Open Access Article
Open Access ArticleIn vivo evaluation of the subchronic systemic toxicity of akermanite bioceramic for bone regeneration following ISO standard methods
Nan Ma†
ac,
Bing Ma†b,
Yanling Zhoub,
Haibo Zhuc,
Ying Zhouc,
Zhiguang Huanb,
Peiji Wang*a and
Jiang Chang *b
*b
aDepartment of Hand Surgery, Second Affiliated Hospital of Suzhou University, Suzhou 215000, China
bState Key Laboratory of High Performance Ceramics and Superfine Microstructure, Shanghai Institute of Ceramics, Chinese Academy of Sciences, 1295 Dingxi Road, Shanghai 200050, China. E-mail: jchang@mail.sic.ac.cn
cDepartment of Orthopaedics, Xuhui Central Hospital of Shanghai, Shanghai 200031, China
First published on 4th June 2019
Abstract
Although the akermanite (Ca2MgSi2O7) bioceramic has been confirmed to possess favorable osteogenic activity, until now little was known about its in vivo subchronic systemic toxicity, which is important for determining the biocompatibility and the clinical applications of the material in bone implants. In this study, the subchronic systemic toxicity of akermanite bioceramic was for the first time investigated according to well-accepted ISO standard methods. Following the method, healthy adult Wistar rats were injected with certain amounts of extracts of akermanite bioceramic that was intended to simulate the ionic product during the degradation of the material when implanted into the body. At day 28 after injection, the general body conditions, blood cytology, blood biochemistry and histology of all important organs of the rats were examined. The results showed that there was no significant difference in the hemoglobin concentration, red blood cell count, platelet count and white blood cell count between the rats with injection of akermanite bioceramic extracts and the saline control. The indicators of liver function, including aspartate aminotransferase and alkaline phosphatase, and kidney function, including blood urea nitrogen and creatinine, did not show significant difference between the two groups (P > 0.05). In addition, the results of histological examination showed that the extract of akermanite bioceramic did not cause any pathological changes to important organs such as the heart, liver and kidneys. These findings demonstrated that the ionic product derived from the degradation of akermanite bioceramic did not cause in vivo subchronic systemic toxicity. The results of the current study provided more strengthened evidence for the biosafety of akermanite bioceramic and suggest that this material with desirable biocompatibility may be a potential candidate for orthopedic clinical applications.
1 Introduction
Although autologous and allogeneic bones are considered as good choices for bone repair, their source is limited and transplantation of these bones may cause serious shortcomings such as infections and immune rejection.1–5 Therefore, some synthetic materials are employed for bone repair in clinical practice. Calcium phosphate bioceramics are similar to natural bone tissues in the inorganic composition (hydroxyapatite) and have favorable biocompatibility, making them widely used for bone repair in clinical practice. The osteogenic activity of sintered calcium phosphate bioceramics still needs improvement, and in some case the degradability of the material is poor.6–10 Therefore, investigation of degradable bioceramics with favorable osteogenic activity has always been a focus in the field of biomaterials for bone repair.It is well known that silicon is an important trace element for bone regeneration and repair, which can up-regulate the expression of osteogenesis-related genes, activate the osteogenesis related signaling pathways, and promote the osteogenic differentiation of bone marrow mesenchymal stem cells.11–14 There has been great progress in the development of silicate-based bioactive ceramics in the past decade. Many studies have confirmed that these ceramics can release Si ions to significantly promote the proliferation and differentiation of bone cells.15–17 Compared with calcium phosphate ceramics, silicate based bioceramics have significantly better osteogenic activity and in vivo degradability, making them have great potential for more efficient bone repair.18–23
Akermanite (Ca2MgSi2O7) is a typical type of silicate based bioceramics, which was capable releasing calcium, magnesium and silicate ions in physiological environment, and some previous studies have demonstrated that implantation of porous akermanite in the injured femur of rabbits could promote the bone regeneration and angiogenesis as compared to β-TCP treated rabbits.24–26 These results suggested that akermanite bioceramic could consider as a good candidate for bone repair with good bioactivity. However, it should be noted that these studies have been focused on the effects of the material on the osteogenic differentiation and bone regeneration, whereas little is known about the subchronic systemic toxicity of degraded products of these material, which is crucial for its future clinical application. Different from the test on bone-forming ability of an implant, the subchronic systemic toxicity test is intended to evaluate the possible effects of the absorption, distribution, or metabolism of products originating from the material, involving parts of the body or organs not in direct contact with the material. Considering the fact that akermanite bioceramic is biodegradable and its ionic biodegradation products, including Ca, Mg and Si ions, are likely to be involved in circulation, concerns may rise over its influence on blood and organ function. It has been reported that silicate or magnesium at a high concentration may inhibit the cell proliferation and even induce cell apoptosis, and overloading of these ionsmay cause damage on lung, cardiovascular system, liver, kidney and other organs.27–33 Thus, although akermanite has favorable osteogenicity, the distribution and subchonic influence of degraded products of akermanite are still poorly understood, and more in-depth studies are needed to confirm its biological safety.
This study therefore aimed to investigate the in vivo subchronic systemic toxicity of akermanite according to the methods that are recommended in ISO 10993-11:2017 and ISO 10993-12:2012. The extracts of akermanite was injected into rats by peritoneal injection daily, and blood cytology, blood biochemistry and histology of different organs were examined over 28 days. Our findings may provide guidance on the subchronic systemic toxicity and biosafty on akermanite based bioceramic, and together with the results that have demonstrate the in vivo bone forming ability of the material, the outcome of the present study may provide a wide scope of view on the safety of akermanite bioceramic for its potential application in bone repair and regeneration.
2 Materials and methods
2.1 Materials
Akermanite powders with the powder size between 25 and 40 μm were purchased from Huaqiao New Materials Company, China.Healthy adult Wistar rats were provided by the Experimental Animal Center of the Sixth People's Hospital in Shanghai. All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of Shanghai Jiao Tong University (Shanghai, China) and approved by the Animal Ethics Committee of Shanghai Jiao Tong University School of Medicine (Shanghai, China).
2.2 Preparation of akermanite extracts
Extracts of akermanite were prepared following the method described in 10.3 of the standard ISO 10993-12:2012 (Biological evaluation of medical devices – Part 12: Sample preparation and reference materials). Briefly, akermanite powders were soaked in normal saline at a ratio of 0.2 g mL−1 at 121 °C for 1 h. Then the supernatant was collected and sterilized by filtration through a filter (0.22 μm, Millipore).2.3 Injection of akermanite extracts into rats
The injection of akermanite extracts was performed according to the method described in 3.8 of the standard ISO 10993-11:2017 (Biological evaluation of medical devices – Part 11: Tests for systemic toxicity). Healthy adult Wistar rats weighing from 180 to 220 g (n = 40) (male: n = 20; female: n = 20) were randomly divided into 2 groups: akermanite group (AKE; male: n = 10; female: n = 10) and control group (male: n = 10; female: n = 10). In AKE group, the akermanite extracts were injected at an extracts volume/rat weight ratio of 20 mL kg−1 by peritoneal injection once a day for 28 days. In control group, normal saline was injected at the same conditions. After each injection, the general conditions (survival, food/water intake and body weight) and toxic reactions were monitored. All the animals were weighed once every 3 days.2.4 Examination of blood cytology and biochemistry
At the end of injection, blood was collected from the heart and processed for cytological and biochemical examinations (reagents were purchased from MaiRui Medical Co., Ltd). Following parameters were detected: hemoglobin concentration (HCG), red blood cell count (RBC), platelet count (PLT), white blood cell (WBC) count, WBC subsets, hematocrit, aspartate aminotransferase (AST), alanine aminotransferase (ALT), total protein (TP), albumin (ALB), ratio of albumin to globulin (A/G), globulin (GLB), AKP, blood urea nitrogen (BUN), creatinine (CRE), urea (UREA), blood glucose (GLU), total cholesterol (CHOL), triglyceride (TG), potassium (K), sodium (Na), chlorine (Cl) and calcium (Ca).2.5 Autopsy and histological examination
At the end of injection, animals were sacrificed, followed by autopsy. The main organs were weighed (brain, lungs, heart, liver, spleen, stomach, kidneys and gonads), and the pathological changes were recorded. Then, these organs were fixed in 10% formalin, embedded in paraffin and sectioned for HE staining. Sections were observed under a light microscope. In addition, the ratio of organ weight to body weight was calculated.Sections were prepared as above mentioned, and the ion distribution was observed by scanning electron microscope (SEM, SU8220, HITACHI, Japan) equipped with an energy dispersive spectrometer (EDS).
2.6 Statistical analysis
Data are expressed as mean ± standard deviation (SD) and compared with t test between groups. Credibility of 95% was employed to determine the statistical significance.3 Results
3.1 The properties of akermanite extracts
The akermanite particles and the extracts of akermanite are shown in Fig. 1A and B, and it can be seen that the particles were white and the akermanite extracts were colorless and transparent. The ion concentrations of akermanite extracts and normal saline were shown in Table 1. The concentrations of Ca, Mg and Si ions of akermanite extracts were 60.20, 0.52 and 32.05 ppm, respectively, which were much higher than those of normal saline.| Ca | Mg | Si | |
|---|---|---|---|
| Akermanite extracts | 60.2 | 0.52 | 32.05 |
| Normal saline | 0.28 | 0.022 | 0.13 |
3.2 General observation and body weight of animals
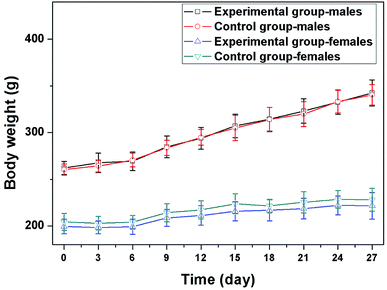
All the rats survived during the test, and no obvious toxic reactions were observed during the overall study period. The body weight was measured once every 3 days, and the results were displayed in Fig. 2. The body weight increased gradually in control group. In AK group, there was no loss of appetite, and the body weight also increased over time. There was no significant difference in the body weight between groups (P > 0.05).3.3 Blood cytology assay
At the end of study, rats were sacrificed and the blood was collected from the heart for cytological examination. It can be seen that there were no obvious reductions in the HCG, RBC, PLT, WBC, WBC subsets and hematocrit after injection. In addition, the indexes of HCG, RBC, PLT, WBC, WBC subsets and hematocrit did show significant difference between the two groups (P > 0.05) (Table 2).| Control group | AKE group | ||||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| WBC | 109 L−1 | 5.77 ± 2.55 | 4.61 ± 1.43 | 5.10 ± 1.43 | 3.65 ± 1.70 |
| RBC | 1012 L−1 | 7.60 ± 0.44 | 6.41 ± 0.57 | 7.02 ± 0.89 | 6.44 ± 0.54 |
| HGB | g L−1 | 145.14 ± 9.14 | 126.17 ± 8.66 | 134.92 ± 10.49 | 127.29 ± 10.59 |
| HCT | % | 43.07 ± 2.70 | 35.87 ± 3.10 | 39.78 ± 5.17 | 36.21 ± 3.02 |
| MCV | fl | 56.69 ± 2.40 | 56.00 ± 0.61 | 56.64 ± 1.50 | 56.19 ± 1.66 |
| PLT | 109 L−1 | 1218.57 ± 224.37 | 798.50 ± 558.42 | 916.00 ± 468.88 | 482.86 ± 502.42 |
| LYMP | % | 64.61 ± 5.95 | 69.02 ± 5.95 | 63.00 ± 10.66 | 73.67 ± 11.73 |
| MONO | % | 14.47 ± 4.41 | 16.28 ± 3.69 | 18.71 ± 8.35 | 12.50 ± 6.40 |
| NEUT | % | 19.47 ± 6.15 | 11.78 ± 2.39 | 16.52 ± 5.46 | 12.10 ± 5.93 |
| EO | % | 1.39 ± 1.45 | 2.28 ± 2.00 | 1.64 ± 1.39 | 2.04 ± 1.41 |
3.4 Serum biochemistry assay
At the end of study, rats were sacrificed and the blood was collected from the heart for biochemical examination. There were no significant increases in the AST, ALT, AKP, TP, ALB, A/G, GLB, BUN, CRE, UREA, blood lipids, GLU, K, Na, Cl and Ca. In addition, significant differences in these parameters were not seen between groups (P > 0.05) (Table 3).| Control group | AKE group | ||||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| AST | U L−1 | 222.5 ± 99.3 | 211.3 ± 148.7 | 183.0 ± 55.7 | 160.9 ± 39.2 |
| ALT | U L−1 | 48.9 ± 9.4 | 73.5 ± 78.4 | 53.2 ± 17.8 | 43.0 ± 14.8 |
| TP | g L−1 | 62.9 ± 5.3 | 67.3 ± 4.9 | 62.1 ± 3.8 | 66.7 ± 4.2 |
| ALB | g L−1 | 37.9 ± 2.6 | 40.4 ± 4.1 | 38.8 ± 2.7 | 41.2 ± 2.4 |
| A/G | % | 1.5 ± 0.2 | 1.5 ± 0.2 | 1.7 ± 0.1 | 1.6 ± 0.2 |
| GLOB | g L−1 | 25.0 ± 3.9 | 26.9 ± 1.9 | 23.3 ± 1.7 | 25.5 ± 3.3 |
| AKP | U L−1 | 263.1 ± 66.2 | 181.4 ± 70.3 | 280.8 ± 63.7 | 154.3 ± 47.5 |
| BUN | mmol L−1 | 6.3 ± 0.6 | 5.5 ± 0.8 | 6.8 ± 0.5 | 6.2 ± 0.9 |
| CREA | mmol L−1 | 15.8 ± 7.4 | 23.1 ± 3.8 | 14.5 ± 9.2 | 21.9 ± 2.2 |
| URCA | mmol L−1 | 325.4 ± 108.4 | 248.5 ± 75.4 | 303.5 ± 96.6 | 251.9 ± 140.9 |
| GLU | mmol L−1 | 13.5 ± 4.5 | 8.7 ± 2.0 | 13.1 ± 4.3 | 10.9 ± 5.9 |
| CHOL | mmol L−1 | 2.2 ± 0.3 | 2.2 ± 0.4 | 1.9 ± 0.2 | 2.1 ± 0.2 |
| TRIG | mmol L−1 | 1.4 ± 0.3 | 0.8 ± 0.1 | 1.8 ± 0.8 | 0.9 ± 0.3 |
| K | mmol L−1 | 9.6 ± 1.9 | 7.9 ± 1.6 | 9.7 ± 3.3 | 7.7 ± 1.8 |
| NA | mmol L−1 | 142.2 ± 2.2 | 141.9 ± 1.6 | 143.2 ± 3.5 | 143.3 ± 3.0 |
| CL | mmol L−1 | 100.8 ± 2.1 | 101.3 ± 1.3 | 101.7 ± 2.1 | 103.3 ± 1.6 |
3.5 Organ to body weight ratios
After 28 days, rats were sacrificed, and the brain, lungs, heart, liver, spleen, stomach, kidneys and gonads were harvested for pathological examination. Macroscopic examination showed no congestion, hemorrhage, edema and necrosis. Each organ was weighed and the organ to body weight ratio was calculated. As shown in Table 4, there was no significant difference in the organ to body weight ratio between two groups (P > 0.05).| Control group | AKE group | ||||
|---|---|---|---|---|---|
| Male | Female | Male | Female | ||
| Liver/body | % | 3.25 ± 0.22 | 3.03 ± 0.23 | 3.46 ± 0.31 | 3.23 ± 0.23 |
| Spleen/body | % | 0.28 ± 0.029 | 0.31 ± 0.021 | 0.32 ± 0.028 | 0.33 ± 0.026 |
| Kidney/body | % | 0.65 ± 0.036 | 0.72 ± 0.051 | 0.68 ± 0.041 | 0.73 ± 0.032 |
| Gonad/body | % | 0.95 ± 0.002 | 0.036 ± 0.0081 | 0.96 ± 0.021 | 0.039 ± 0.016 |
| Brain/body | % | 0.49 ± 0.16 | 0.68 ± 0.21 | 0.52 ± 0.16 | 0.66 ± 0.24 |
| Heart/body | % | 0.46 ± 0.23 | 0.48 ± 0.26 | 0.48 ± 0.31 | 0.49 ± 0.32 |
| Lung/body | % | 0.42 ± 0.28 | 0.52 ± 0.18 | 0.46 ± 0.21 | 0.56 ± 0.13 |
| Stomach/body | % | 0.53 ± 0.11 | 0.56 ± 0.28 | 0.59 ± 0.21 | 0.58 ± 0.31 |
3.6 Pathological examinations
The main organs of the rats, including liver, renal and heart, were collected after their sacrifice and fixed in 10% formalin, and then were processed for HE staining. The results of pathological examinations of the organs were presented in Fig. 3. In AKE group and control group, pathological changes were not observed in all main organs such as liver, renal, and heart. In both groups, liver capsule was complete, the hepatic lobules displayed normal structure, hepatocytes had normal morphology, and there were no aggregation of inflammatory cells and cell necrosis (Fig. 3A and B). The renal capsule was complete, and the structure of renal cortex and medulla was normal (Fig. 3C and D). The heart structure was complete, and cardiomyocytes displayed continuous and regular arrangement. The nucleus was clear and identifiable, and there were no cell congestion, edema and necrosis (Fig. 3E and F).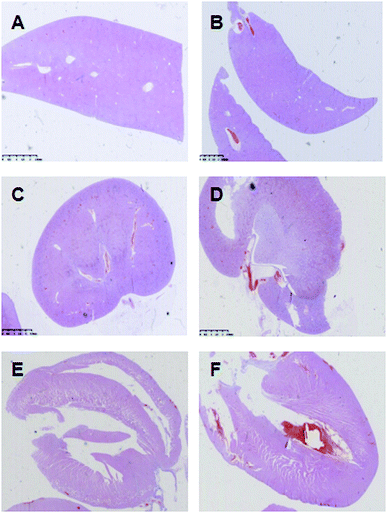 | ||
| Fig. 3 Pathological result of liver (A and B), renal (C and D) and heart (E and F) of the control group (A, C and E) and the AKE group (B, D and F) of rats as observed by HE staining. | ||
The elemental distribution of Ca, Mg and Si in the organs of rats in AKE group and control group are shown in Fig. 4. At the end of the study (28 days), Ca, Mg and Si were not found in the heart, liver and kidney of rats in both groups.
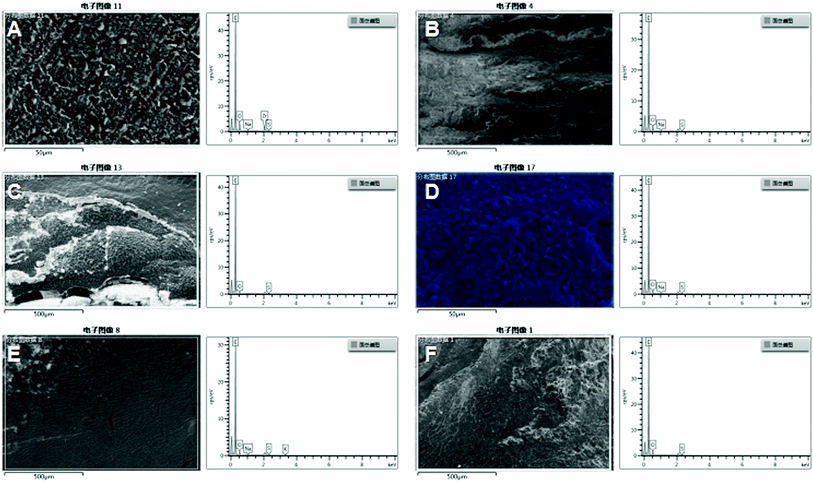 | ||
| Fig. 4 The elemental concentrations of Ca, Mg and Si in the organs of rats including liver, renal, and heart of the control group (A, C, and E) and the AKE group (B, D, and F). | ||
4 Discussion
The implants that are to be used for bone repair should have favorable biocompatibility and safety. In this sense, the metabolic products of an implanted biodegradable biomaterial should be safely excreted from the human body and should not be accumulated in organs and tissues, which is important to the avoidance of the toxic reactions of the body to implants.A variety of studies have shown that akermanite bioceramics can release some ions (such as Si and Mg) to promote the bone regeneration during its degradation in vivo.24,25 However, until now little is known about the subchronic systemic toxicity of akermanite bioceramics, including vital signs, blood cytology, serum biochemistry, pathological examination of organs, making the biocompatibility of the material still not well illuminated. ISO 10993-11:2017 is a well-established standard with authority that provides the instruction for the evaluation of subchronic systemic toxicity of medical devices, including bone implants. Therefore, in this study, for the first time the subchronic systemic toxicity of akermanite bioceramics according to ISO 10993-11:2017 was systemically investigated in rats, which may provide evidence on the biological safety of akermanite bioceramic.
In general, all the rats survived throughout the study, and of specially interest, the ionic degradation product of akermanite bioceramic did not cause any significant adverse effect on the main cellular components of blood. According to previous studies, it has been reported that overloading of Si and Mg in blood at a high concentration may cause damage to RBC, WBC, PLT and HCG and inhibit the proliferation of these cells, resulting in the reduction in RBC, WBC, WBC subsets, including hematocrit and HCG, which may lead to tissue hypoxia, low immune status and functional disorder of body.27,28 In present study, it was found that the extract of akermanite bioceramics did not cause any significant influence on the features of RBC, WBC, PLT and HCG, as there were no remarkable difference between the groups. These findings indicate that the Si and Mg released by akermanite bioceramics did not affect the metabolism of cells in the blood, indicating that the degradation of the material would not cause significant toxicity to blood cells. It is reasonable to believe that the good compatibility of akermanite bioceramic could be attributed to the fact that the ionic concentration of its degradation product was relatively low, which was well tolerated by the blood cells.
In some previous studies it has been demonstrated that Si and Mg in daily intake can be effectively metabolized in the liver and expelled through the stool and urine. However, overdose Si or Mg through circulation may cause damage to the liver and kidney.34,35 Namely, it may cause hepatocyte necrosis and rupture, leading to the release of AST, ALT and ALP into the blood, which cause increases in blood enzymes. In the present study, it was found that the injection of the extract of akermanite bioceramic by peritoneal injection did not cause significant increases in the ALT, AST, ALP, urea and serum creatinine (parameters related to kidney function), suggesting that the Si and Mg released from akermanite bioceramic were well below the toxic dose, and thus can be safely and effectively metabolized in vivo and would not cause toxicity to the liver and kidney.
Once a biodegradable bone graft is implanted, in common sense there will be concern raising over the potential accumulation of its degradation product in organs, which may have toxic effects. In the present study, important organs including the heart, live and kidney were both macroscopically and microscopically examined after injection of the extract of akermanite bioceramics, and pathological phenomenon such as congestion, hemorrhage, hyperplasia, edema and necrosis were not observed in these organs. These results suggest that ionic degradation products did not cause damage to important organs. In addition, Si and Mg were not detectable in liver and kidney after 28 days of continuous injection of the extract of akermanite bioceramic. Considering the fact that liver and kidney are known to be responsible for the metabolism of Si and Mg, it is reasonable to believe that the metabolic products of akermanite are normally metabolized without either accumulating or causing damage to these organs.
Taken together, the results of the present study demonstrated that the ionic degradation products of akermanite bioceramic did not cause any adverse effect on structure and function of the blood cells and all important organs, and were safely metabolized by the body. According to the instruction of the ISO standard related to the evaluation of the subchronic systemic toxicity of an implant, we believe that the results may provide strengthened evidence that akermanite bioceramics possess good biosafety in terms of subchronic systemic toxicity. The results of the present study, together with those of previous studies that have demonstrated the outstanding osteogenetic bioactivity of akermanite bioceramic, would further confirm that the material would be a bioactive bone graft material with desirable biosafety for orthopedic applications.
5 Conclusions
In the present study, the subchronic systemic toxicity of the ionic degradation products of akermanite bioceramic was investigated according to the instruction of ISO standard. It was found that the injection of the ionic extract of akermanite bioceramics did not induce any significant adverse effect on the numbers and function of main blood cells. In addition, no pathological change was observed in all important organs of the animals after injection of the extracts, and the functions of the liver and kidney as the main metabolizing organs were not affected. These results demonstrated that akermanite bioceramic did not have any subchronic systemic toxicity, and considering its well-accepted osteogenic bioactivity, showed great potential as bioactive bone graft materials for orthopedic applications.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors wish to express their thanks to National Key R&D Program of China (No. 2018YFB1105600), the National Natural Science Foundation of China (No. 81671830), Medical Project of Suzhou (No. Szxk201802), and Science Development Plan of Suzhou (No: SZS201720) for financial support.References
- J. Breeze, J. Patel, M. S. Dover and R. W. Williams, Br. J. Oral Maxillofac. Surg., 2017, 55, 830–833 CrossRef CAS PubMed.
- M. K. Sen and T. Miclau, Injury, 2007, 38, S75–S80 CrossRef PubMed.
- A. Sakkas, F. Wilde, M. Heufelder, K. Winter and A. Schramm, Int. J. Implant Dent., 2017, 3, 23 CrossRef PubMed.
- A. Sakkas, A. Schramm, K. Winter and F. Wilde, Risk factors for post-operative complications after procedures for autologous bone augmentation from different donor sites, J. Cranio Maxill. Surg., 2018, 46, 312–322 CrossRef PubMed.
- R. Windhager, G. M. Hobusch and M. Matzner, Orthopade, 2017, 46, 656–664 CrossRef CAS PubMed.
- S. V. Dorozhkin, Biomatter, 2011, 1(1), 3–56 CrossRef PubMed.
- H. P. Stallmann, C. Faber, A. L. Bronckers, A. V. Nieuw Amerongen and P. I. Wuisman, J. Antimicrob. Chemother., 2004, 54, 472–476 CrossRef CAS PubMed.
- A. Matsumine, K. Takegami, K. Asanuma, T. Matsubara, Y. Nakamura, A. Uchida and A. Sudo, Int. J. Clin. Oncol., 2011, 16, 101–108 CrossRef PubMed.
- K. Na, S. W. Kim, B. K. Sun, B. G. Woo, H. N. Yang, H. M. Chung and K. H. Park, Biomaterials, 2007, 28, 2631–2637 CrossRef CAS PubMed.
- Y. Mizushima, T. Ikoma, J. Tanaka, K. Hoshi, T. Ishihara, Y. Ogawa and A. Ueno, J. Controlled Release, 2006, 110, 260–265 CrossRef CAS PubMed.
- D. Correa, A. Almirall, R. García-Carrodeguas, L. A. dos Santos, A. H. De Aza, J. Parra and J. A. Delgado, J. Biomed. Mater. Res., Part A, 2014, 102, 3693–3703 CrossRef PubMed.
- X. Y. Yang, M. Liu, Y. Zhao, H. Y. Jia, S. Z. Xu, X. G. Li, X. Y. Chen, F. Zhang, C. Y. Gao and Z. R. Gou, J. Mater. Chem. B, 2014, 2, 3830–3838 RSC.
- A. A. Eid, K. A. Hussein, L. N. Niu, G. H. Li, I. Watanabe, M. Al-Shabrawey, D. H. Pashley and F. R. Tay, Acta Biomater., 2014, 10, 3327–3334 CrossRef CAS PubMed.
- J. Lu, J. Wei, Y. Yan, H. Li, J. Jia, S. Wei, H. Guo, T. Xiao and C. Liu, J. Mater. Sci.: Mater. Med., 2011, 22, 607–615 CrossRef CAS PubMed.
- K. L. Lin, L. G. Xia, H. Y. Li, H. B. Pan, Y. Xu, W. W. Lu, Z. Zhang and J. Chang, Biomaterials, 2013, 34, 10028–10042 CrossRef CAS PubMed.
- C. Wang, K. L. Lin and J. Chang, Biomaterials, 2013, 34, 64–77 CrossRef CAS PubMed.
- S. F. Xu, K. L. Lin, Z. Wang, J. Chang, L. Wang, J. X. Lu and C. Q. Ning, Biomaterials, 2008, 29, 2588–2596 CrossRef CAS PubMed.
- C. T. Wu and J. Chang, J. Biomater. Appl., 2006, 21, 119–129 CrossRef CAS PubMed.
- C. T. Wu and J. Chang, J. Biomed. Mater. Res., Part B, 2007, 83, 153–160 CrossRef PubMed.
- H. L. Sun, C. T. Wu, K. R. Dai, J. Chang and T. T. Tang, Biomaterials, 2006, 27, 5651–5657 CrossRef CAS PubMed.
- Q. H. Liu, L. Cen, S. Yin, L. Chen, G. P. Liu, J. Chang and L. Cui, Biomaterials, 2008, 29, 4792–4799 CrossRef CAS PubMed.
- Y. Huang, X. G. Jin, X. L. Zhang, H. L. Sun, J. W. Tu, T. T. Tang, J. Chang and K. R. Dai, Biomaterials, 2009, 30, 5041–5048 CrossRef CAS PubMed.
- M. Diba, O. M. Goudouri, F. Tapia and A. R. Boccaccini, Curr. Opin. Solid State Mater. Sci., 2014, 18, 147–167 CrossRef CAS.
- C. T. Wu, J. Chang, S. Y. Ni and J. Y. Wang, J. Biomed. Mater. Res., Part A, 2006, 76, 73–80 CrossRef PubMed.
- W. Y. Zhai, H. X. Lu, L. Chen, X. T. Lin, Y. Huang, K. R. Dai, K. Naoki, G. P. Chen and J. Chang, Acta Biomater., 2012, 8, 341–349 CrossRef CAS PubMed.
- Y. Huang, X. G. Jin, X. L. Zhang, H. L. Sun, J. W. Tu, T. T. Tang, J. Chang and K. R. Dai, Biomaterials, 2009, 30, 5041–5048 CrossRef CAS PubMed.
- M. Y. Shie, S. J. Ding and H. C. Chang, Acta Biomater., 2011, 7, 2604–2614 CrossRef CAS PubMed.
- T. Yu, D. Hubbard, A. Ray and H. Ghandehari, J. Controlled Release, 2011, 163, 46–54 CrossRef PubMed.
- R. Kumar, I. Roy, T. Y. Ohulchanskky, L. A. Vathy, E. J. Bergey, M. Sajjad and P. N. Prasad, ACS Nano, 2010, 4, 699–708 CrossRef CAS PubMed.
- H. J. Eom and J. Choi, Toxicol. In Vitro, 2009, 23, 1326–1332 CrossRef CAS PubMed.
- J. S. Kim, T. J. Yoon, K. N. Yu, B. G. Kim, S. J. Park, H. W. Kim, K. H. Lee, S. B. Park, J. K. Lee and M. H. Cho, Toxicol. Sci., 2005, 89, 338–347 CrossRef PubMed.
- M. Amin, E. M. Ewais, Y. M. Ahmed, E. A. Ashor, U. Hess and K. Rezwan, Bioceram. Dev. Appl., 2016, 6(1), 088 Search PubMed.
- E. M. Ewais, A. Moustafa, K. Pardun and K. Rezwan, J. Mater. Sci., 2015, 5, 21–36 CAS.
- A. Nemmar, P. Yuvaraju, S. Beegam, J. Yasin, E. E. Kazzam and B. H. Ali, Int. J. Nanomed., 2016, 11, 919–927 CrossRef CAS PubMed.
- R. Swaminathan, Clin. Biochem. Rev., 2003, 24, 47–66 CAS.
Footnote |
| † The two authors contributed to the work equally. |
| This journal is © The Royal Society of Chemistry 2019 |