Open Access Article
Open Access ArticlePHB/PCL fibrous membranes modified with SiO2@TiO2-based core@shell composite nanoparticles for hydrophobic and antibacterial applications
Xinghuan Lina,
Shanshan Lia,
Joonhoo Jungb,
Wei Maa,
Lin Lia,
Xuehong Ren *a,
Yuyu Sun
*a,
Yuyu Sun b and
Tung-Shi Huangc
b and
Tung-Shi Huangc
aKey Laboratory of Eco-textiles of Ministry of Education, College of Textiles and Clothing, Jiangnan University, Wuxi, Jiangsu 214122, China
bDepartment of Chemistry, University of Massachusetts Lowell, Lowell, 01854, USA
cDepartment of Poultry Science, Auburn 36849, AL, USA. E-mail: xhren@jiangnan.edu.cn
First published on 25th July 2019
Abstract
In order to prepare multifunctional fibrous membranes with hydrophobicity, antibacterial properties and UV resistance, we used silica and titanium dioxide for preparing SiO2@TiO2 nanoparticles (SiO2@TiO2 NPs) to create roughness on the fibrous membranes surfaces. The introduction of TiO2 was used for improving UV resistance. N-Halamine precursor and silane precursor were introduced to modify SiO2@TiO2 NPs to synthesize SiO2@TiO2-based core@shell composite nanoparticles. The hydrophobic antibacterial fibrous membranes were prepared by a dip-pad process of electrospun biodegradable polyhydroxybutyrate/poly-ε-caprolactone (PHB/PCL) with the synthesized SiO2@TiO2-based core@shell composite nanoparticles. TEM, SEM and FT-IR were used to characterize the synthesized SiO2@TiO2-based core@shell composite nanoparticles and the hydrophobic antibacterial fibrous membranes. The fibrous membranes not only showed excellent hydrophobicity with an average water contact angle of 144° ± 1°, but also appreciable air permeability. The chlorinated fibrous membranes could inactivate all S. aureus and E. coli O157:H7 after 5 min and 60 min of contact, respectively. In addition, the chlorinated fibrous membranes exhibited outstanding cell compatibility with 102.1% of cell viability. Therefore, the prepared hydrophobic antibacterial degradable fibrous membranes may have great potential application for packaging materials.
1 Introduction
Due to a heightened awareness of environmental issues, there is an increasing demand on biodegradable materials as potential replacements of traditional polymeric materials, such as polypropylene (PP), polyethylene (PE), and polystyrene (PS), to reduce waste accumulation.1–3 Furthermore, there is a growing demand for global healthcare issues in regard to microbial infections in a variety of areas such as food packing, medical devices, textile materials and biomedical materials.4–6 Therefore, the development of environmental-friendly alternative materials with efficient antibacterial properties is highly desirable.Due to their biodegradability and biocompatibility, biodegradable materials have been widely investigated concerning potential applications as food packaging and biomedical materials.7–9 However, the properties of the biodegradable materials for food packing and medical materials still show shortcomings, especially in respect to antibacterial properties. Therefore, there is an urgent need to impart antimicrobial performance to improve their range of uses. Recently, antimicrobial agents, such as inorganic metals and their oxides, organic macromolecules, including chitosan compounds and quaternary ammonium compounds, and N-halamine have been widely used.10–17 Among these, N-halamine materials have extensively drawn attention due to their excellent antibacterial efficacies, broad-spectrum sterilization and easy regenerability.18,19 Aside from antibacterial properties, high hydrophobicity of material surfaces is one of the most important characteristics for packaging materials. On one hand, materials applied for food packaging with excellent hydrophobicity can block the ingress of moisture and oxygen to slow down bacterial growth and food spoilage.20 On the other hand, materials with prominent hydrophobicity can exhibit a self-cleaning function and may improve their antimicrobial properties. At present, a salient hydrophobic surface is mainly prepared by building a nano-structured surface and reducing surface energy on the substrate.21
Nanomaterial due to its small size and large specific surface area has many physical and chemical properties.22–24 Electrospinning is a well-known and cost-effective technique to produce nanofibers from various polymers. The resulting electrospun nanofibers are useful in many applications such as biomedical, filter and energy related materials.25–27
Over the last few years, nanoparticles including inorganic particles, metal particles and others have been synthesized to prepare different shapes and sizes with outstanding properties.28,29 Subsequently, core@shell nanoparticles have attracted considerable attention for nanotechnology fields owing to chemical and colloidal stabilities, adjustable physical properties and controllability of interparticle interactions within assemblies.30,31
In our previous study,32,33 we found that synthesized N-halamine siloxane compounds had poor stability under UV irradiation, because its Si-alkyl group bond easily decomposed which in return led to decreased chlorine content. Therefore, in this study, we used TiO2 and SiO2 for building SiO2@TiO2 NPs, which SiO2 acted as a core for it is chemically inert, does not affect redox reactions at the core surface and can easily be modified with many other compounds,34 while TiO2 as a UV light protecting agent acted as the shell owing to its unique physical properties under UV light irradiation.16,35 To improve the hydrophobicity, we grafted the N-halamine precursors and silane precursors onto the SiO2@TiO2 NPs to build multilevel nanostructures. Polyhydroxybutyrate/poly-ε-caprolactone (PHB/PCL) served as a biodegradable substrate that could be electrospun into nanofibers. Subsequently, the synthesized N-halamine containing SiO2@TiO2-based core@shell composite nanoparticles were deposited onto these fibrous membranes and chlorinated. Results demonstrated that the obtained membranes exhibited remarkable antimicrobial activity against S. aureus and E. coli O157:H7, respectively, outstanding hydrophobicity and appreciable air permeability. Moreover, the chlorinated fibrous membranes displayed excellent UVA light and storage stability. Besides, the active chlorine of that chlorinated fibrous membranes could easily be recovered.
2 Results and discussion
2.1 Characterization of SiO2@TiO2-based core@shell composite nanoparticles
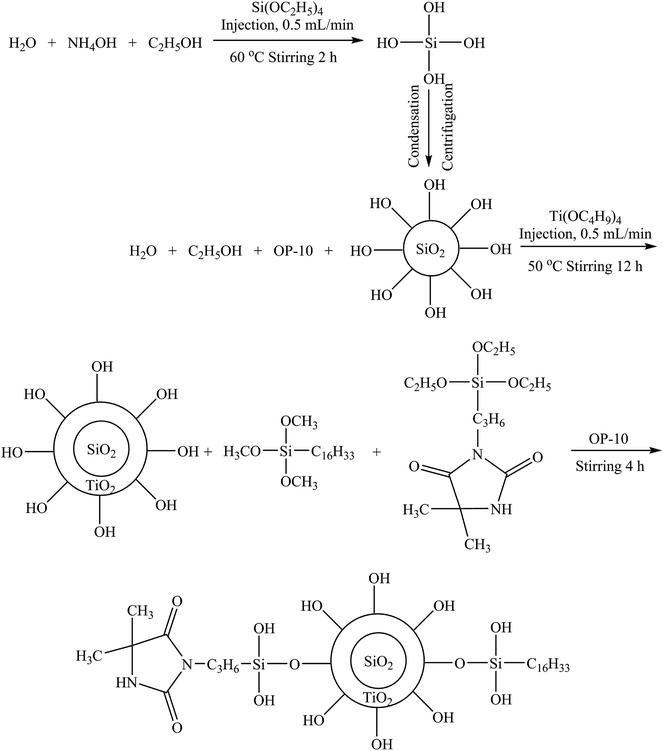
The surface morphologies and structures of the nanoparticles were observed by TEM and FT-IR and the results are shown in Fig. 1. It can be seen that the size of the prepared SiO2 NPs (Fig. 1A) was relatively uniform; the average diameter was determined to be 129.6 ± 10 nm, while the average diameter of SiO2@TiO2 NPs (Fig. 1B) was 274.5 ± 16 nm. It could be inferred that the thickness of shell (TiO2) was 60 ± 5 nm. Upon modification with HDTMS and SPH, the size of SiO2@TiO2-based core@shell composite nanoparticles (Fig. 1C) was not visibly changed, which might due to the fact that HDTMS and SPH are rather small compounds compared to SiO2@TiO2 NPs.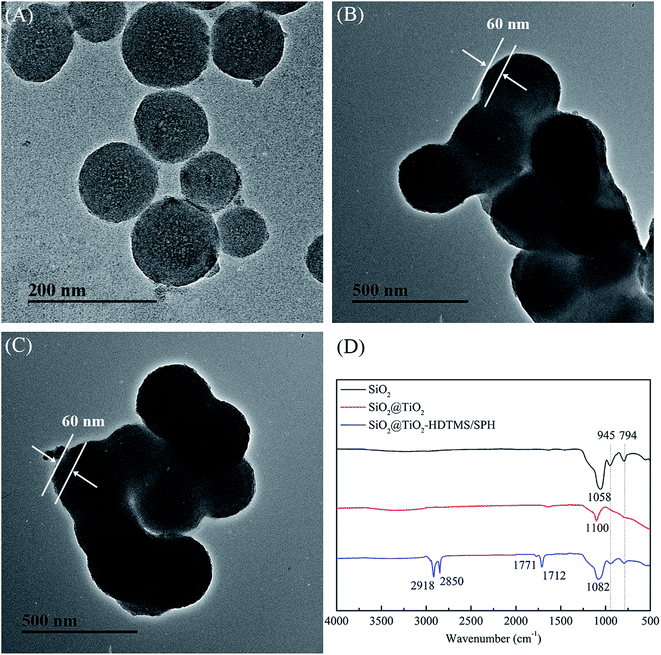 | ||
| Fig. 1 TEM images of (A) SiO2 NPs, (B) SiO2@TiO2 NPs and (C) SiO2@TiO2-based core@shell composite nanoparticles; (D) FT-IR spectra of nanoparticles. | ||
FT-IR spectra (Fig. 1D) show the bands at around 1058, 945 and 794 cm−1 owing to asymmetric stretching vibration of Si–O–Si, stretching vibration of Si–O–H, and a symmetric stretching vibration of Si–O–Si, respectively.36,37 The band at 1100 cm−1 in the spectra of SiO2@TiO2 NPs was assigned to the stretching vibration of Ti–O.16 In contrast to SiO2@TiO2 NPs, SiO2@TiO2-based core@shell composite nanoparticles had a few new bands at 2915 and 2850 cm−1 attributed to the –CH3 and –CH2– bending vibration of the HDTMS, while 1771 and 1712 cm−1 can be ascribed to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration of the SPH.38
O stretching vibration of the SPH.38
2.2 Characterization of composite fibrous membranes
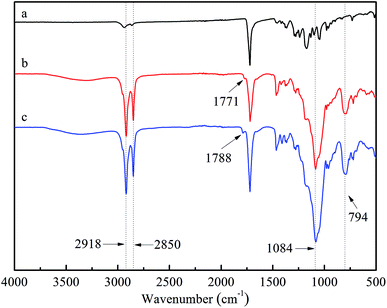
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, asymmetric stretching vibration of Si–O–Si and a symmetric stretching vibration of Si–O–Si on SiO2@TiO2-based core@shell composite nanoparticles, respectively.36,37 Besides, the intensities of –CH3 and –CH2– peak at 2918 and 2850 cm−1 in spectra (b) and (c) were obviously stronger than those in (a), which was mainly attributed to the long alkyl chains of HDTMS.38 After chlorination, the characteristic vibrational band of C
O, asymmetric stretching vibration of Si–O–Si and a symmetric stretching vibration of Si–O–Si on SiO2@TiO2-based core@shell composite nanoparticles, respectively.36,37 Besides, the intensities of –CH3 and –CH2– peak at 2918 and 2850 cm−1 in spectra (b) and (c) were obviously stronger than those in (a), which was mainly attributed to the long alkyl chains of HDTMS.38 After chlorination, the characteristic vibrational band of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O shifted from 1771 cm−1 to 1788 cm−1, which was ascribed to the transformation of N–H bond to N–Cl bond.39
O shifted from 1771 cm−1 to 1788 cm−1, which was ascribed to the transformation of N–H bond to N–Cl bond.39
2.3 Thermal analysis
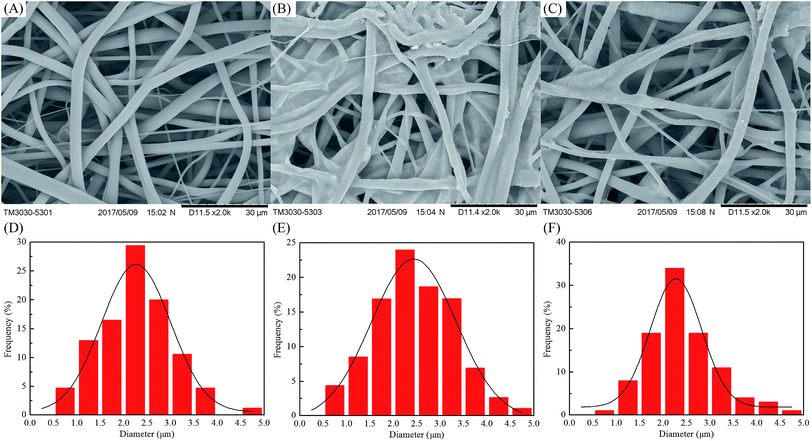
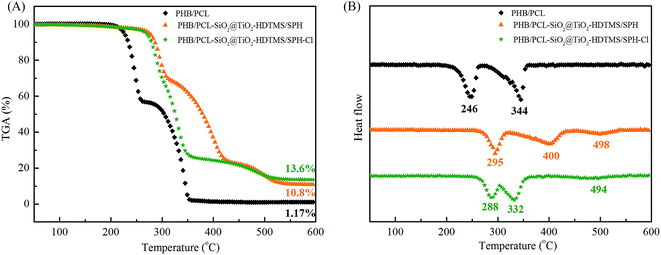
The TGA and DTG of fibrous membranes are shown in Fig. 4. There were two degradation steps that became apparent in the TGA curves for PHB/PCL fibrous membranes (Fig. 4A). PHB was the first to degrade with its weight loss temperature ranging from 250 to 300 °C.26 And PCL was the second to degrade with its weight loss temperature ranging from 350 to 450 °C. The main decomposition temperature of PHB and PCL of PHB/PCL–SiO2@TiO2–HDTMS/SPH fibrous membranes was higher than these in curve of PHB/PCL fibrous membranes, which might be due to cross-linking of SiO2@TiO2 NPs at the surface of PHB/PCL fibrous membranes. There was a new degradation step of PHB/PCL–SiO2@TiO2–HDTMS/SPH fibrous membranes which was ascribed to SPH, and its weight loss occurred at 450 °C to 550 °C.40 After chlorination, the main degradation temperature of PHB, PCL and SPH decreased. It is possible that the N–Cl bonds of SPH broke and further accelerated the thermal decomposition of fibrous membranes via a free radical process.15 The remaining residual mass of PHB/PCL fibrous membranes was 1.17% at 600 °C, while it was 10.8% and 13.6% at 600 °C, respectively, before and after chlorination in case of PHB/PCL–SiO2@TiO2–HDTMS/SPH fibrous membranes. The final residual in case of PHB/PCL fibrous membranes at 600 °C was just carbon, while in case of PHB/PCL–SiO2@TiO2–HDTMS/SPH fibrous membranes at 600 °C it included nitrogen, silica and titanium.2.4 Hydrophobicity and air permeability
The contact angles and air permeability of fibrous membranes were determined and the results are shown in Table 1. The surface of PHB/PCL fibrous membranes was quickly wetted with 2 μL of distilled water, while the surface of PHB/PCL–SiO2@TiO2–HDTMS/SPH fibrous membranes exhibited outstanding hydrophobicity with a contact angle up to 144° ± 1°. There were no significant differences in contact angle before and after chlorination. The PHB/PCL fibrous membranes displayed excellent air permeability (54.6 ± 2.6 mm s−1), whereas the air permeability of the PHB/PCL–SiO2@TiO2–HDTMS/SPH fibrous membranes declined to 44.8 ± 1.3 mm s−1. After chlorination, the PHB/PCL–SiO2@TiO2–HDTMS/SPH fibrous membranes showed good air permeability of 50.9 ± 1.7 mm s−1. Thus, the improvement in hydrophobicity of PHB/PCL fibrous membranes did not compromise the air permeability of the membranes after surface modified. In addition, the chlorination process made no difference to hydrophobicity and air permeability. Therefore, in conclusion the fibrous membranes had good hydrophobicity and air permeability and may have a great potential for applications as textile and biomedical materials.2.5 Antibacterial efficacy
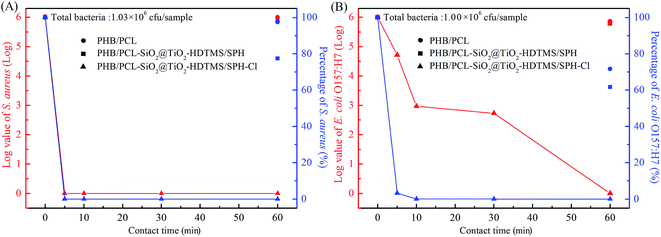
The antibacterial efficacy of fibrous membranes against S. aureus and E. coli O157:H7 were tested and the results are displayed in Fig. 5. The initial populations of S. aureus and E. coli O157:H7 were 1.03 × 106 CFU per sample and 1.00 × 106 CFU per sample. Clearly, the PHB/PCL fibrous membranes (Fig. 5A) had poor antibacterial property against S. aureus with log value of 0.011 (2.43%) reduction within 60 min. Similarly, the PHB/PCL fibrous membranes (Fig. 5B) only caused 0.145![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log reduction of E. coli O157:H7 within 60 min. After modified with SiO2@TiO2-based core@shell composite nanoparticles, the bactericidal efficacies of fibrous membranes against S. aureus and E. coli O157:H7 improved slightly with log reductions of 0.111 and 0.210, respectively, which might be attributed to the addition of TiO2.35 In contrast, as expected, the bactericidal percentages of the chlorinated membranes against S. aureus and E. coli O157:H7 were dramatically increased with the extension of contact time. After chlorination, N–H bonds of SPH were converted to N–Cl bonds, which were able to release oxidative halogens (Cl+) to attracted to bacterial organisms, and then destroyed the cell membrane of bacteria by oxidizing thiol groups or halogenating amino groups in proteins.41 From Fig. 5A and B, it can be seen that the chlorinated fibrous membranes inactivated 100% of S. aureus and E. coli O157:H7 within 5 min and 60 min of contact time, respectively. The antibacterial efficiency of the chlorinated fibrous membranes against S. aureus was significantly higher than E. coli O157:H7. The different shapes and surface structures of bacteria might cause them to adhere to the samples at different degrees, which was in accordance with results reported by other research groups.32,42,43
log reduction of E. coli O157:H7 within 60 min. After modified with SiO2@TiO2-based core@shell composite nanoparticles, the bactericidal efficacies of fibrous membranes against S. aureus and E. coli O157:H7 improved slightly with log reductions of 0.111 and 0.210, respectively, which might be attributed to the addition of TiO2.35 In contrast, as expected, the bactericidal percentages of the chlorinated membranes against S. aureus and E. coli O157:H7 were dramatically increased with the extension of contact time. After chlorination, N–H bonds of SPH were converted to N–Cl bonds, which were able to release oxidative halogens (Cl+) to attracted to bacterial organisms, and then destroyed the cell membrane of bacteria by oxidizing thiol groups or halogenating amino groups in proteins.41 From Fig. 5A and B, it can be seen that the chlorinated fibrous membranes inactivated 100% of S. aureus and E. coli O157:H7 within 5 min and 60 min of contact time, respectively. The antibacterial efficiency of the chlorinated fibrous membranes against S. aureus was significantly higher than E. coli O157:H7. The different shapes and surface structures of bacteria might cause them to adhere to the samples at different degrees, which was in accordance with results reported by other research groups.32,42,43
2.6 Cytotoxicity test
The results of cytotoxicity tests of fibrous membranes were shown in Fig. 6. The cell viability cultured in extract solution of PHB/PCL fibrous membranes declined dramatically to 69.6% compared with the control sample. According to the International Standard Organization (ISO/EN 10993-5), the reduction of cell viability by more than 30% is considered a cytotoxic effect.44 In this study, PHB/PCL fibrous membranes showed some degree of cytotoxic effect after 24 h incubation. After modified with SiO2@TiO2-based core@shell composite nanoparticles, the fibrous membranes showed good cell compatibility with 95.3% of cell viability. After chlorination, the cell viability of PHB/PCL–SiO2@TiO2–HDTMS/SPH fibrous membranes increased to 102.1%, which was higher than that of the control. The result is consistent with the previous report that low concentration of oxidative chlorine on the surface after deoxidation transferred into chloride containing Cl− and these compounds could promote cell growth.45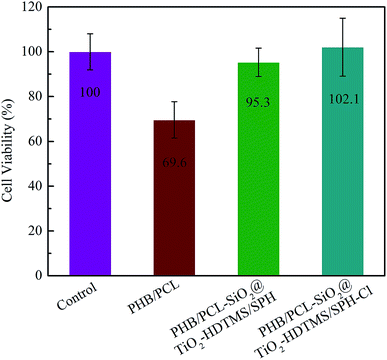 | ||
| Fig. 6 Cell viability of rat skin fibroblasts on control and different fibrous membranes after 24 h incubation. | ||
2.7 UVA light stability and storage stability
The PHB/PCL–SiO2@TiO2–HDTMS/SPH–Cl fibrous membranes were evaluated for UVA light and storage stability, and the results are displayed in Fig. 7. From Fig. 7A, it can be seen that the active chlorine content decreased with the extension of irradiation time, and only 0.04 wt% (10.8% of the initial active chlorine content) of active chlorine content remained after 24 h exposure. The loss of active chlorine was attributed to the decomposition of N–Cl bonds.46 After rechlorination, the active chlorine of the samples was 0.26 wt%, suggesting that 70% of the initial active chlorine content (Cl+% = 0.37 wt%) could be regained. The unrecovered chlorine content was ascribed to decomposition of Si–C bond.33,47 N-Halamine siloxane compound had poor UVA light stability because Si-alkyl group bond could break and result in the loss of the hydantoin ring under UV irradiation.32,33 The introduction of TiO2 in building the core@shell structure and further modification of the fibrous membranes could significantly increase UVA light stability. TiO2 is composed of a full valence band and a vacant conduction band, which allows the electrons to become transitional between the valence band and the conduction band under UV light irradiation with wavelengths less than 387.5 nm.16,35 Thus, TiO2 played an important role as a UV light protecting agent to guard the structure of SPH from decomposition, improving the UVA light stability of the chlorinated fibrous membranes. | ||
| Fig. 7 UVA light stability (A) and storage stability (B) of chlorinated PHB/PCL–SiO2@TiO2–HDTMS/SPH fibrous membranes. | ||
Fig. 7B shows that there was a slight decrease in the chlorine content over storage time when the samples were kept in the dark. After 60 days, the active chlorine content of the chlorinated PHB/PCL–SiO2@TiO2–HDTMS/SPH fibrous membranes was 0.30%, and thus 83.3% of the original chlorine content (0.37%), which still had efficient antibacterial effect.48 After rechlorination, almost all of the active chlorine of the fibrous membranes was recovered. The above results indicated that the N–Cl bond of the chlorinated PHB/PCL–SiO2@TiO2–HDTMS/SPH fibrous membranes was relatively stable under dark condition. Overall, the good UVA light and storage stability as well as their chlorine rechargeability make the prepared antimicrobial fibrous membranes useful materials for numerous practical applications.
3 Experimental
3.1 Materials and characterization
Polyhydroxybutyrate (PHB, Mn = 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) was provided by Tianjin Green Bio Materials Co., Ltd (Green Bio), China. 5,5-Dimethylhydantoin (DMH) was purchased from Hebei Yaguang Fine Chemical Co. Ltd. Octyl phenol ethoxylates-10 (OP-10) from Shengyu Chemical Co., Ltd, Shanghai, China. Poly-ε-caprolactone (PCL, Mn = 80
000 g mol−1) was provided by Tianjin Green Bio Materials Co., Ltd (Green Bio), China. 5,5-Dimethylhydantoin (DMH) was purchased from Hebei Yaguang Fine Chemical Co. Ltd. Octyl phenol ethoxylates-10 (OP-10) from Shengyu Chemical Co., Ltd, Shanghai, China. Poly-ε-caprolactone (PCL, Mn = 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1), tetra-n-butyl titanate (TBT), hexadecyltrimethoxysilane (HDTMS) and (3-chloropropyl) triethoxysilane were purchased from J&K Technology Co., Ltd, Shanghai, China. Tetraethoxysilane (TEOS), sodium hydroxide (NaOH), sodium thiosulfate (Na2S2O3), sodium hypochlorite (10% chlorine content), azobis(isobutyronitrile) (AIBN), potassium iodide (KI), chloroform (CHCl3), N,N-dimethylformamide (DMF), ethanol and acetic acid were purchased from Sinopharm Chemical Reagent Co., Ltd, Shanghai, China. Bacteria of S. aureus ATCC 6538 and E. coli O157:H7 ATCC 43895 (American Type Culture Collection, Rockville, MD) were used in this study.
000 g mol−1), tetra-n-butyl titanate (TBT), hexadecyltrimethoxysilane (HDTMS) and (3-chloropropyl) triethoxysilane were purchased from J&K Technology Co., Ltd, Shanghai, China. Tetraethoxysilane (TEOS), sodium hydroxide (NaOH), sodium thiosulfate (Na2S2O3), sodium hypochlorite (10% chlorine content), azobis(isobutyronitrile) (AIBN), potassium iodide (KI), chloroform (CHCl3), N,N-dimethylformamide (DMF), ethanol and acetic acid were purchased from Sinopharm Chemical Reagent Co., Ltd, Shanghai, China. Bacteria of S. aureus ATCC 6538 and E. coli O157:H7 ATCC 43895 (American Type Culture Collection, Rockville, MD) were used in this study.
The morphologies of membranes were characterized by scanning electron microscopy (TM3030, Hitachi High Technologies, Japan) and the average diameter and size distribution of fibers from the SEM images were determined by a nanomeasurer software (Department of Chemistry, Fudan University, Shanghai, China). Fourier transform infrared (FT-IR) spectra were tested by a NEXUS 470 spectrometer (Nicolet Instrument Corporation, USA). Transmission electron microscope (TEM) were characterized by a JEM 2100 TEM microscope operated at 200 kV (Hitachi High Technologies, Japan), and the samples (c = 0.1–0.2 g L−1) were obtained by dipping copper 400-mesh carrier grids. Thermogravimetric analysis (TGA) was tested by a Q500 TGA (TA Instruments Co. Ltd., USA) via heating 5 mg sample from room temperature to 600 °C at a rate of 10 °C min. The air permeability was tested with a water vapor transmission tester (YG 461E, Ningbo Textile Instrument Co. Ltd., China).
3.2 Preparation of PHB/PCL electrospun fibrous membranes
Ten percent (w/v) of PHB/PCL (w/w, 40/60) were dissolved in chloroform at 45 °C for 3–5 h under stirring. The final solution was electrospun at room temperature to make fibrous membranes. And the parameters were as followed: 20 kV of voltage, 1.5 mL h−1 of flow rate and 20 cm of tip-to-collector distance.3.3 Synthesis of 5,5-dimethyl-3-(3′-triethoxysilylpropyl)hydantoin (SPH)
5,5-Dimethyl-3-(3′-triethoxysilylpropyl)hydantoin (SPH) was synthesized according to S. D. Worley reported.49 Hydantoin sodium salt was prepared by mixing DMH with an equimolar quantity of NaOH in ethanol to react for 10 min at 90 °C. After drying at 45 °C for 2 days, the prepared hydantoin sodium salt was dissolved in DMF, and subsequently (3-chloropropyl)triethoxysilane was added with stirring at 95 °C for 10 h. The SPH was obtained through filtration and evaporation of DMF.3.4 Synthesis of SiO2@TiO2-based core@shell composite nanoparticles
Colloidal silica nanoparticles (SiO2 NPs) were prepared according to the protocol of previous studies.32 First, a mixture (100 mL) of ethanol, H2O and 10% NH4OH (v/v/v, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was stirred vigorously to form a homogeneous solution. Then, a stock solution containing 10 mL TEOS and 10 mL ethanol was added into the mixture at a rate of 0.5 mL min−1 with a constant pressure funnel. After mixing vigorously, the mixture solution was vigorously stirring at 60 °C for 2 h. Finally, the SiO2 NPs were separated by centrifugation.
1) was stirred vigorously to form a homogeneous solution. Then, a stock solution containing 10 mL TEOS and 10 mL ethanol was added into the mixture at a rate of 0.5 mL min−1 with a constant pressure funnel. After mixing vigorously, the mixture solution was vigorously stirring at 60 °C for 2 h. Finally, the SiO2 NPs were separated by centrifugation.
In the next step the SiO2 NPs were dispersed uniformly in 100 mL ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (19
H2O (19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) in an ultrasonic bath for 30 min, and 0.5 g OP-10 was added into the mixture. Another solution containing 10 mL TTB and 10 mL ethanol was injected into the above mixture at a rate of 0.5 mL min−1 with a constant pressure funnel. The resulting mix was stirred vigorously at 50 °C for 12 h. Finally, the SiO2@TiO2 NPs were separated by centrifugation.
1, v/v) in an ultrasonic bath for 30 min, and 0.5 g OP-10 was added into the mixture. Another solution containing 10 mL TTB and 10 mL ethanol was injected into the above mixture at a rate of 0.5 mL min−1 with a constant pressure funnel. The resulting mix was stirred vigorously at 50 °C for 12 h. Finally, the SiO2@TiO2 NPs were separated by centrifugation.
The prepared SiO2@TiO2 NPs were dispersed uniformly in the 100 mL ethanol solution and 0.5 g OP-10 was added to the dispersion in an ultrasonic bath for 30 min. Then, 8 wt% SPH, and 12 wt% HDTMS were added with vigorously stirring at room temperature for 4 h. The SiO2@TiO2-based core@shell composite nanoparticles were separated by centrifugation, washed 2 times with ethanol, and dried at 45 °C. In Scheme 1 the process is schematically illustrated.
3.5 Preparation of hydrophobic antibacterial fibrous membranes
Ten percent (w/v) of SiO2@TiO2-based core@shell composite nanoparticles and one percent (w/v) AIBN were dissolved in the ethanol solution in an ultrasonic bath for 30 min. The PHB/PCL fibrous membranes were dipped in the above colloidal solution, followed by two dips (30 s of each dip) and two pads (wet pick-up 75–80%). Then, the fibrous membranes were dried at 60 °C for 1 h. The treated fibrous membranes were washed thoroughly with ethanol and dried. And these membranes were called PHB/PCL–SiO2@TiO2–HDTMS/SPH. Then, fibrous membranes were soaked in a sodium hypochlorite solution (10 wt%, pH 7) at room temperature for 1 h to render antimicrobial properties. The chlorinated fibrous membranes were washed thoroughly and dried at 45 °C for 1 h to remove unbound chlorine. These membranes were called PHB/PCL–SiO2@TiO2–HDTMS/SPH–Cl.3.6 Chlorine content measurement
The active chlorine content on fibrous membranes was tested by a modified iodometric/thiosulfate titration method. The active chlorine weight percent of fibrous membranes was calculated according to the eqn (1):
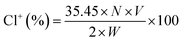 | (1) |
3.7 Biocidal efficacy test
Gram-positive S. aureus (ATCC 6538) and Gram-negative E. coli O157:H7 (ATCC 43895) were used to test the antibacterial properties for samples according to the modified AATCC 100-2004 method. In this test, a certain volume of bacterial suspensions (106–107 CFU) were added to the center between two pieces of fibrous membranes (2.54 × 2.54 cm2) and the sample assemblies were held in place by putting a sterile weight on top to make sufficient contact between the bacteria and the membranes. After exposure to the bacteria for 5, 10, 30 and 60 min, the membranes were quenched with 5.0 mL sterile 0.02 N sodium thiosulfate solutions to remove oxidative chlorine residuals and vortexed to release bacteria from fibrous membranes to solution. 10-fold serial dilutions of the sterilized solutions were prepared with phosphate buffer (100 mM, pH 7), and each dilution was plated on Trypticase soy agar plate. The agar plates were incubated at 37 °C for 24 h, and the bacterial colony counts were calculated for biocidal efficacy analysis.3.8 Cell viability test
In vitro biocompatibility of fibrous membranes was tested on rat skin fibroblasts (ATCC CRL-1213) according to International Standard Organization (ISO/EN 10993-5).44 Rat skin fibroblasts were cultured in a media of Dulbecco's Modified Eagle Medium (DMEM), Fetal Bovine Serum (FBS) and Penstrip (v/v/v, 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at 37 °C under 5% CO2 atmosphere. After culturing 3 generation of cells, an aliquot of 100 μL cell suspension (∼104 cells) was seeded in each well of 96-well plates, and, meanwhile, the membranes were immersed into culture medium at 37 °C under 5% CO2 atmosphere for 24 h to prepare liquid extracts. After 24 h incubation, the culture medium was replaced with liquid extracts. After another 24 h incubation with the extracts, 100 μL of fresh medium and 50 μL of XTT/PMS reagent (v/v, 50
1) at 37 °C under 5% CO2 atmosphere. After culturing 3 generation of cells, an aliquot of 100 μL cell suspension (∼104 cells) was seeded in each well of 96-well plates, and, meanwhile, the membranes were immersed into culture medium at 37 °C under 5% CO2 atmosphere for 24 h to prepare liquid extracts. After 24 h incubation, the culture medium was replaced with liquid extracts. After another 24 h incubation with the extracts, 100 μL of fresh medium and 50 μL of XTT/PMS reagent (v/v, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were replaced to each well and the plates were incubated dark for another 2–4 h. The absorbance of each well at OD490 nm was measured with a reference wavelength of OD690 nm using a microplate reader (Infinity M200 Pro, Tecan). Cells only incubated in culture medium were tested under the same conditions to act as negative controls and data were normalized with this blank control.
1) were replaced to each well and the plates were incubated dark for another 2–4 h. The absorbance of each well at OD490 nm was measured with a reference wavelength of OD690 nm using a microplate reader (Infinity M200 Pro, Tecan). Cells only incubated in culture medium were tested under the same conditions to act as negative controls and data were normalized with this blank control.
3.9 Contact angle measurements
Water contact angle was tested by digital microscope contact angle tester (26700-300, Instrument & Equipment Specialties Inc., USA). 2 μL of distilled water was dropped on the surface of fibrous membranes, and the contact angle was recorded after 1 min for more than 5 times in different positions to calculate the average value for each sample.3.10 UV light stability test
The chlorine stability of the PHB/PCL–SiO2@TiO2–HDTMS/SPH–Cl fibrous membranes under UV light was measured using a model Accelerated Weathering Tester (Q-LAB Company, USA). The PHB/PCL–SiO2@TiO2–HDTMS/SPH–Cl fibrous membranes were placed in a UV chamber (type A, 315–400 nm, 0.89 W, 60 °C). After 1, 2, 4, 8, 12 and 24 h of UV light irradiation, the fibrous membranes were removed from the UV chamber and their chlorine contents were measured immediately. The membranes after irradiation for 24 h were rechlorinated and their chlorine contents again determined by titration.3.11 Storage stability test
The PHB/PCL–SiO2@TiO2–HDTMS/SPH–Cl fibrous membranes were placed into self-sealed bags in a dark environment. After a specific time (10, 20, 30, 45 and 60 d), the chlorine contents in the fibrous membranes were calculated by titration method. After 60 days, the fibrous membranes were rechlorinated. The chlorine contents of rechlorinated fibrous membranes were determined.4 Conclusions
We synthesized SiO2@TiO2-based core@shell composite nanoparticles with the average diameter 274.5 ± 16 nm. SEM and FT-IR results showed that the fibrous membranes surfaces were modified with the SiO2@TiO2-based core@shell composite nanoparticles. The prepared fibrous membranes had excellent hydrophobicity with an average water contact angle of 144° ± 1° and favorable air permeability. The chlorinated fibrous membranes could inactivate 100% S. aureus and E. coli O157:H7 within 5 min and 60 min of contact, respectively. The cytotoxicity test demonstrated that the modified fibrous membranes have no cytotoxicity, and the chlorinated fibrous membranes could promote cell growth. The stability tests indicated that the chlorinated fibrous membranes exhibited excellent UVA light stability and superior storage stability. Therefore, the designed multifunctional fibrous membranes may find many suitable applications in the packaging and biomedical materials industry.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank for the support of the Project of Jiangsu Science and Technological Innovation Team, and the Fundamental Research Funds for the Central Universities (No. JUSRP51722B, No. JUSRP11806), the National First-Class Discipline Program of Light Industry Technology and Engineering (LITE2018-2), and 111 Projects (B17021).References
- L. S. Montagna, A. L. Catto, M. M. D. Forte, E. Chiellini, A. Corti, A. Morelli and R. M. C. Santana, Polym. Degrad. Stab., 2015, 120, 186–192 CrossRef CAS.
- A. A. Shah, F. Hasan, A. Hameed and S. Ahmed, Biotechnol. Adv., 2008, 26, 246–265 CrossRef CAS PubMed.
- L. S. Montagna, T. L. D. Montanheiro, A. C. Borges, C. Y. Koga-Ito, A. P. Lemes and M. C. Rezende, J. Appl. Polym. Sci., 2017, 134, 44234–44242 Search PubMed.
- M. B. Patel, S. A. Patel, A. Ray and R. M. Patel, J. Appl. Polym. Sci., 2003, 89, 895–900 CrossRef CAS.
- E. S. Park, H. J. Lee, H. Y. Park, M. N. Kim, K. H. Chung and J. S. Yoon, J. Appl. Polym. Sci., 2001, 80, 728–736 CrossRef CAS.
- L. Kou, J. Liang, X. H. Ren, H. B. Kocer, S. D. Worley, R. M. Broughton and T. S. Huang, Colloids Surf., A, 2009, 345, 88–94 CrossRef CAS.
- E. I. Shishatskaya, T. G. Volova, A. P. Puzyr, O. A. Mogilnaya and S. N. Efremov, J. Mater. Sci.: Mater. Med., 2004, 15, 719–728 CrossRef CAS PubMed.
- J. M. Lagaron and E. Nunez, J. Plast. Film Sheeting, 2012, 28, 79–89 CrossRef CAS.
- J. G. Fernandes, D. M. Correia, G. Botelho, J. Padrao, F. Dourado, C. Ribeiro, S. Lanceros-Mendez and V. Sencadas, Polym. Test., 2014, 34, 64–71 CrossRef CAS.
- D. Guldiren and S. Aydin, Mater. Sci. Eng., C, 2017, 78, 826–832 CrossRef CAS PubMed.
- T. Uchimaru, S. Tsuzuki, L. Chen and J. Mizukado, J. Fluorine Chem., 2017, 194, 33–39 CrossRef CAS.
- M. L. Yin, X. L. Chen, R. Li, D. Huang, X. Y. Fan, X. H. Ren and T. S. Huang, J. Appl. Polym. Sci., 2016, 133, 44204–44211 Search PubMed.
- X. H. Ren, L. Kou, J. Liang, S. D. Worley, Y. M. Tzou and T. S. Huang, Cellulose, 2008, 15, 593–598 CrossRef CAS.
- X. H. Ren, L. Kou, H. B. Kocer, C. Y. Zhu, S. D. Worley, R. M. Broughton and T. S. Huang, Colloids Surf., A, 2008, 317, 711–716 CrossRef CAS.
- R. Li, P. Hu, X. H. Ren, S. D. Worley and T. S. Huang, Carbohydr. Polym., 2013, 92, 534–539 CrossRef CAS PubMed.
- J. Li, Y. Liu, Z. M. Jiang, K. K. Ma, X. H. Ren and T. S. Huang, Ind. Eng. Chem. Res., 2014, 53, 13058–13064 CrossRef CAS.
- H. B. Kocer, S. D. Worley, R. M. Broughton and T. S. Huang, React. Funct. Polym., 2011, 71, 561–568 CrossRef CAS.
- I. Cerkez, H. B. Kocer, S. D. Worley, R. M. Broughton and T. S. Huang, Cellulose, 2012, 19, 959–966 CrossRef CAS.
- Y. Liu, Q. H. He, R. Li, D. Huang, X. H. Ren and T. S. Huang, Fibers Polym., 2016, 17, 2035–2040 CrossRef CAS.
- A. Nesic, J. Ruzic, M. Gordic, S. Ostojic, D. Micic and A. Onjia, Composites, Part B, 2017, 110, 56–61 CrossRef CAS.
- M. Nosonovsky and B. Bhushan, Microelectron. Eng., 2007, 84, 382–386 CrossRef.
- A. I. Safonov, S. V. Starinskii, V. S. Sulyaeva, N. I. Timoshenko and E. Y. Gatapova, Tech. Phys. Lett., 2017, 43, 159–161 CrossRef CAS.
- R. Li, M. M. Sun, Z. M. Jiang, X. H. Ren and T. S. Huang, Fibers Polym., 2014, 15, 234–240 CrossRef CAS.
- X. G. Zhang, H. Y. Wang, Z. J. Liu, Y. X. Zhu, S. Q. Wu, C. J. Wang and Y. J. Zhu, Appl. Surf. Sci., 2017, 396, 1580–1588 CrossRef CAS.
- F. Kayaci, O. C. O. Umu, T. Tekinay and T. Uyar, J. Agric. Food Chem., 2013, 61, 3901–3908 CrossRef CAS PubMed.
- X. Y. Fan, Q. Y. Jiang, Z. Sun, G. Li, X. H. Ren, J. Liang and T. S. Huang, Fibers Polym., 2015, 16, 1751–1758 CrossRef CAS.
- S. H. Park, S. M. Lee, H. S. Lim, J. T. Han, D. R. Lee, H. S. Shin, Y. J. Jeong, J. Kim and J. H. Cho, ACS Appl. Mater. Interfaces, 2010, 2, 658–662 CrossRef CAS PubMed.
- J. E. Q. Quinsaat, F. A. Nuesch, H. Hofmann and D. M. Opris, RSC Adv., 2013, 3, 6964–6971 RSC.
- M. Meng, H. W. He, J. Xiao, P. Zhao, J. L. Xie and Z. S. Lu, J. Colloid Interface Sci., 2016, 461, 369–375 CrossRef CAS PubMed.
- C. R. Li, X. O. Zhang and Z. X. Cao, Science, 2005, 310, 236 Search PubMed.
- J. P. Cook and D. D. Evanoff, Abstr. Pap. Am. Chem. Soc., 2014, 247 Search PubMed.
- X. H. Lin, Y. M. Li, Y. Liu, L. Li, X. H. Ren, Y. Y. Sun and T. S. Huang, J. Ind. Eng. Chem., 2018, 63, 303–311 CrossRef CAS.
- H. B. Kocer, A. Akdag, S. D. Worley, O. Acevedo, R. M. Broughton and Y. N. Wu, ACS Appl. Mater. Interfaces, 2010, 2, 2456–2464 CrossRef CAS PubMed.
- V. De Matteis, L. Rizzello, M. P. Di Bello and R. Rinaldi, J. Nanopart. Res., 2017, 19, 196–210 CrossRef.
- J. Li, R. Li, J. M. Du, X. H. Ren, S. D. Worley and T. S. Huang, Cellulose, 2013, 20, 2151–2161 CrossRef CAS.
- Y. Zhao, Y. W. Tang, X. G. Wang and T. Lin, Appl. Surf. Sci., 2010, 256, 6736–6742 CrossRef CAS.
- S. Lee, Y. C. Cha, H. J. Hwang, J. W. Moon and I. S. Han, Mater. Lett., 2007, 61, 3130–3133 CrossRef CAS.
- C. Chaikeaw and K. Srikulkit, J. Sol-Gel Sci. Technol., 2017, 81, 774–781 CrossRef CAS.
- Y. Chen, L. Wang, H. J. Yu, Q. Shi and X. C. Dong, J. Mater. Sci., 2007, 42, 4018–4024 CrossRef CAS.
- Y. F. Wang, L. Li, Y. Liu, X. H. Ren and J. Liang, Mater. Sci. Eng., C, 2016, 69, 1075–1080 CrossRef CAS PubMed.
- X. L. Li, Y. Liu, Z. M. Jiang, R. Li, X. H. Ren and T. S. Huang, Cellulose, 2015, 22, 3609–3617 CrossRef CAS.
- M. F. Richter, B. S. Drown, A. P. Riley, A. Garcia, T. Shirai, R. L. Svec and P. J. Hergenrother, Nature, 2017, 545, 299–304 CrossRef CAS PubMed.
- X. Lin, X. Fan, R. Li, Z. Li, T. Ren, X. Ren and T. S. Huang, Polym. Adv. Technol., 2017, 29, 481–489 CrossRef.
- I. 10993-5, International Organization for Standardization Geneve, Switzerland, 2009.
- R. Li, J. F. Dou, Q. Y. Jiang, J. Li, Z. W. Xie, J. Liang and X. H. Ren, Chem. Eng. J., 2014, 248, 264–272 CrossRef CAS.
- X. Y. Fan, X. H. Ren, T. S. Huang and Y. Y. Sun, RSC Adv., 2016, 6, 42600–42610 RSC.
- Y. Liu, J. Li, L. Li, S. McFarland, X. H. Ren, O. Acevedo and T. S. Huang, ACS Appl. Mater. Interfaces, 2016, 8, 3516–3523 CrossRef CAS PubMed.
- X. H. Ren, A. Akdag, H. B. Kocer, S. D. Worley, R. M. Broughton and T. S. Huang, Carbohydr. Polym., 2009, 78, 220–226 CrossRef CAS.
- S. D. Worley, Y. Chen, J. W. Wang and R. Wu, Surf. Coat. Int., Part B, 2005, 88, 93–99 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2019 |