Open Access Article
Open Access ArticleA cascade reaction-based switch-on fluorescent sensor for Ce(IV) ions in real samples†
Xiaojing Laia,
Ruixiang Wang*a,
Jinhui Lia,
Guanyinsheng Qiu *b and
Jin-Biao Liu
*b and
Jin-Biao Liu *a
*a
aSchool of Metallurgy and Chemical Engineering, Jiangxi University of Science and Technology, 86 Hongqi Road, Ganzhou 341000, China. E-mail: liujinbiao@jxust.edu.cn; wrx9022@163.com
bCollege of Biological, Chemical Science and Engineering, Jiaxing University, 118 Jiahang Road, Jiaxing 314001, China. E-mail: 11110220028@fudan.edu.cn
First published on 16th July 2019
Abstract
We herein report a cascade reaction-based switch-on fluorescence sensor for Ce(IV) ions. The mechanism is based on the ability of Ce(IV) ions to oxidize β-naphthol, which condenses with o-phenylenediamine to generate fluorescent benzo[a]phenazine. The sensor achieved a 200-fold fluorescence enhancement and was successfully applied to real sample detection.
Cerium (Ce) is the most abundant rare-Earth element and has been widely used as polishing powders, fluorescent powders, magnets, catalysts and a ceramic colorant in industry.1,2 Although Ce(III) is the most abundant type of cerium in the Earth, Ce(IV) can be more easily extracted and separated from cerium fluorocarbonate mineral. Furthermore, the oxidation processes to convert Ce(III) to Ce(IV) during the post-treatment stage further increases the exposure of Ce(IV) to the community.3–5 High concentration of Ce is known to be an environmental hazard and to have acute toxicity to seeds.6,7 Prolonged exposure to Ce(IV) ions will cause malfunctions in the human body, particularly with the circulation systems, immune systems and central nervous systems.8 Therefore, accurate and rapid determination of Ce(IV) ion is of significant interest for both environmental and clinical applications.
Over the centuries, instrument-based methods have been employed for detection of the Ce(IV) ion, such as inductively coupled plasma atomic emission spectroscopy (ICP-AES), inductively coupled plasma mass spectrometry (ICP-MS), radiochemical neutron activation, and electrochemical methods.9,10 However, these methods generally have drawbacks including high cost, long processing time, complicated sample preparation and instrumental complexity. Meanwhile, fluorescent sensors are powerful tools for detecting environmentally and biologically significant analytes in the presence of interfering matrices, by virtue of their simplicity and high sensitivity.11–20 To date, a wide variety of fluorescent sensors have been developed for monitoring Ce ions.21–25 However, these methods are typically limited by the uses of toxic inorganic nanomaterials, low sensitivity and low selectivity. Therefore, the development of a fast, sensitive and efficient sensing platform for Ce(IV) ions is highly desirable.
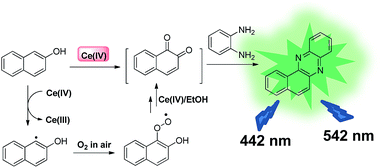
Over the past years, fluorescence properties of benzo[a]phenazine and its derivatives have received considerable attention in organic synthesis, materials and analytical chemistry.26–30 Inspired by their promising properties, we herein developed a novel Ce(IV) probe based on Ce(IV)-promoted oxidation/condensation/cyclization to generate highly fluorescent benzo[a]phenazine. It is noteworthy that the Ce(IV) ion has been extensively studied as an oxidant in various chemical reactions.31 In this work, the Ce(IV) ion is used as a single-electron oxidant to facilitate the reaction with OPD, followed by the generation of a fluorescent product.32 In the presence of Ce(IV) ions, BNO is oxidized into naphthalene-1,2-dione and then condensed with o-phenylenediamine (OPD) to form fluorescent benzo[a]phenazine (Scheme 1). To our knowledge, this is the first detection method using the Ce(IV) ion based on the Ce(IV)-mediated cascade reaction.
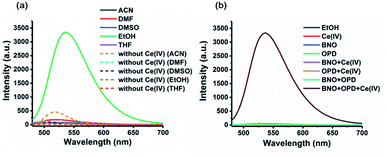
This sensing system is comprised of β-naphthol (BNO) and o-phenylenediamine (OPD). First, we investigated the fluorescence changes of a mixture of BNO and OPD in organic solvent in the presence of Ce(IV) ions. To study solvent effects, a series of organic solvents, including acetonitrile (ACN), dimethylformamide (DMF), dimethyl sulfoxide (DMSO), ethanol (EtOH), and tetrahydrofuran (THF) were used. BNO (50 μM) was first mixed with Ce(IV) (120 μM), followed by adding OPD (40 μM) for 3 h at room temperature (rt). The result showed that a strong fluorescence enhancement was observed in EtOH (Fig. 1a).
A series of control experiments were performed to study the role of different components in this sensing system. The results showed that no fluorescence enhancement was found in the absence of any of the components (Fig. 1b). Moreover, UV-vis absorption spectra exhibited a strong absorption band between 300 nm and 400 nm, but only when all components were present (Fig. S1†). Furthermore, when the reaction was carried out at millimolar levels in the detection system, the final product benzo[a]phenazine was obtained with structure confirmed by 1H/13C NMR and melting point (Fig. S2†). To verify the reaction mechanism, the naphthalene-1,2-dione intermediate also was isolated and characterized by NMR (Fig. S3†). Therefore, the proposed method is capable of sensing Ce(IV) ions.
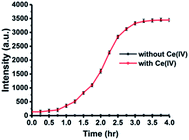
Considering the importance of incubation time in a sensing system, we next investigated the change of fluorescence intensity against different incubation times. In the presence of Ce(IV) ions (120 μM), the fluorescence intensity of the system increased with longer incubation time, reaching a steady state at 3 h. In contrast, only negligible fluorescence enhancement was observed in the absence of Ce(IV) ions. Therefore, 3 h was chosen as the optimized incubation time for subsequent experiments (Fig. 2).
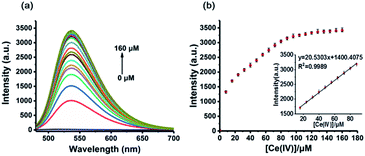
The sensitivity of an analytical method is very important for its application. So, we examined fluorescence responses of our method to various concentrations of Ce(IV) ions. The system containing BNO (50 μM) was incubated with indicated concentrations of Ce(IV) ions at rt for 1 h and followed by adding OPD (40 μM) for 3 h. As shown in Fig. 3a, the fluorescence intensity of the system increased with the concentration of Ce(IV) (8–160 μM). Upon the addition of Ce(IV) ions, the sensor displayed more than a 200-fold fluorescence enhancement. A good linear relationship was established in a range of 16–88 μM, and the limit of detection (LOD) was calculated as 1.23 μM, according to LOD = 3σ/s.
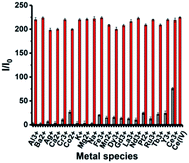
To verify the good selectivity of this method, a range of common metal ions (Al3+, Ba2+, Ag+, Ca2+, Cr3+, Co2+, K+, Mg2+, Na+, Fe3+, Mn2+) and rare-Earth metal ions (Dy3+, Gd3+, La3+, Nd3+, Tb3+, Y3+, Ce3+) were investigated. The results showed that only Ce(IV) ions triggered a significant fluorescence enhancement, and encouragingly even Ce3+ only slightly enhanced the fluorescence of the system (Fig. 4). However, other common and rare-Earth metal ions exhibited low fluorescence enhancement. To demonstrate the specificity of the sensor to Ce(IV) ions, a competition experiment was carried out by adding Ce(IV) ions (120 μM) to a system having the same concentration of other cations. No significant fluorescence change was observed, indicating that this reaction-based method possess great potential to detect Ce(IV) ions even in the presence of other common interfering cations. These results demonstrated high selectivity of this method towards the Ce(IV) ion, which can be attributed to the special single-electron oxidation property of the Ce(IV) ion.
Encouraged by the excellent sensitivity and selectivity of this sensing system, we next investigated its ability to detect Ce(IV) ions in real samples. First, a water sample from the Ganjing River (pH = 7.9) was collected and used in this work. Upon the addition of a water sample containing Ce(IV) ions (16 μM) into the sensing system, an obvious fluorescence enhancement was observed with a RSD of 0.92% (Fig. 5a). Moreover, we further explored its application to the detection of Ce(IV) ions in a lateritic soil sample collected from Ganzhou (pH = 5.4). The samples were pre-treated according to a reported method.33 The results showed that Ce(IV) ions (24 μM) could induce a significant luminescence enhancement with a RSD of 0.93% (Fig. 5b). Taken together, we have successfully demonstrated the applicability of this sensing system to detect Ce(IV) ions in the presence of those complex matrices.
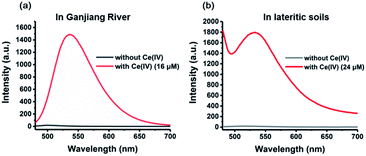 | ||
| Fig. 5 Emission spectra of the sensing system with and without Ce(IV) ions in (a) a water sample from the Ganjiang River and (b) lateritic soil samples. | ||
Conclusions
In this work, we report a switch-on fluorescence system for the detection of Ce(IV) ions. A cascade reaction-based platform is based on the ability of Ce(IV) to oxidize BNO, followed by condensation/cyclization with OPD to form fluorescent benzo[a]phenazine. After optimization, the probe exhibited a linear range of 8–160 μM, with a limit of detection of 1.23 μM at a signal-to-noise ratio (S/N) of 3. The system selectively detected Ce(IV) ions over 18 cations and showed a more than 200-fold enhancement of the fluorescence signal. We also successfully demonstrated its applicability for the detection of Ce(IV) ions in real samples, including river water from the Ganjiang River and lateritic soil from Ganzhou. It is anticipated that further optimization could lead to the development of an improved sensor with great potential for the in situ monitoring of Ce(IV) ions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Natural Science Foundation of China (No. 21762018 and 51564021), and the Program of Qingjiang Excellent Young Talents of Jiangxi University of Science and Technology.Notes and references
- M. R. Abedi, H. A. Zamani, M. R. Ganjali and P. Norouzi, Int. J. Environ. Anal. Chem., 2008, 88, 353–362 CrossRef CAS.
- J. B. Hedrick and S. P. Sinha, J. Alloys Compd., 1994, 207–208, 377–382 CrossRef CAS.
- F. L. Moore, Anal. Chem., 1969, 41, 1658–1661 CrossRef CAS.
- L. Xinghua, X. HUANG, Z. Zhaowu, L. Zhiqi and L. Ying, J. Rare Earths, 2009, 27, 119–122 CrossRef.
- D. Zhang, W. Wang, Y. Deng, J. Zhang, H. Zhao and J. Chen, Chem. Eng. J., 2012, 179, 19–25 CrossRef CAS.
- A. García, R. Espinosa, L. Delgado, E. Casals, E. González, V. Puntes, C. Barata, X. Font and A. Sánchez, Desalination, 2011, 269, 136–141 CrossRef.
- F. R. Cassee, E. C. van Balen, C. Singh, D. Green, H. Muijser, J. Weinstein and K. Dreher, Crit. Rev. Toxicol., 2011, 41, 213–229 CrossRef.
- W. Cheng, S. S. Park, W. Tang, B. S. You and B. H. Koo, J. Rare Earths, 2010, 28, 785–789 CrossRef CAS.
- Y. Hamajima, M. Koba, K. Endo and H. Nakahara, J. Radioanal. Nucl. Chem., 1985, 89, 315–321 CrossRef CAS.
- V. K. Gupta, A. K. Singh and B. Gupta, Anal. Chim. Acta, 2006, 575, 198–204 CrossRef CAS PubMed.
- M. H. Lee, J. S. Kim and J. L. Sessler, Chem. Soc. Rev., 2015, 44, 4185–4191 RSC.
- T. Ueno and T. Nagano, Nat. Methods, 2011, 8, 642–645 CrossRef CAS.
- R. Wang, X. Lai, G. Qiu and J.-B. Liu, Chin. J. Org. Chem., 2019, 39, 952–960 CrossRef.
- S.-L. Yao, S.-J. Liu, X.-M. Tian, T.-F. Zheng, C. Cao, C.-Y. Niu, Y.-Q. Chen, J.-L. Chen, H. Huang and H.-R. Wen, Inorg. Chem., 2019, 58, 3578–3581 CrossRef CAS.
- C. Wu, K. J. Wu, T. S. Kang, H. D. Wang, C. H. Leung, J. B. Liu and D. L. Ma, Sci. Rep., 2018, 8, 12467 CrossRef.
- C. Wu, J.-B. Liu, G. Li, S.-Y. Wong, Q.-B. Han, C.-H. Leung and D.-L. Ma, Dyes Pigm., 2018, 159, 479–482 CrossRef CAS.
- C. Wu, K. Vellaisamy, G. Yang, Z. Z. Dong, C. H. Leung, J. B. Liu and D. L. Ma, Dalton Trans., 2017, 46, 6677–6682 RSC.
- C. Wu, G. Li, Q. B. Han, R. J. Pei, J. B. Liu, D. L. Ma and C. H. Leung, Dalton Trans., 2017, 46, 17074–17079 RSC.
- J.-B. Liu, C. Yang, C.-N. Ko, K. Vellaisamy, B. Yang, M.-Y. Lee, C.-H. Leung and D.-L. Ma, Sens. Actuators, B, 2017, 243, 971–976 CrossRef CAS.
- C. N. Ko, C. Wu, G. Li, C. H. Leung, J. B. Liu and D. L. Ma, Anal. Chim. Acta, 2017, 984, 193–201 CrossRef CAS.
- F. Salehnia, F. Faridbod, A. S. Dezfuli, M. R. Ganjali and P. Norouzi, J. Fluoresc., 2017, 27, 331–338 CrossRef CAS.
- M. K. Rofouei, N. Tajarrod, M. Masteri-Farahani and R. Zadmard, J. Fluoresc., 2015, 25, 1855–1866 CrossRef CAS.
- H. Cui, Q. Zhang, A. Myint, X. Ge and L. Liu, J. Photochem. Photobiol., A, 2006, 181, 238–245 CrossRef CAS.
- Y. Wang, F. Yan, D. Kong, F. Zu, Z. Bai, J. Xu and L. Chen, Desalin. Water Treat., 2018, 107, 147–154 CrossRef CAS.
- P. Ghosh, A. D. Shukla and A. Das, Tetrahedron Lett., 2002, 43, 7419–7422 CrossRef CAS.
- P. Saluja, A. Chaudhary and J. M. Khurana, Tetrahedron Lett., 2014, 55, 3431–3435 CrossRef CAS.
- R. Sivakumar, S. Naveenraj and S. Anandan, J. Lumin., 2011, 131, 2195–2201 CrossRef CAS.
- C. E. M. Carvalho, I. M. Brinn, A. V. Pinto and M. d. C. F. R. Pinto, J. Photochem. Photobiol., A, 2000, 136, 25–33 CrossRef CAS.
- P. Singh, A. Baheti and K. R. J. Thomas, J. Org. Chem., 2011, 76, 6134–6145 CrossRef CAS.
- J. M. Khurana, A. Chaudhary, A. Lumb and B. Nand, Green Chem., 2012, 14, 2321–2327 RSC.
- A. K. Das, Coord. Chem. Rev., 2001, 213, 307–325 CrossRef CAS.
- V. Nair and A. Deepthi, Chem. Rev., 2007, 107, 1862–1891 CrossRef CAS.
- M. R. Abedi, H. A. Zamani, M. R. Ganjali and P. Norouzi, Int. J. Environ. Anal. Chem., 2008, 88, 353–362 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ra03776d |
| This journal is © The Royal Society of Chemistry 2019 |