probes utilizing benzenesulfonyl ester or boronate ester chemistry, the selectivity of probe 41 among ROS is unique. Which made probe 41 could be as a useful tool for biological detection. Experiments indicated that the probe could detect endogenous H2O2 produced by RAW264.7 macrophages and A431 human epidermal cancer cells. The design strategy used here applied not only to fluorescein derivatives, but also to other fluorophore. In 2014, Hideki Sumimoto research group99 also designed and synthesized a fluorescent probe 42 (Fig. 25) with fluorescein as the nucleus based on the response mechanism of phenyldiethyl ketone, introducing a phage targeted positioning group benzyl guanine. It resulted in the formation of a fluorophore–protein conjugate that reacts rapidly with H2O2. Probe 42 rapidly reacted with H2O2 and showed a 9-fold increase in fluorescence intensity. Which successfully applied to monitor endogenous H2O2 produced during phagocytosis of RAW264.7 macrophages in real time.
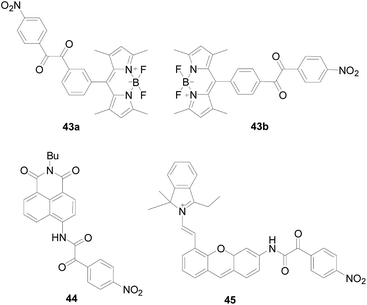
Chen Jianbo and colleagues100 designed new probes 43a and 43b (Fig. 26) using BODIPY as the fluorophore and benzil as the H2O2 recognition group. Investigating the fluorescent properties of 43a and 43b, finding that 43a and 43b successfully turn on highly green-emitting fluorescence, which satisfies “off–on” fluorescent probes to decrease background interferences. In addition, when they are reacting with excess H2O2 for 60 minutes, respectively, the fluorescence intensity of 43a increased by 78 times, much higher than that of 43b (70 times). The authors believe that the above results are because 43a owns a more pronounced steric effect and weaker capacity of the electron withdrawing. Inspired by much better response of 43a to H2O2, a further step was took to investigate other analytical capabilities of 43a. Since the carboxyl product of 43a reacts with H2O2 to increase the adsorption capacity and sensing efficiency, probe 43a exhibits a fast response (30 s) and high sensitivity (0.94 ppb) to H2O2 vapor, and has a high fluorescence quantum yield of 0.43–0.56. Probe 43a is the first fluorescent probe could be successfully used for H2O2 vapor detection and has been used for the visual detection of H2O2 of vapor by naked-eye and fluorescence imaging in living cells. Almost at the same time, Sabrina Heng's team reported a probe101 with the same structure as 43b and performed spectral analysis, selectivity and cell imaging experiments. The probe is highly selective for H2O2, and oocytes stained with probe showed a minor increase in fluorescence when treated with H2O2.
Zhang Xiaoling's group102 published a ratio type fluorescent probe 44 (Fig. 26) for the targeted detection of H2O2 in the endoplasmic reticulum. The α-ketoamide in the probe was converted to an amine after reaction with H2O2, the new fluorescence emission peak gradually increased at 540 nm, and the peak at 465 nm gradually decreased. The probe 44 can quantitatively detected the content of H2O2 by the ratio method with highly selectivity, and eliminated the error caused by the probe concentration and environment. And realizes the detection of exogenous H2O2 in HeLa cells.
Using the same mechanism, Bo Tang's research group103 developed a near-infrared fluorescent probe 45 (Fig. 26), which integration a unique reaction site α-ketoamide into a hemi-cyanine fluorophore. Probe 45 is a fluorescence “off–on” probe regulated by an ICT mechanism. Upon reaction with H2O2, hemi-cyanine-NH2 was released and showed significant fluorescence enhancement attributing to the ICT process. Probe 45 has a high reaction rate and high specificity, and the fluorescence intensity at 704 nm in the fluorescence spectrum increases as the concentration of H2O2 increases, whereas other ROS or RNS no. The use of probe 45 confirmed the formation of H2O2 during ischemia-reperfusion injury at both cell organ levels.
In general, the above-mentioned benzil-responsive fluorescent H2O2 probe reacts more rapidly, and both used a nitro group as an electron-withdrawing group to enhance the reactivity of the probe. However, this type of ratio-type near-infrared probe is not mature enough, and there is room for further research.
5. o-Benzenediol based fluorescent H2O2 probes
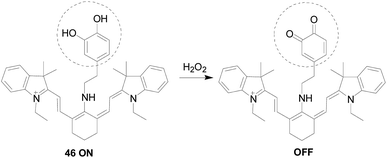
The o-diphenolic hydroxyl group is also a good response group for H2O2.Hankley's team104 introduced the synthesis and biological applications of a novel H2O2 probe 46 (Fig. 27). The o-diphenolic hydroxyl in the probe structure reacted with H2O2 to oxidize to o-quinone, which was a fluorescence quenching reaction, and the fluorescence intensity at 630 nm gradually decreased with the colour change from blue to purple of solution. Only a small fluorescence response was observed in the emission spectrum except for physiologically relevant levels of H2O2. As the GSH in the solution added, the fluorescence was turn on. Therefore, this “on–off–on” type of probe can monitor intracellular H2O2 oxidative stress and thiol-reduction repair processes simply and directly, and has good permeability and specificity in cells, negligible background fluorescence and cells toxicity.
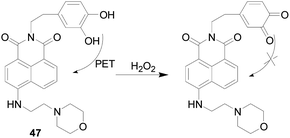
As a further study in this field, in 2017, Manoj Kumar and colleagues105 reported a lysosomal targeting H2O2 fluorescent probe 47 (Fig. 28), which comprised a catechol responsive group and a naphthalimide fluorophore. In that catechol can undergo PET effects to the naphthalimide core resulting in weak molecular fluorescence in aqueous solution. Upon addition of H2O2 to the aqueous solution of probe 47, the PET effects were inhibited and the emission band at 537 nm was enhanced. There was no obviously fluorescent response to other ROS/RNS like NO, TBHP, ·OH, and OONO− and bio-thiols like Cys, GSH and Hcy. The morpholino group in the probe 47 acted as a lysosomes-targeting group can be used for endogenous detection of H2O2 in living cell lysosomes, and can also be used to detect H2O2 in brain tissues and living nematodes.
6. Enamine-based fluorescent probes for H2O2
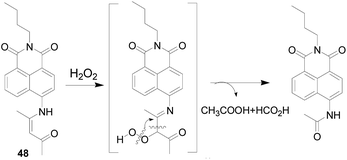
In 2018, Wang Peng's group106 used H2O2 to promote the carbon–carbon double bond cleavage, and prepared a probe 48 (Fig. 29) with an enamine group as a reactive group. After the reaction of the compound 48 with H2O2, a peroxide intermediate formed, which is then converted to a stable amide with blue fluorescence emission with good sensitivity. The response of probe 48 to H2O2 was nearly 10–30 times than that of control group and other interference factors, such as various amino acids (Met, Glu, Cys, GSH) and ROS (TBPH, ClO−, ·OH, NO, ONOO−). Properly designed probes have great potential to monitor H2O2 in cells, mouse liver sections and tumor tissues using single-photon and two-photon fluorescence imaging with low cytotoxicity.7. Other types probes for H2O2
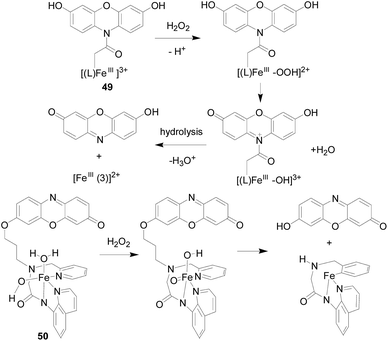
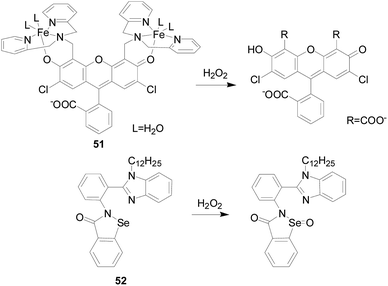
Yutaka Hitomi's team107 developed a metal-based probe 49 (Fig. 30), which produced a fluorescent response faster than an organic molecular-based fluorescent probe, showing significant fluorescence enhancement in 20 seconds. The Fe complex in this probe reacted rapidly with H2O2 to form a cationic amide group by three routes. Hydrolysis of the cationic amide group by an iron(III)-bound hydroxo group or an unbound water molecule finally gives resorufin and a hydrolyzed iron complex, [FeIII(3)]2+. Resorufin produces significant red fluorescence. The authors applied it to the detection of H2O2 produced during the enzymatic reaction. Since probe 49 may be hydrolyzed by amidate under cell culture conditions, which hinders its application to fluorescence imaging of intracellular H2O2. In order to fully enhance the stability of the metal complex H2O2 probe to visualize intracellular H2O2, the group developed a new iron complex-based probe 50 (ref. 108) (Fig. 30). The group employs the nonheme iron-based peroxidase mimetic Fe(III) mpaq (mpaq = 2-[N-methyl-N-pyridin-2-ylmethyl]-amino-N′quinolin-8-yl-acetamido) as a H2O2-responsive component, in which the iron complex reacts rapidly with H2O2 to form an oxidant substance that converts the closely appended o-alkyl resorufin moiety to resorufin. Probe 50 showed a strong enhancement in fluorescence intensity in response to H2O2, which was much stronger than other oxidants such as ·OtBu, O2−, NO and ClO−. Experiments have shown that probe 50 is suitable for H2O2 imaging produced by EGF stimulation.Youngmin You's team109 developed a new fluorescent probe 51 (Fig. 31) for H2O2 based on the cleavage of a paramagnetic Fe ionophore from a fluorophore. By dissociation of the Fe-ionophore, probes 51 reacts with H2O2 produces 2,7-dichloro-fluorescein, rather than other oxidants derived from H2O2 (e.g., ·OH). Almost no response was observed for O2−, HOCl, t-BuOOH, NO, 1O2, and only a limited increase in the fluorescence intensity was observed in the cases of t-BuOOH and ·OH. Moreover, the probe 51 is suitable for live cell imaging and has the ability to detect intracellular H2O2 in lysosomes.
Xiaoqi Yu's research group110 designed and synthesized a “turn-on” type fluorescent probe 52 containing selenium. Under the action of H2O2, the selenium atom was oxidized to selenium oxide, and the effect of PET itself was inhibited, forming aggregates and AIE-type fluorescence occurred. The fluorescence intensity of probe 52 was proportional to the H2O2 concentration, more than 110-fold fluorescence enhancement was observed after treatment with 200 μM H2O2 for 1 h at ambient temperature. Other ROS/RNS, including ClO−, TBPH, ONOO−, ·OtBu, ·OH, 1O2, and O2−, could not cause the fluorescence change of the probe 52.
8. Concluding and outlook
The production, decomposition and diffusion of hydrogen peroxide are unstable in different cells. It is important to develop a highly sensitive hydrogen peroxide probe for real-time in situ detection of H2O2 in cells or organisms.111 The development of fluorescent probes provides a versatile and valuable way of thinking about the processes behind many biological phenomena in living systems. Among the most H2O2 probes, the development of fluorescent probes is the most extensive because of their better biocompatibility and higher sensitivity.This review summarizes several commonly used small molecule types of H2O2 fluorescent probes, such as based on aryl borate or boric acid, sulfonate, benzoyl and the like. These fluorescent probes are able to overcome the nature of hydrogen peroxide itself and have high sensitivity and specificity for H2O2. The probes developed in recent years combined with confocal microscopy and micro-spectral detection technology have been able to perform “real-time, visible, quantitative” detection of hydrogen peroxide in living cells and tissues. In spite of that, the development of probes requires further consideration of the following issues. Since the same recognition moiety that adapts to different fluorophores will exhibit specificity for different targets, an in-depth study can be performed based on the structure–reactivity relationship of the reported probes to derive intrinsic rules that guide the targeted design and synthesis of the probe. Although the detection of endogenous H2O2 in vivo can be obtained, the imaging depth is still limited due to the light penetrating ability and the stability of the probe. Due to the low background interference and weak photo-bleaching effects, ratiometric and TP-probes have great bio-imaging potential for H2O2 and can be used as sensing platforms, but this only helps solve some of the problems, not all. In general, these issues not only provide researchers with challenges, but also the opportunity to enter new research areas.
Therefore, in the development of the probe, considering the relatively low concentration in steady state of H2O2 in the body, it needs to be designed from both chemical and biological aspects. On the one hand, we need to find more reaction mechanisms (currently based on oxidation reactions). On the other hand, in addition to considering high selectivity and sensitivity, low auto-oxidation, high optical stability, low cytotoxicity, good solubility, cell permeability, and low interference of biological environmental substances need to be considered. In addition, since redox reactions are involved in the maintenance of normal function and health of cells, it is also a research focus of fluorescent probes to study redox probes of cellular redox biology by monitoring changes in reversible fluorescence over time.
In summary, the search for probes that better reflect the physiological and pathological functions of H2O2 in cells and organisms will be one of the important hotspots and difficult areas of current life science research. We believe that with the development and further integration of novel small molecule fluorescent probes in related fields such as pharmacy, medicine and life sciences, the physiological and pathological functions of H2O2 in biological systems will be better explained and understood. Hydrogen peroxide probes will have broader application prospects in the fields of pathophysiology, disease diagnosis and treatment.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21305079 and 21606147), the Academy of Science and Technology Project of Shandong Academy of Medical Sciences (No. 2017-55 and 2018-19), Key Projects of Industrial Science and Technology Plan in Qiannan Prefecture (2017) 11, project of Haixi Science and Technology Bureau of Qinghai Province 2017-Q4.Notes and references
- C. Nathan and A. Cunningham-Bussel, Nat. Rev. Immunol., 2013, 13, 349–361 CrossRef CAS PubMed.
- S. J. Dixon and B. R. Stockwell, Nat. Chem. Biol., 2014, 10, 9–17 CrossRef CAS PubMed.
- C. C. Winterbourn, Nat. Chem. Biol., 2008, 4, 278–286 CrossRef CAS PubMed.
- H. Wu, Q. Song, G. Ran, X. Lu and B. Xu, TrAC, Trends Anal. Chem., 2011, 30, 133–141 CrossRef CAS.
- F. L. Muller, M. S. Lustgarten, Y. Jang, A. Richardson and H. Van Remmen, Free Radical Biol. Med., 2007, 43, 477–503 CrossRef CAS PubMed.
- J. J. Hu, N. K. Wong, S. Ye, X. Chen, M. Y. Lu, A. Q. Zhao, Y. Guo, A. C. Ma, A. Y. Leung, J. Shen and D. Yang, J. Am. Chem. Soc., 2015, 137, 6837–6843 CrossRef CAS PubMed.
- A. A. Alfadda and R. Sallam, J. Biomed. Biotechnol., 2012, 2012, 936486 Search PubMed.
- T. F. Brewer, F. J. Garcia, C. S. Onak, K. S. Carroll and C. J. Chang, Annu. Rev. Biochem., 2015, 84, 765–790 CrossRef CAS PubMed.
- A. Kaur, M. A. Haghighatbin, C. F. Hogan and E. J. New, Chem. Commun., 2015, 51, 10510–10513 RSC.
- L. A. Sena and N. S. Chandel, Mol. Cell, 2012, 48, 158–167 CrossRef CAS PubMed.
- C. C. Winterbourn, Biochim. Biophys. Acta, 2014, 1840, 730–738 CrossRef CAS PubMed.
- A. Gomes, E. Fernandes and J. L. F. C. Lima, J. Biochem. Biophys. Methods, 2005, 65, 45–80 CrossRef CAS PubMed.
- P. D. Ray, B. W. Huang and Y. Tsuji, Cell. Signalling, 2012, 24, 981–990 CrossRef CAS PubMed.
- N. Kumar, V. Bhalla and M. Kumar, Coord. Chem. Rev., 2013, 257, 2335–2347 CrossRef CAS.
- X. Sun, Q. Xu, G. Kim, S. E. Flower, J. P. Lowe, J. Yoon, J. S. Fossey, X. Qian, S. D. Bull and T. D. James, Chem. Sci., 2014, 5, 3368 RSC.
- X. Zhou, Y. Kwon, G. Kim, J. H. Ryu and J. Yoon, Biosens. Bioelectron., 2015, 64, 285–291 CrossRef CAS PubMed.
- Z. Lou, P. Li and K. Han, Acc. Chem. Res., 2015, 48, 1358–1368 CrossRef CAS PubMed.
- K. Araki and K. Inaba, Antioxid. Redox Signaling, 2012, 16, 790–799 CrossRef CAS PubMed.
- B. Chance, H. Sies and A. Boveris, Physiol. Rev., 1979, 59, 527–605 CrossRef CAS PubMed.
- H. C. Dawkes and S. E. Phillips, Curr. Opin. Struct. Biol., 2001, 11, 666–673 CrossRef CAS PubMed.
- J.-J. P. Kim and R. Miura, Eur. J. Biochem., 2004, 271, 483–493 CrossRef CAS PubMed.
- T. Finkel, Curr. Opin. Cell Biol., 2003, 15, 247–254 CrossRef CAS PubMed.
- J. R. Stone and S. Yang, Antioxid. Redox Signaling, 2006, 8, 243–270 CrossRef CAS PubMed.
- P. Niethammer, C. Grabher, A. T. Look and T. J. Mitchison, Nature, 2009, 459, 996–999 CrossRef CAS PubMed.
- C. Behl, J. B. Davis, F. Lesley and D. Schubert, Cell, 1994, 77, 817–827 CrossRef CAS PubMed.
- B. D'Autreaux and M. B. Toledano, Nat. Rev. Mol. Cell Biol., 2007, 8, 813–824 CrossRef PubMed.
- J. R. Stone and S. Yang, Antioxid. Redox Signaling, 2006, 8, 243–270 CrossRef CAS PubMed.
- H. Ohshima, M. Tatemichi and T. Sawa, Arch. Biochem. Biophys., 2003, 417, 3–11 CrossRef CAS PubMed.
- K. J. Barnham, C. L. Masters and A. I. Bush, Nat. Rev. Drug Discovery, 2004, 3, 205–214 CrossRef CAS PubMed.
- A. M. Shah and K. M. Channon, Heart, 2004, 90, 485 CrossRef.
- H. Rath and E. Schönpflug, Bestimmung von Faserbegleitstoffen, 1960 Search PubMed.
- R. Brandt and A. S. Keston, Anal. Biochem., 1965, 11, 6–9 CrossRef CAS PubMed.
- S. L. Hempel, G. R. Buettner, Y. Q. O'Malley, D. A. Wessels and D. M. Flaherty, Free Radical Biol. Med., 1999, 27, 146–159 CrossRef CAS PubMed.
- H. G. Kuivila and A. G. Armour, J. Am. Chem. Soc., 1957, 79, 5659–5662 CrossRef CAS.
- L. C. Lo and C. Y. Chu, Chem. Commun., 2003, 2728 RSC.
- M. C. Y. Chang, A. Pralle, E. Y. Isacoff and C. J. Chang, J. Am. Chem. Soc., 2004, 126, 15392–15393 CrossRef CAS PubMed.
- A. E. Albers, V. S. Okreglak and C. J. Chang, J. Am. Chem. Soc., 2006, 128, 9640–9641 CrossRef CAS PubMed.
- E. W. Miller, O. Tulyathan, E. Y. Isacoff and C. J. Chang, Nat. Chem. Biol., 2007, 3, 263–267 CrossRef CAS PubMed.
- B. C. Dickinson and C. J. Chang, J. Am. Chem. Soc., 2008, 130, 9638–9639 CrossRef CAS PubMed.
- A. E. Albers, B. C. Dickinson, E. W. Miller and C. J. Chang, Bioorg. Med. Chem. Lett., 2008, 18, 5948–5950 CrossRef CAS PubMed.
- C. Y. Ang, S. Y. Tan, S. Wu, Q. Qu, M. F. E. Wong, Z. Luo, P.-Z. Li, S. Tamil Selvan and Y. Zhao, J. Mater. Chem. C, 2016, 4, 2761–2774 RSC.
- J. Dong, Z. Zhao, R. Liu, H. Zhang, Y. Wu and X. Ba, RSC Adv., 2017, 7, 55067–55073 RSC.
- L. Du, M. Li, S. Zheng and B. Wang, Tetrahedron Lett., 2008, 49, 3045–3048 CrossRef CAS PubMed.
- L. Du, N. Ni, M. Li and B. Wang, Tetrahedron Lett., 2010, 51, 1152–1154 CrossRef CAS PubMed.
- J. Xu, Q. Li, Y. Yue, Y. Guo and S. Shao, Biosens. Bioelectron., 2014, 56, 58–63 CrossRef CAS PubMed.
- J. Xu, Y. Zhang, H. Yu, X. Gao and S. Shao, Anal. Chem., 2016, 88, 1455–1461 CrossRef CAS PubMed.
- Y. Liu, J. Niu, J. Nie, F. Meng and W. Lin, New J. Chem., 2017, 41, 3320–3325 RSC.
- J. Han, C. Chu, G. Cao, W. Mao, S. Wang, Z. Zhao, M. Gao, H. Ye and X. Xu, Bioorg. Chem., 2018, 81, 362–366 CrossRef CAS PubMed.
- Y. Chen, X. Shi, Z. Lu, X. Wang and Z. Wang, Anal. Chem., 2017, 89, 5278–5284 CrossRef CAS PubMed.
- J. Liu, J. Ren, X. Bao, W. Gao, C. Wu and Y. Zhao, Anal. Chem., 2016, 88, 5865–5870 CrossRef CAS PubMed.
- J. Liu, S. Zhou, J. Ren, C. Wu and Y. Zhao, Analyst, 2017, 142, 4522–4528 RSC.
- W. Zhang, W. Liu, P. Li, F. Huang, H. Wang and B. Tang, Anal. Chem., 2015, 87, 9825–9828 CrossRef CAS PubMed.
- Z. Song, R. T. Kwok, D. Ding, H. Nie, J. W. Lam, B. Liu and B. Z. Tang, Chem. Commun., 2016, 52, 10076–10079 RSC.
- J. Liu, C. Zhang, J. Dong, J. Zhu, C. Shen, G. Yang and X. Zhang, New J. Chem., 2017, 41, 4733–4737 RSC.
- M. Ren, B. Deng, K. Zhou, X. Kong, J. Y. Wang and W. Lin, Anal. Chem., 2017, 89, 552–555 CrossRef CAS PubMed.
- X. Wang, Y. Huang, W. Lv, C. Li, W. Zeng, Y. Zhang and X. Feng, Anal. Methods, 2017, 9, 1872–1875 RSC.
- F. Dai, F. Jin, Y. Long, X. Jin and B. Zhou, Free Radical Res., 2018, 1–151 Search PubMed.
- Z. Zhou, Y. Li, W. Su, B. Gu, H. Xu, C. Wu, P. Yin, H. Li and Y. Zhang, Sens. Actuators, B, 2019, 280, 120–128 CrossRef CAS.
- L. Zhou, H. Ding, W. Zhao and S. Hu, Spectrochim. Acta, Part A, 2019, 206, 529–534 CrossRef CAS PubMed.
- X. Liu, L. He, L. Yang, Y. Geng, L. Yang and X. Song, Sens. Actuators, B, 2018, 259, 803–808 CrossRef CAS.
- X. Liu, H. Tian, L. Yang, Y. Su, M. Guo and X. Song, Sens. Actuators, B, 2018, 255, 1160–1165 CrossRef CAS.
- J. Wang, W. Zhu, G. Niu, G. Jiang, Q. Chen, P. Gao, Y. Li, G. Zhang, X. Fan and B. Z. Tang, Chem. Commun., 2018, 54, 13957–13960 RSC.
- F. Xu, W. Tang, S. Kang, J. Song and X. Duan, Dyes Pigm., 2018, 153, 61–66 CrossRef CAS.
- L. Yang, J. Y. Niu, R. Sun, Y. J. Xu and J. F. Ge, Analyst, 2018, 143, 1813–1819 RSC.
- N. Zhang, B. Dong, X. Kong, C. Wang, W. Song and W. Lin, J. Fluoresc., 2018, 28, 681–687 CrossRef CAS PubMed.
- X. Liang, X. Xu, D. Qiao, Z. Yin and L. Shang, Chem. - Asian J., 2017, 12, 3187–3194 CrossRef CAS PubMed.
- F. Xu, H. Li, Q. Yao, J. Fan, J. Wang and X. Peng, J. Mater. Chem. B, 2016, 4, 7363–7367 RSC.
- H. Xiao, P. Li, X. Hu, X. Shi, W. Zhang and B. Tang, Chem. Sci., 2016, 7, 6153–6159 RSC.
- Y. Shen, F. Yan, X. Huang, X. Zhang, Y. Zhang, C. Zhang, J. Jin, H. Li and S. Yao, RSC Adv., 2016, 6, 88096–88103 RSC.
- J. Shanmugapriya, K. Rajaguru, G. Sivaraman, S. Muthusubramanian and N. Bhuvanesh, RSC Adv., 2016, 6, 85838–85843 RSC.
- C. Chung, D. Srikun, C. S. Lim, C. J. Chang and B. R. Cho, Chem. Commun., 2011, 47, 9618–9620 RSC.
- M. Ren, B. Deng, J. Y. Wang, X. Kong, Z. R. Liu, K. Zhou, L. He and W. Lin, Biosens. Bioelectron., 2016, 79, 237–243 CrossRef CAS PubMed.
- Q. Ma, X. li, J. Zhang, X. Zhu, L. Zhou and H. Liu, Anal. Methods, 2017, 9, 4558–4565 RSC.
- H. Li, Q. Yao, J. Fan, J. Du, J. Wang and X. Peng, Biosens. Bioelectron., 2017, 94, 536–543 CrossRef CAS PubMed.
- G. Masanta, C. H. Heo, C. S. Lim, S. K. Bae, B. R. Cho and H. M. Kim, Chem. Commun., 2012, 48, 3518–3520 RSC.
- C. S. Lim, M. K. Cho, M. Y. Park and H. M. Kim, ChemistryOpen, 2018, 7, 53–56 CrossRef CAS PubMed.
- Y. Lu, X. Shi, W. Fan, C. A. Black, Z. Lu and C. Fan, Spectrochim. Acta, Part A, 2018, 190, 353–359 CrossRef CAS PubMed.
- M. Kumar, N. Kumar, V. Bhalla, P. R. Sharma and Y. Qurishi, Chem. Commun., 2012, 48, 4719–4721 RSC.
- D. Srikun, E. W. Miller, D. W. Domaille and C. J. Chang, J. Am. Chem. Soc., 2008, 130, 4596–4597 CrossRef CAS PubMed.
- G. Wu, F. Zeng, C. Yu, S. Wu and W. Li, J. Mater. Chem. B, 2014, 2, 8528–8537 RSC.
- S.-L. Zhong, J. Zhang, Q. Hu, W.-X. Chen, T.-F. Ma and J.-H. Zhao, Asian Pac. J. Cancer Prev., 2014, 20, 2254 Search PubMed.
- N. Velusamy, N. Thirumalaivasan, K. N. Bobba, A. Podder, S. P. Wu and S. Bhuniya, J. Photochem. Photobiol., B, 2018, 191, 99–106 CrossRef PubMed.
- L. He, X. Liu, Y. Zhang, L. Yang, Q. Fang, Y. Geng, W. Chen and X. Song, Sens. Actuators, B, 2018, 276, 247–253 CrossRef CAS.
- L. Tang, M. Tian, H. Chen, X. Yan, K. Zhong and Y. Bian, Dyes Pigm., 2018, 158, 482–489 CrossRef CAS.
- J. Hou, M. Qian, H. Zhao, Y. Li, Y. Liao, G. Han, Z. Xu, F. Wang, Y. Song and Y. Liu, Anal. Chim. Acta, 2018, 1024, 169–176 CrossRef CAS PubMed.
- K. Xu, L. He, X. Yang, Y. Yang and W. Lin, Analyst, 2018, 143, 3555–3559 RSC.
- Y. Shen, X. Zhang, Y. Zhang, Y. Wu, C. Zhang, Y. Chen, J. Jin and H. Li, Sens. Actuators, B, 2018, 255, 42–48 CrossRef CAS.
- X. Lu, M. Zhao, P. Chen, Q. Fan, W. Wang and W. Huang, J. Mater. Chem. B, 2018, 6, 4531–4538 RSC.
- A. Sikora, J. Zielonka, M. Lopez, J. Joseph and B. Kalyanaraman, Free Radical Biol. Med., 2009, 47, 1401–1407 CrossRef CAS PubMed.
- J. Zielonka, A. Sikora, M. Hardy, J. Joseph, B. P. Dranka and B. Kalyanaraman, Chem. Res. Toxicol., 2012, 25, 1793–1799 Search PubMed.
- J. Zhou, Y. Li, J. Shen, Q. Li, R. Wang, Y. Xu and X. Qian, RSC Adv., 2014, 4, 51589–51592 RSC.
- H. Maeda, Y. Fukuyasu, S. Yoshida, M. Fukuda, K. Saeki, H. Matsuno, Y. Yamauchi, K. Yoshida, K. Hirata and K. Miyamoto, Angew. Chem., Int. Ed. Engl., 2004, 43, 2389–2391 CrossRef CAS PubMed.
- K. Xu, B. Tang, H. Huang, G. Yang, Z. Chen, P. Li and L. An, Chem. Commun., 2005, 5974–5976 RSC.
- K. Xu, F. Liu, H. Wang, S. Wang, L. Wang and B. Tang, Sci. China, Ser. B: Chem., 2009, 52, 734–740 CrossRef CAS.
- J. Heo, C.-K. Lim, Y. Kim, H.-J. Cho, Y.-D. Lee, J.-h. Maeng, D.-R. Ahn, S. Lee, J. Bang, S. Y. Park and S. Kim, Chem. Commun., 2016, 52, 1131–1134 RSC.
- H. Guo, G. Chen, M. Gao, R. Wang, Y. Liu and F. Yu, Anal. Chem., 2019, 91, 1203–1210 CrossRef CAS PubMed.
- Y. Sawaki and C. S. Foote, J. Am. Chem. Soc., 1979, 101(21), 6292–6296 CrossRef CAS.
- M. Abo, Y. Urano, K. Hanaoka, T. Terai, T. Komatsu and T. Nagano, J. Am. Chem. Soc., 2011, 133, 10629–10637 CrossRef CAS PubMed.
- M. Abo, R. Minakami, K. Miyano, M. Kamiya, T. Nagano, Y. Urano and H. Sumimoto, Anal. Chem., 2014, 86, 5983–5990 CrossRef CAS PubMed.
- B. Li, J.-B. Chen, Y. Xiong, X. Yang, C. Zhao and J. Sun, Sens. Actuators, B, 2018, 268, 475–484 CrossRef CAS.
- M. S. Purdey, H. J. McLennan, M. L. Sutton-McDowall, D. W. Drumm, X. Zhang, P. K. Capon, S. Heng, J. G. Thompson and A. D. Abell, Sens. Actuators, B, 2018, 262, 750–757 CrossRef CAS.
- C. Gao, Y. Tian, R. Zhang, J. Jing and X. Zhang, Anal. Chem., 2017, 89, 12945–12950 CrossRef CAS PubMed.
- X. Xie, X. Yang, T. Wu, Y. Li, M. Li, Q. Tan, X. Wang and B. Tang, Anal. Chem., 2016, 88, 8019–8025 CrossRef CAS PubMed.
- F. Yu, P. Li, P. Song, B. Wang, J. Zhao and K. Han, Chem. Commun., 2012, 48, 4980–4982 RSC.
- S. I. Reja, M. Gupta, N. Gupta, V. Bhalla, P. Ohri, G. Kaur and M. Kumar, Chem. Commun., 2017, 53, 3701–3704 RSC.
- N. Li, J. Huang, Q. Wang, Y. Gu and P. Wang, Sens. Actuators, B, 2018, 254, 411–416 CrossRef CAS.
- Y. Hitomi, T. Takeyasu, T. Funabiki and M. Kodera, Anal. Chem., 2011, 83, 9213–9216 CrossRef CAS PubMed.
- Y. Hitomi, T. Takeyasu and M. Kodera, Chem. Commun., 2013, 49, 9929–9931 RSC.
- D. Song, J. M. Lim, S. Cho, S.-J. Park, J. Cho, D. Kang, S. G. Rhee, Y. You and W. Nam, Chem. Commun., 2012, 48, 5449–5451 RSC.
- Y. X. Liao, K. Li, M. Y. Wu, T. Wu and X. Q. Yu, Org. Biomol. Chem., 2014, 12, 3004–3008 RSC.
- H. Guo, H. Aleyasin, B. C. Dickinson, R. E. Haskew-Layton and R. R. Ratan, Cell & Bioscience, 2014, 4, 64 Search PubMed.
| This journal is © The Royal Society of Chemistry 2019 |