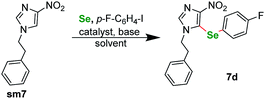Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Copper-catalyzed direct C–H arylselenation of 4-nitro-pyrazoles and other heterocycles with selenium powder and aryl iodides. Access to unsymmetrical heteroaryl selenides†
Michał Jakubczyk a,
Satenik Mkrtchyan*a,
Izabela D. Madurab,
Paulina H. Marekbc and
Viktor O. Iaroshenko
a,
Satenik Mkrtchyan*a,
Izabela D. Madurab,
Paulina H. Marekbc and
Viktor O. Iaroshenko *a
*a
aLaboratory of Homogeneous Catalysis and Molecular Design at the Centre of Molecular and Macromolecular Studies, Polish Academy of Sciences, Sienkiewicza 112, PL-90-363 Łodź, Poland. E-mail: iva108@gmail.com; viktori@cbmm.lodz.pl
bDepartment of Inorganic Chemistry, Faculty of Chemistry, Warsaw University of Technology, Noakowskiego 3, 00-664, Warsaw, Poland
cFaculty of Chemistry, University of Warsaw, Pasteura 1, 02-093 Warsaw, Poland
First published on 13th August 2019
Abstract
A one-pot, Cu-catalyzed direct C–H arylselenation protocol using elemental Se and aryl iodides was developed for nitro-substituted, N-alkylated pyrazoles, imidazoles and other heterocycles including 4H-chromen-4-one. This general and concise method allows one to obtain a large number of unsymmetrical heteroaryl selenides bearing a variety of substituents. The presence of the nitro group was confirmed to be essential for the C–H activation and can also be used for further functionalisation and manipulation. Several examples of heteroannulated benzoselenazines were also synthesized using the developed synthetic protocol.
Introduction
Organoselenium compounds have been known for many years to have biological activity that can be useful in medicine.1 Their therapeutic properties include antitumor,2 antioxidant,3,4 antiinflammatory, antiviral, antimicrobial and neuroprotective action.5 Among these, the antioxidant effect seems to be the most important mode of action recognized for organoselenium therapeutics as the oxidative stress is a symptom and often the cause of many diseases. Mammalian organisms are equipped with glutathione peroxidases (GPx)6,7 – a family of antioxidant enzymes that naturally contain selenium in their active site in the form of a selenocysteine8 (Sec) residue. One of the most thoroughly studied synthetic drugs that demonstrates the so called GPx-like activity9 – ebselen, is often considered a comparative standard when investigating other organoselenium drug candidates.10–12Diorganyl selenides receive much attention, both due to their biological activity13–17 and their value in synthetic chemistry.18–24 Non-cyclic diorganyl selenides can be grouped, according to the structure of the organyl substituents, as symmetrical and unsymmetrical compounds. This nominal division, present in the literature comes from the varying difficulty and complexity of the synthetic methodology. In a historical context, sodium or potassium selenides (Na2Se, K2Se) or polyselenides (Na2Sex or K2Sex) can be reacted with bromides to yield symmetrical dialkyl selenides25–27 or with arenediazonium salts to prepare symmetrical diaryl selenides.28–34 In a more modern context, TM-catalyzed cross-coupling reactions are one of the most common methods for the preparation of aryl chalcogenides.35 Particularly, copper-catalyzed methods utilizing: elemental selenium with aryl halides,36–38 potassium selenocyanates with aryl halides,39,40 triarylbismuthanes with elemental selenium41 or boronic acids with diaryl diselenides,42 elemental selenium43 or selenourea44 – can be applied. Some of these methods yield also diselenides as by- or main-products, depending on the reaction conditions. Unsymmetrical diaryl selenides can be obtained by similar methods from elemental selenium,36,38 diselenides45–48 and selenols49,50 as a selenium source. Additionally, two other methods have been developed. Nucleophilic substitution of bromine in PhSeBr by mild nucleophiles (arylboronic acids, arylsiloxanes, and arylstannanes) catalyzed by alumina-supported copper catalyst51 and a transition metal-free, base promoted reaction of arylhydrazines with diaryl diselenides.52
Unsymmetrical aryl-Se-heteroaryl compounds represent yet another advanced challenge as synthetic targets. Structures bearing pyridine and thiophen moieties at selenium are available by previously mentioned methods from diselenides.53–55 Special attention should be paid to those reagents in the context of unsymmetrical heteroaryl selenides' synthesis. In a recent review, Arsenyan summarizes the progress in this matter, listing all the possible synthetic pathways to obtain aryl-Se-heteroaryl compounds (bearing a variety of pharmacophores) from diaryl diselenides.56 These transformations include nucleophilic and electrophilic reactions (synthons RSe+ and RSe−), reactions involving radicals (RSe˙), copper catalysis, single electron transfer (SET) reactions (photochemical reactions) and direct heteroaryl selenation of activated C(sp2)–H bond.
The C–H bond is the most widespread structural fragment in organic chemistry, and its functionalization has been the subject of intensive studies.67–71 In recent years, TM-catalyzed C–H activation reactions emerged as one of the most important methodologies in modern organic chemistry. This applies also to selenation. The TM-catalyzed direct arylselenation of C–H bonds is one of the most efficient methods for the synthesis of unsymmetrical diaryl selenides. The to-date developed protocols involve the use of diselenides (palladium,63 rhodium,72 ruthenium64,73 iron oxide,74 silver,75,76 and copper65,66,77 catalyzed) or ArSeCl (ruthenium78 catalyzed) as selenium source. There are also a few very recent examples of copper-catalyzed one-pot three-component procedures involving Se powder, which can be considered an obvious step in methodology development, since diselenides are easily obtained from aryl iodides and selenium powder in similar catalytic conditions.37
Wu et al. obtained a library of (phenylseleno)-1H-indoles selecting CuO as the best catalyst.61 Their protocol is also suitable for the formation of an intramolecular C–Se–C bond. The authors pointed out that the free NH group of the indol was critical as the N-substituted starting materials did not undergo arylselenation in selected conditions. However, Guo et al. showed that also N-substituted indoles can undergo C–H arylselenation in specific conditions.59 In the same paper, the authors revisit the selenation of imidazo[1,2-a]pyridines, previously submitted to reaction with 4-coumarinyl triflates.60 2-(2-Bromophenyl)imidazo[1,2-a]pyridines were also explored as starting materials in an intramolecular variant of the reaction by Wang et al.58 In another paper, Wu et al. proposed conditions for C–H arylselenation of 2-phenyl- and 2-aryl-1,3,4-oxadiazoles,62 a scaffold found in many drugs.79 Slightly different conditions for the same starting materials were also selected by Braga et al.57
Since we have our long-standing interest in TM-catalyzed C–H activation, we were curious if a general, practical and concise approach could be realized for the arylselenation of other heterocycles – widening the scope of the starting materials. The regioselectivity of C–H activation reactions of more complex functionalized substrates containing two or more reactive C–H bonds is an important topic of current research.67–71,80–90 The regioselectivity of such reactions is controlled by the presence of functional groups in the substrate. This includes directing substituents (carbonyl, cyano, etc.),80–83,85,88–90 halogen substituents (mostly fluorine or chlorine),80–83,85,88–93 and ring heteroatoms (namely – sulfur, nitrogen, and oxygen). The use of the nitro group as a regio-directing substituent in C–H activations has been investigated previously94,95 also by our group in the context of arylation of nitro-pyrazoles,96 nitro-imidazoles97 and other nitro-heteroarenes.98 Herein we describe a one-pot method to obtain unsymmetrical diorganyl selenides from C–H activated heterocycles (including nitro-substituted), aryl iodides and elemental selenium powder.
Results and discussion
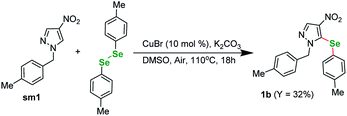
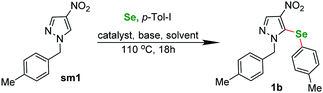
In order to confirm the validity of the literature protocols we started our investigations by performing a test reaction between a N-substituted nitro-heterocycle and diphenyl diselenide (Scheme 1). Basing on our previous research on TM-catalyzed C–H functionalization of nitro-substituted (hetero)arenes we selected pyrazole sm1 as a model compound for this experiment. The conditions were selected following the literature on reactions involving diaryl diselenides and our experience. The reaction between sm1 (1 equiv.) and 1,2-di-p-tolyldiselane (1 equiv.) catalyzed by CuBr (0, 1 equiv.) under basic conditions (3 equiv. K2CO3) in DMSO under air gave the expected product in 32% yield after 18 h in 110 °C. The selenation occurred exclusively at the C(5) position of the pyrazole ring.With this encouraging result in hand we moved to a three-component reaction. The above conditions applied to the same starting material sm1 in reaction with 4-iodotoluene and selenium powder gave the expected product in 38% yield (Table 1, entry 1). With this result we began the optimization of the conditions.
| Entry | Catalyst | Base | Solvent | Yield% | Ref. |
|---|---|---|---|---|---|
| a Reaction conditions unless specified otherwise: 1 equiv. sm1, 2 equiv. aryl iodide, 3 equiv. selenium powder (100 mesh), 4 equiv. base, catalyst (10 mol%), 1 mL dry solvent. Performed in a Teflon screw cap-sealed pressure tube.b Ligand added – Phen (10 mol%).c Reaction loaded in glovebox under argon. | |||||
| 1 | CuBr | K2CO3 | DMSO | 38 | |
| 2 | CuI | KHCO3 | DMSO | 23 | 57 |
| 3 | CuI | None | DMF | NR | 58 |
| 4 | Cu(OAc)2 | KOH | DMF | 30b | 59 |
| 5 | CuO | None | DMF | NR | 60 |
| 6 | CuO | Na3PO4·12H2O | DMSO | 46c | 61 |
| 7 | CuCl2 | Na2CO3 | DMF | 39c | 62 |
| 8 | CuI | K2CO3 | DMSO | 14c | 37 |
| 9 | Pd(OAc)2 | K2CO3 | DMSO | 51c | 63 |
| 10 | PdCl2(PPh3)2 | K2CO3 | DMSO | 55c | |
| 11 | [Ru(p-cymene)Cl2]2 | K2CO3 | DCE | 27 | 64 |
| 12 | CuI | None | DMSO | NR | 65 |
| 13 | CuO | K2CO3 | DMF | 43c | 66 |
| 14 | CuBr2 | K2CO3 | DMF | 45c | 66 |
| 15 | CuBr2 | K2CO3 | DMA | 40c | |
| 16 | CuBr2 | K2CO3 | Toluene | 9c | |
| 17 | CuBr2 | K2CO3 | DMSO | 74c | |
| 18 | CuBr2 | K2CO3 | DMSO | 83 | |
| 19 | CuCl2 | K2CO3 | DMSO | 63 | |
| 20 | CuBr2 | KOH | DMSO | 70 | |
| 21 | NiCl2(PPh3)2 | K2CO3 | DMSO | Tracec | |
| 22 | ReOCl3(PPh3)2 | K2CO3 | DMSO | 15c | |
| 23 | Co(OAc)2 | K2CO3 | DMSO | 10c | |
| 24 | FeCl2 | K2CO3 | DMSO | 9c | |
| 25 | AgOAc | K2CO3 | DMSO | 12c | |
| 26 | PtCl2(bpy) | K2CO3 | DMSO | 29c | |
At start we applied the conditions matching those known in the literature to work for selenation of heteroaryls with elemental selenium. Braga et al.57 tested many variants involving CuI and a handful of other copper salts for the selenation of 1,3,4-oxadiazoles, for most of which he obtained quite satisfactory results. In our case however, the combination of CuI and KHCO3 gave only 23% yield (Table 1, entry 2). Following the procedure optimized by Wang et al.58 we skipped the base and switched to DMF, this combination also was not optimal (Table 1, entry 3). This is not however surprising, since the authors of the mentioned work assumed a radical pathway in their reaction involving a derivative of imidazo[1,2-a]pyridine.
The same substrates were investigated by Guo et al. twice, in combination with aryl iodides and coumarinyl triflates. Application of similar conditions did not raise the yield significantly (Table 1, entries 4 and 5). Another set of conditions, following the work of Wu and Wu et al.61 pointed at CuO in the combination with strong base in DMSO under argon (46% yield, Table 1, entry 6). This prompted us to apply Cu(II) salt again (Table 1, entry 7). A few other combinations were tried (entries 8, 12, 13, 19) with moderate success, but only after switching to CuBr2 the yields raised to higher levels (entries 14–18 and 20). Finally, the optimal conditions were found (entry 18). Beside the copper salt, the choice of the base seems to be less important than the choice of the solvent and aerobic conditions. This indicates that an oxidant might be consumed during the course of the reaction, since both – DMSO and O2 in air can act as oxidizing agents. A handful of other conditions were tested, including catalysts based on more expensive transition metals, known to work in C–H activation protocols. Among those, only the use of Pd(II) salts yielded more than 50% of the target product.
Having acquired the optimal conditions for our starting material, we moved on to the scope assessment. 4-Nitropyrazole sm1 was submitted to reaction with a number of substituted iodobenzene derivatives (Table 2, entries 1–9). The results were mostly satisfactory, although in some cases the duration and temperature of the reaction had to be slightly extended. In order to test the influence of the alkyl substituent at N(1) position we submitted derivatives with phenethyl-(sm2), phenylpropyl-(sm3) and butyl-(sm4) substituents to the reaction. It is of note, for the 4-nitropyrozoles in question the reactions with o-fluoroiodobenzene all gave similar results (yield = 71–77%, Table 2, entries 6, 11, 16, 20). In the case of m-fluoroiodobenzene the yields are slightly lower and range from 67 to 76% (Table 2, entries 7, 12, 17, 21), what can be attributed to a different inductive EWG effect.
| Entry | Structure | R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) |
Number | Time/h | Temp./°C | Yield/% |
|---|---|---|---|---|---|---|
| a Reaction conditions unless specified otherwise: 1 equiv. sm1–sm5, 2 equiv. aryl iodide, 3 equiv. selenium powder (100 mesh), 4 equiv. K2CO3, CuBr2 (10 mol%), 1 mL dry DMSO. Performed in a Teflon screw cap-sealed pressure tube, loaded in air. | ||||||
| 1 |  |
m-Me | 1a | 24 | 115 | 77 |
| 2 | p-Me | 1b | 25 | 115 | 83 | |
| 3 | p-Et | 1c | 24 | 115 | 85 | |
| 4 | m-CF3 | 1d | 26 | 115 | 80 | |
| 5 | p-CF3 | 1e | 25 | 115 | 70 | |
| 6 | o-F | 1f | 25 | 115 | 77 | |
| 7 | m-F | 1g | 25 | 115 | 79 | |
| 8 | p-F | 1h | 35 | 115 | 81 | |
| 9 | p-Br | 1i | 25 | 115 | 64 | |
| 10 |  |
m-CF3 | 2a | 30 | 115 | 83 |
| 11 | o-F | 2b | 30 | 115 | 71 | |
| 12 | m-F | 2c | 25 | 115 | 82 | |
| 13 | p-Cl | 2d | 25 | 115 | 79 | |
| 14 | p-MeO | 2e | 25 | 115 | 72 | |
| 15 | 2-(3-Br-py) | 2f | 25 | 115 | 56 | |
| 16 |  |
o-F | 3a | 26 | 115 | 75 |
| 17 | m-F | 3b | 26 | 115 | 80 | |
| 18 | p-F | 3c | 26 | 115 | 86 | |
| 19 |  |
o-Me | 4a | 24 | 115 | 69 |
| 20 | o-F | 4b | 24 | 115 | 74 | |
| 21 | m-F | 4c | 24 | 115 | 81 | |
| 22 | p-F | 4d | 30 | 115 | 79 | |
| 23 | 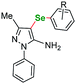 |
m-Me | 5a | 24 | 110 | 79 |
| 24 | p-Me | 5b | 24 | 110 | 89 | |
| 25 | p-Et | 5c | 24 | 110 | 83 | |
| 26 | m-CF3 | 5d | 24 | 110 | 76 | |
| 27 | p-CF3 | 5e | 17 | 110 | 85 | |
| 28 | o-F | 5f | 18 | 110 | 80 | |
| 29 | m-F | 5g | 19 | 110 | 79 | |
| 30 | p-F | 5h | 17 | 110 | 74 | |
| 31 | o-Br | 5i | 22 | 110 | 66 | |
| 32 | p-Cl | 5j | 24 | 110 | 71 | |
| 33 | m-NO2 | 5k | 24 | 110 | 80 | |
| 34 | p-MeO | 5l | 24 | 110 | 75 | |
| 35 | o-CH3OOC | 5m | 20 | 110 | 65 | |
It is worth to mention that the removal or exchange of the pyrazole C(4)-nitro substituent makes the starting materials unreactive in our protocol. The unsubstituted 1-phenethyl-1H-pyrazole as well as the ethyl 1-phenethyl-1H-pyrazole-4-carboxylate derivatives gave only faint traces of the products or no reaction at all when submitted to the reaction with a variety of phenyl iodides. This result shows the impact of the directing effect of the nitro group. The same situation is true for 4-nitro N-phenyl pyrazoles. Derivatives with p-tolyl- and 4-fluorophenyl-substituents both gave negative results what indicates that electron-withdrawing substituents at the N(1) position can also render the starting material unreactive at the C(5) position. Therefore, as a next step we submitted an electron rich 3-methyl-1-phenyl-1H-pyrazole-5-amine (sm5) that has only the C(4)–H bond available for transformation. As expected, this starting material underwent the C–H activation under the optimized conditions much easier. This allowed for a broader scope of aryl iodides to be tested, however all the attempts gave very satisfactory results. Lower yields were recorded only for o-Br and o-CH3OOC substituted aryl iodides, what could be caused by the volume of those substituents (Table 2, entries 31 and 35 respectively).
Following our previous work, we were eager to test also 4-nitro imidazole derivatives within our protocol. The first attempts however were disappointing in terms of the yield. We conducted a short optimization again (Table 3), for starting material sm7. The prolongation of the reaction course and raising the temperature had only limited influence on the reaction yield. CuBr2 remained the best catalyst. Finally, switching to Cs2CO3 as base and DMA as solvent raised the yield to about 68% (Table 3, entry 8). Similarly as for pyrazoles, the selenation occurred exclusively at the C(5) position of the imidazole ring. We also conducted several experiments regarding N-substitution of 4-nitroimidazoles. Similarly as for pyrazoles, starting material with p-tolyl- substituent at the N(1) position gave only trace product. We also submitted to the protocol two N-methyl derivatives. 1-Methyl-4-nitro-1H-imidazole did not react at all, whereas 1,2-dimethyl-4-nitro-1H-imidazole gave only trace product.
| Entry | Catalyst | Base | Solvent | Temp./°C | Time/h | Yield/% |
|---|---|---|---|---|---|---|
| a Reaction conditions unless specified otherwise: 1 equiv. sm7, 2 equiv. aryl iodide, 3 equiv. selenium powder (100 mesh), 4 equiv. base, catalyst (10 mol%), 1 mL dry solvent. Performed in a Teflon screw cap-sealed pressure tube.b Loaded in glovebox under argon. | ||||||
| 1 | CuBr2 | K2CO3 | DMSO | 110 | 18 | 10 |
| 2 | CuBr2 | K2CO3 | DMSO | 125 | 96 | 18 |
| 3 | CuBr2 | K2CO3 | DMSO | 130 | 40 | 14 |
| 4 | CuBr2 | K2CO3 | Toluene | 130 | 40 | NR |
| 5 | CuBr2 | K2CO3 | DMF | 130 | 40 | 22 |
| 6 | CuBr2 | K2CO3 | DMA | 130 | 40 | 34 |
| 7 | CuBr2 | Cs2CO3 | DMSO | 115 | 24 | 49 |
| 8 | CuBr2 | Cs2CO3 | DMA | 115 | 24 | 68 |
| 9 | CuBr2 | Li2CO3 | DMSO | 115 | 40 | NR |
| 10 | CuI | K2CO3 | DMSO | 115 | 40 | NR |
| 11 | Cu(OAc)2 | K2CO3 | DMSO | 115 | 40 | Trace |
| 12 | NiCl2·6H2O | K2CO3 | DMSO | 115 | 40 | NR |
| 13 | AgOAc | K2CO3 | DMSO | 115 | 40 | Trace |
| 14 | PdCl2(PPh3)2 | K2CO3 | DMSO | 115 | 40 | Tracea |
To the best of our knowledge there are only a few reports of compounds structurally similar to ours that contain the pyrazole or imidazole structural moiety, all of them were obtained from diselenides as selenium source. Perin and Schumacher et al. developed an oxidant promoted mild protocol using potassium peroxymonosulfate (Oxone) to conduct direct selenation of N-unsubstituted pyrazoles.99 Yan et al. successfully selenated a series of N-phenyl substituted pyrasoles via I2 mediated protocol.100 Zhang and Zhong et al. used a FeBr3/I2 complex to introduce phenyl- and benzyl-selanyl groups into a series of N-phenyl aminopyrazoles.101 Schiesser et al. performed a lithium/selenium exchange on a N-SEM protected (2-(trimethylsilyl)ethoxymethyl-) imidazole ring as a step in the synthesis of selenofonsartan analogues.102 We tested also benzimidazole derivative sm10 as an example of fused imidazole derivative. The outcome was quite satisfying (Table 4, entry 9–72% yield, entry 10–77% yield). A triazole derivative sm11 was also tested as an example of this family of five-membered heterocycles.
| Entry | Structure | R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) |
Number | Time/h | Temp./°C | Yield/% |
|---|---|---|---|---|---|---|
| a Reaction conditions unless specified otherwise: 1 equiv. sm6–sm12, 2 equiv. aryl iodide, 3 equiv. selenium powder (100 mesh), 4 equiv. K2CO3, CuBr2 (10 mol%), 1 mL dry DMSO. Performed in a Teflon screw cap-sealed Pressure Tube, loaded in air.b Base – Cs2CO3 and solvent – DMA. | ||||||
| 1 | 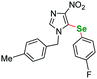 |
6 | 24 | 115 | 65a | |
| 2 | 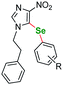 |
p-Me | 7a | 24 | 115 | 86a |
| 3 | o-F | 7b | 30 | 115 | 55a | |
| 4 | m-F | 7c | 24 | 120 | 73a | |
| 5 | p-F | 7d | 30 | 115 | 68a | |
| 6 | 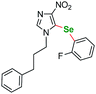 |
8 | 24 | 120 | 60a | |
| 7 |  |
o-F | 9a | 25 | 120 | 57a |
| 8 | p-F | 9b | 30 | 120 | 73a | |
| 9 |  |
3,5-diCF3 | 10a | 24 | 115 | 72 |
| 10 | m-OCF3 | 10b | 24 | 115 | 77 | |
| 11 |  |
11 | 24 | 115 | 67 | |
| 12 |  |
p-Et | 12a | 30 | 120 | 46 |
| 13 | p-F | 12b | 30 | 120 | 48 | |
Chromones and their derivatives are common motifs abundant in biologically active compounds, numerous natural products, and pharmaceuticals.103–105 Following our interest in those scaffolds106,107 we submitted 4H-chromen-4-one (sm12) to aryloselenation to our optimized reaction conditions. The two attempts we undertook gave satisfactory results (Table 4, entries 12 and 13). To the best of our knowledge there are only a few literature reports of selenated chromones (either by direct C–H activation108,109 or via de novo cyclization110,111) none of them obtained with the use of elemental selenium. With respect to the latter methodology we also submitted an enaminone to our protocol however, the reaction of (E)-3-(dimethylamino)-1-(2-hydroxyphenyl)prop-2-en-1-one was unsuccessful. A few other heteroaryl starting materials were tested within the current protocol giving negative results. Among them were: 3-methyl-5-nitro-1-phenyl-1H-pyrazolo[3,4-b]pyridine, 1,3-dimethyl-6-aminouracil, 3,4,5-trimethoxyaniline and 3-nitropyridine.
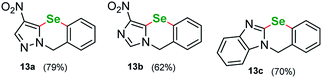
Following other seminal works in the field we were curious to see if our protocol can be used to perform an intramolecular cyclization introducing selenium. We procured a series of heterocycles bearing 2-iodobenzyl- substituent at the N(1) ring atom: 1-(2-iodobenzyl)-1H-pyrazole, 1-(2-iodobenzyl)-4-nitro-1H-pyrazole, ethyl 1-(2-iodobenzyl)-1H-pyrazole-4-carboxylate, 1-(2-iodobenzyl)-1H-imidazole, 1-(2-iodobenzyl)-4-nitro-1H-imidazole, ethyl 1-(2-iodobenzyl)-1H-imidazole-4-carboxylate and 1-(2-iodobenzyl)-1H-benzo[d]imidazole. Similarly, to the parent starting materials tested in the intermolecular attempts, only the nitro-derivatives and benzimidazole successfully reacted giving benzoselenazines 13a, 13b and 13c in 79%, 62% and 70% yield respectively (Fig. 2).
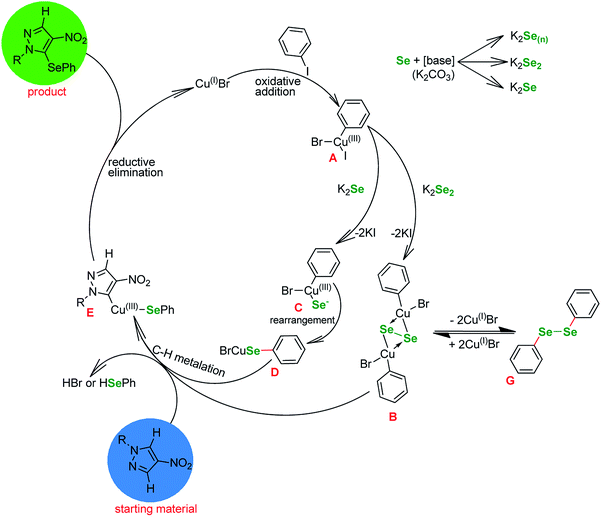
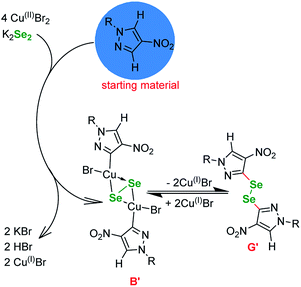
Basing on the literature reports, our observations and the outcome of the optimizations (Tables 1 and 3) we propose the following mechanism (Fig. 1). Since the reaction is not proceeding without base (Table 1, entries 3, 5 and 12) it is safe to assume that the selenium enters the catalytic cycle in a form of a selenide anion (Se2−)61,62 or diselenide anion (Se22−).57 The reduction of elemental selenium with base is a well known process. The first step in the catalytic cycle involving copper is oxidative addition of Cu(I) halide into the I–CAr bond of the phenyl iodide, forming Cu(III) intermediate A.
These species react with the reduced selenium to produce a copper–selenium complex. At this point it is unpractical to distinguish between mono, di and polyselenide anions and whether the Se–Se bond is retained or not. Many authors formally agree on the existence of a square-planar species B, which is a common point with similar to our copper-catalyzed selenations utilizing diselenides, since it can undergo a reversible transformation to diphenyl diselenide G.59 However, at this point it is necessary to depict the transfer of the Se atom (C to D) and the formation of Se–C bond. Next, the selenium–copper complex enters reaction with the heterocyclic starting material in a metalation step, with the formal extraction of HBr or HSePh species. The intermediate E undergoes reductive elimination to give the desired product and regenerate the catalyst.
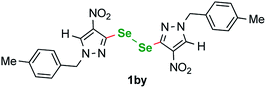
We have also detected an unusual byproduct in the crude mixtures from reactions 1a–1i. Diselenide 1by (Fig. 3) was isolated during the chromatographic purification of products 1e and 1g from the crude mixtures in 10% and 8% yield. The presence of this diselenide can be explained as follows (Fig. 4). The starting material is oxidized by Cu(II) species forming a copper complex that reacts with K2Se2 to give an analogous to B square-planar species B′, that can also undergo a reversible transformation yielding byproduct G′.
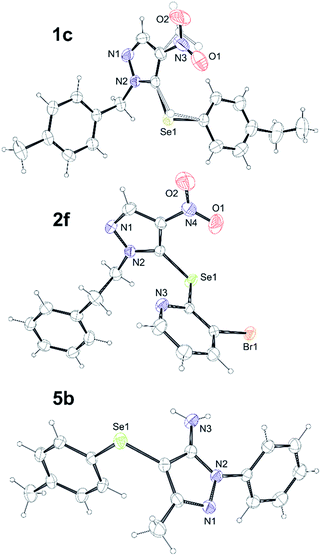
Finally, compounds 1c, 2f, 5b, 5d, 5f, 5i, 5k and 5m were obtained as single crystals suitable for X-ray diffraction analysis. X-ray crystallographic analyses of the title structures helped us to corroborate the structural constitution of the samples and determined the position of Se–Ar substituent (See Fig. 1 in ESI†). The representative structures of compounds 1c, 2f and 5b are depicted in the Fig. 5.
Conclusions
We have proposed a general method to obtain unsymmetrical heteroaryl-aryl selenides containing 4-nitropyrazole, 4-nitroimidazole and a few other scaffolds by copper-catalyzed direct C–H selenation of N-substituted heteroaryls with iodobenzenes and elemental selenium. The scope and limitations of our methodology has been assessed in respect to both organic starting materials. A plausible mechanism has been proposed in relation to the previous research. The established reaction conditions work very well for 4-nitropyrasole derivatives and acceptably good for 4-nitroimidazoles. A total of 48 compounds have been prepared, majority of them bearing easily transformable nitro group and a few of them substituted with a similarly useful groups in the benzene ring. The protocol is also useful for closing a benzoselenazine ring from appropriate starting materials.Conflicts of interest
Authors declare no conflict of interest.Acknowledgements
This research program is supported by two grants from Polish National Center of Sciences (NCN): Marie Skłodowska-Curie POLONEZ 2 grant (Nr. 2016/21/P/ST5/00630) obtained by Dr Satenik Mkrtchyan and SONATA 10 (Nr. 2015/19/D/ST5/02774) obtained by Dr Viktor O. Iaroshenko. We also would like to acknowledge the Operational Project of Education Development 2014–2020 co-financed by European Social Fund (Paulina H. Marek).Notes and references
- E. R. T. Tiekink, Dalton Trans., 2012, 41, 6390–6395 RSC.
- D. Manna, G. Roy and G. Mugesh, Acc. Chem. Res., 2013, 46, 2706–2715 CrossRef CAS PubMed.
- L. Engman, M. Karlsson Ott, V. P. Singh, J. Poon, J. Yan, S. Kumar, S. Kumar and X. Lu, Angew. Chem., Int. Ed., 2016, 55, 3729–3733 CrossRef PubMed.
- W. Hassan, M. Jan, J. Rafique, D. Back, E. Waczuk, A. Braga, J. Da Rocha, T. Frizon, S. Saba and R. Canto, Molecules, 2015, 20, 10095–10109 CrossRef PubMed.
- F. A. R. Barbosa, R. F. S. Canto, S. Saba, J. Rafique and A. L. Braga, Bioorg. Med. Chem., 2016, 24, 5762–5770 CrossRef CAS PubMed.
- F. L. Muller, M. S. Lustgarten, Y. Jang, A. Richardson and H. Van Remmen, Free Radical Biol. Med., 2007, 43, 477–503 CrossRef CAS PubMed.
- O. Epp, R. Ladenstein and A. Wendel, Eur. J. Biochem., 1983, 133, 51–69 CrossRef CAS PubMed.
- R. Longtin, J. Natl. Cancer Inst., 2004, 96, 504–505 CrossRef PubMed.
- K. P. Bhabak and G. Mugesh, Acc. Chem. Res., 2010, 43, 1408–1419 CrossRef CAS PubMed.
- T. Schewe, Gen. Pharmacol., 1995, 26, 1153–1169 CrossRef CAS PubMed.
- H. Sies, Methods Enzymol., 1994, 234, 476–482 CAS.
- H. Sies, Free Radical Biol. Med., 1993, 14, 313–323 CrossRef CAS PubMed.
- E. Domínguez-Álvarez, M. Álvarez-Pérez, J. Handzlik, M. Marć and W. Ali, Molecules, 2018, 23, 628 CrossRef PubMed.
- B. K. Sarma and G. Mugesh, Org. Biomol. Chem., 2008, 6, 965–974 RSC.
- C. W. Nogueira, G. Zeni and J. B. T. Rocha, Chem. Rev., 2004, 104, 6255–6286 CrossRef CAS PubMed.
- G. Mugesh and H. B. Singh, Chem. Soc. Rev., 2000, 29, 347–357 RSC.
- G. Mugesh, W. W. Du Mont and H. Sies, Chem. Rev., 2001, 101, 2125–2179 CrossRef CAS PubMed.
- A. J. Cresswell, S. T.-C. Eey and S. E. Denmark, Nat. Chem., 2015, 7, 146–152 CrossRef CAS PubMed.
- M. Godoi, M. W. Paixão and A. L. Braga, Dalton Trans., 2011, 40, 11347–11355 RSC.
- J. V. Comasseto, L. W. Ling, N. Petragnani and H. A. Stefani, Synthesis, 1997, 373–403 CrossRef CAS.
- T. G. Back, Organoselenium Chemistry: A Practical Approach, Oxford University Press, Oxford UK, 1999 Search PubMed.
- A. L. Braga, D. S. Lütke, F. Vargas and R. C. Braga, Synlett, 2006, 2006, 1453–1466 CrossRef.
- A. L. Braga, F. Vargas, J. A. Sehnem and R. C. Braga, J. Org. Chem., 2005, 70, 9021–9024 CrossRef CAS PubMed.
- A. Krief and L. Hevesi, Organoselenium Chemistry I, Springer Berlin Heidelberg, Berlin, Heidelberg, 2011 Search PubMed.
- A. Krief and M. Derock, Tetrahedron Lett., 2002, 43, 3083–3086 CrossRef CAS.
- A. Krief and M. Derock, Synlett, 2005, 2005, 1755–1757 CrossRef.
- A. Krief, M. Trabelsi, W. Dumont and M. Derock, Synlett, 2004, 2004, 1751–1754 CrossRef.
- J. Młochowski and L. Syper, in Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, Ltd, Chichester, UK, 2001 Search PubMed.
- H. M. Leicester, Org. Synth., 1943, 2, 238 Search PubMed.
- R. Lesser and R. Weiß, Ber. Dtsch. Chem. Ges., 1914, 47, 2510–2525 CrossRef CAS.
- W. Dilthey, L. Neuhaus, E. Reis and W. Schommer, J. Prakt. Chem., 1929, 124, 81–126 CrossRef.
- O. Behaghel and K. Hofmann, Ber. Dtsch. Chem. Ges., 1939, 72, 697–712 CrossRef.
- A. Schoeller, Ber. Dtsch. Chem. Ges., 1919, 52, 1517–1518 CrossRef.
- D. L. J. Clive and H. Cheng, Synth. Commun., 2003, 33, 1951–1961 CrossRef CAS.
- A. Krief, in Comprehensive Organometallic Chemistry II, Elsevier, 1995, pp. 515–569 Search PubMed.
- N. Taniguchi, Synlett, 2005, 2005, 1687–1690 CrossRef.
- Y. Li, C. Nie, H. Wang, X. Li, F. Verpoort and C. Duan, Eur. J. Org. Chem., 2011, 2011, 7331–7338 CrossRef CAS.
- N. Taniguchi, Tetrahedron, 2012, 68, 10510–10515 CrossRef CAS.
- K. H. V. Reddy, V. P. Reddy, B. Madhav, J. Shankar and Y. V. D. Nageswar, Synlett, 2011, 2011, 1268–1272 CrossRef.
- A. V. Kumar, V. P. Reddy, C. S. Reddy and K. R. Rao, Tetrahedron Lett., 2011, 52, 3978–3981 CrossRef CAS.
- M. Matsumura, H. Kumagai, Y. Murata, N. Kakusawa and S. Yasuike, J. Organomet. Chem., 2016, 807, 11–16 CrossRef CAS.
- A. Kumar and S. Kumar, Tetrahedron, 2014, 70, 1763–1772 CrossRef CAS.
- J. T. Yu, H. Guo, Y. Yi, H. Fei and Y. Jiang, Adv. Synth. Catal., 2014, 356, 749–752 CrossRef CAS.
- V. P. Reddy, A. V. Kumar and K. R. Rao, J. Org. Chem., 2010, 75, 8720–8723 CrossRef CAS PubMed.
- K. Swapna, S. N. Murthy and Y. V. D. Nageswar, Eur. J. Org. Chem., 2011, 2011, 1940–1946 CrossRef.
- B. Movassagh and Z. Hosseinzadeh, Synlett, 2016, 27, 777–781 CrossRef CAS.
- Y. Li, H. Wang, X. Li, T. Chen and D. Zhao, Tetrahedron, 2010, 66, 8583–8586 CrossRef CAS.
- A. Kumar, B. S. Bhakuni, C. D. Prasad, S. Kumar and S. Kumar, Tetrahedron, 2013, 69, 5383–5392 CrossRef CAS.
- R. K. Gujadhur and D. Venkataraman, Tetrahedron Lett., 2003, 44, 81–84 CrossRef CAS.
- H. Suzuki, H. Abe and A. Osuka, Chem. Lett., 2006, 10, 151–152 CrossRef.
- S. Bhadra, A. Saha and B. C. Ranu, J. Org. Chem., 2010, 75, 4864–4867 CrossRef CAS PubMed.
- T. Taniguchi, A. Murata, M. Takeda, T. Mizuno, A. Nomoto and A. Ogawa, Eur. J. Org. Chem., 2017, 2017, 4928–4934 CrossRef CAS.
- N. Taniguchi and T. Onami, J. Org. Chem., 2004, 69, 915–920 CrossRef CAS PubMed.
- B. Mohan, C. Yoon, S. Jang and K. H. Park, ChemCatChem, 2015, 7, 405–412 CrossRef CAS.
- S. Kumar and L. Engman, J. Org. Chem., 2006, 71, 5400–5403 CrossRef CAS PubMed.
- A. Ivanova and P. Arsenyan, Coord. Chem. Rev., 2018, 370, 55–68 CrossRef CAS.
- M. M. Peterle, M. R. Scheide, L. T. Silva, S. Saba, J. Rafique and A. L. Braga, ChemistrySelect, 2018, 3, 13191–13196 CrossRef CAS.
- P. Sun, M. Jiang, W. Wei, Y. Min, W. Zhang, W. Li, D. Yang and H. Wang, J. Org. Chem., 2017, 82, 2906–2913 CrossRef CAS PubMed.
- T. Guo, Z. Dong, P. Zhang, W. Xing and L. Li, Tetrahedron Lett., 2018, 59, 2554–2558 CrossRef CAS.
- T. Guo, X. N. Wei, H. Y. Wang, Y. L. Zhu, Y. H. Zhao and Y. C. Ma, Org. Biomol. Chem., 2017, 15, 9455–9464 RSC.
- D. Luo, G. Wu, H. Yang, M. Liu, W. Gao, X. Huang, J. Chen and H. Wu, J. Org. Chem., 2016, 81, 4485–4493 CrossRef CAS PubMed.
- D. Hu, M. Liu, H. Wu, W. Gao and G. Wu, Org. Chem. Front., 2018, 5, 1352–1355 RSC.
- M. Iwasaki, Y. Tsuchiya, K. Nakajima and Y. Nishihara, Org. Lett., 2014, 16, 4920–4923 CrossRef CAS PubMed.
- S. Dana, A. Mandal, H. Sahoo and M. Baidya, Org. Lett., 2017, 19, 1902–1905 CrossRef CAS PubMed.
- V. G. Ricordi, S. Thurow, F. Penteado, R. F. Schumacher, G. Perin, E. J. Lenardão and D. Alves, Adv. Synth. Catal., 2015, 357, 933–939 CrossRef CAS.
- A. R. Rosario, K. K. Casola, C. E. S. Oliveira and G. Zeni, Adv. Synth. Catal., 2013, 355, 2960–2966 CrossRef CAS.
- L. Ackermann, in Modern Arylation Methods, Wiley-VCH, 2009, p. 543 Search PubMed.
- G. Dyker, in Handbook of C–H Transformations: Applications in Organic Synthesis, Wiley-VCH, 2005, p. 661 Search PubMed.
- T. Brückl, R. D. Baxter, Y. Ishihara and P. S. Baran, Acc. Chem. Res., 2012, 45, 826–839 CrossRef PubMed.
- J. Roger, A. L. Gottumukkala and H. Doucet, ChemCatChem, 2010, 2, 20–40 CrossRef CAS.
- N. Kuhl, M. N. Hopkinson, J. Wencel-Delord and F. Glorius, Angew. Chem., Int. Ed., 2012, 51, 10236–10254 CrossRef CAS PubMed.
- S. Yu, B. Wan and X. Li, Org. Lett., 2015, 17, 58–61 CrossRef CAS PubMed.
- X. Fang, Z. Weng, Y. Song, W. Ma, L. Ackermann and L. Gu, Eur. J. Org. Chem., 2019, 41–45 Search PubMed.
- J. Rafique, S. Saba, T. E. A. Frizon and A. L. Braga, ChemistrySelect, 2018, 3, 328–334 CrossRef CAS.
- W. Ma, H. Dong, D. Wang and L. Ackermann, Adv. Synth. Catal., 2017, 359, 966–973 CrossRef CAS.
- A. Thupyai, C. Pimpasri and S. Yotphan, Org. Biomol. Chem., 2018, 16, 424–432 RSC.
- A. Mandal, H. Sahoo and M. Baidya, Org. Lett., 2016, 18, 3202–3205 CrossRef CAS PubMed.
- S. Shu, Z. Fan, Q. Yao and A. Zhang, J. Org. Chem., 2016, 81, 5263–5269 CrossRef CAS PubMed.
- K. D. Patel, S. M. Prajapati, S. N. Panchal and H. D. Patel, Synth. Commun., 2014, 44, 1859–1875 CrossRef CAS.
- O. Daugulis, H. Q. Do and D. Shabashov, Acc. Chem. Res., 2009, 42, 1074–1086 CrossRef CAS PubMed.
- D. A. Colby, R. G. Bergman and J. A. Ellman, Chem. Rev., 2010, 110, 624–655 CrossRef CAS PubMed.
- P. B. Arockiam, C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879–5918 CrossRef CAS PubMed.
- O. Baudoin, Chem. Soc. Rev., 2011, 40, 4902–4911 RSC.
- A. V. Gulevich, F. S. Melkonyan, D. Sarkar and V. Gevorgyan, J. Am. Chem. Soc., 2012, 134, 5528–5531 CrossRef CAS PubMed.
- C. Huang, N. Ghavtadze, B. Godoi and V. Gevorgyan, Chem.–Eur. J., 2012, 18, 9789–9792 CrossRef CAS PubMed.
- D. Lapointe, T. Markiewicz, C. J. Whipp, A. Toderian and K. Fagnou, J. Org. Chem., 2011, 76, 749–759 CrossRef CAS PubMed.
- I. V. Seregin and V. Gevorgyan, Chem. Soc. Rev., 2007, 36, 1173–1193 RSC.
- N. Ghavtadze, F. S. Melkonyan, A. V. Gulevich, C. Huang and V. Gevorgyan, Nat. Chem., 2014, 6, 122–125 CrossRef CAS PubMed.
- T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147–1169 CrossRef CAS PubMed.
- D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174–238 CrossRef CAS PubMed.
- E. Ben-Ari, M. Gandelman, H. Rozenberg, L. J. W. Shimon and D. Milstein, J. Am. Chem. Soc., 2003, 125, 4714–4715 CrossRef CAS PubMed.
- B. Liégault, I. Petrov, S. I. Gorelsky and K. Fagnou, J. Org. Chem., 2010, 75, 1047–1060 CrossRef PubMed.
- J.-W. Gu, S.-Z. Zhu, P. Li, H. Zhang, G. Zhao, Y. Ying and Y.-M. He, Tetrahedron, 2010, 66, 8387–8391 Search PubMed.
- P. Guo, J. M. Joo, S. Rakshit and D. Sames, J. Am. Chem. Soc., 2011, 133, 16338–16341 CrossRef CAS PubMed.
- L. Caron, L. C. Campeau and K. Fagnou, Org. Lett., 2008, 10, 4533–4536 CrossRef CAS PubMed.
- V. O. Iaroshenko, A. Gevorgyan, O. Davydova, A. Villinger and P. Langer, J. Org. Chem., 2014, 79, 2906–2915 CrossRef CAS PubMed.
- V. O. Iaroshenko, A. Gevorgyan, S. Mkrtchyan, K. Arakelyan, T. Grigoryan, J. Yedoyan, A. Villinger and P. Langer, J. Org. Chem., 2015, 80, 2103–2119 CrossRef CAS PubMed.
- V. O. Iaroshenko, A. Gevorgyan, S. Mkrtchyan, T. Grigoryan, E. Movsisyan, A. Villinger and P. Langer, ChemCatChem, 2015, 7, 316–324 CrossRef CAS.
- R. Cargnelutti, A. M. Barcellos, D. Alves, R. F. Schumacher, G. Perin, A. L. Belladona, I. Rodrigues and J. A. Roehrs, Tetrahedron, 2018, 74, 4242–4246 CrossRef.
- J. Wang, Y. Liu and J. Yan, New J. Chem., 2018, 42, 13684–13688 RSC.
- M. Xu, X. H. Zhang and P. Zhong, Synth. Commun., 2012, 42, 3472–3481 CrossRef CAS.
- R. L. Grange, J. Ziogas, J. A. Angus and C. H. Schiesser, Tetrahedron Lett., 2007, 48, 6301–6303 CrossRef CAS.
- G. P. Ellis, in The Chemistry of Heterocyclic Compounds, Wiley, 2008, vol. 31, pp. 1692–1693 Search PubMed.
- J. Garrido, E. Uriarte, A. Gaspar, F. Borges and M. J. Matos, Chem. Rev., 2014, 114, 4960–4992 CrossRef PubMed.
- S. Wetzel, R. S. Bon, K. Kumar and H. Waldmann, Angew. Chem., Int. Ed., 2011, 50, 10800–10826 CrossRef CAS PubMed.
- M. Miliutina, S. A. Ejaz, V. O. Iaroshenko, A. Villinger, J. Iqbal and P. Langer, Org. Biomol. Chem., 2016, 14, 495–502 RSC.
- S. Mkrtchyan and V. O. Iaroshenko, Eur. J. Org. Chem., 2018, 2018, 6867–6875 CrossRef CAS.
- T. Guo, Synth. Commun., 2017, 47, 2053–2061 CrossRef CAS.
- J. Zhu, B. Xu, J. Yu, Y. Ren, J. Wang, P. Xie, C. U. Pittman and A. Zhou, Org. Biomol. Chem., 2018, 16, 5999–6005 RSC.
- S. M. Silva, A. L. Braga, S. Saba, A. R. Schneider, J. Rafique and M. S. Franco, ACS Omega, 2017, 2, 2280–2290 CrossRef.
- B. Godoi, A. Sperança, C. A. Bruning, D. F. Back, P. H. Menezes, C. W. Nogueira and G. Zeni, Adv. Synth. Catal., 2011, 353, 2042–2050 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1922898–1922905. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9ra05004c |
| This journal is © The Royal Society of Chemistry 2019 |