Open Access Article
Open Access ArticleTiO2–Au composite nanofibers for photocatalytic hydrogen evolution
Xiaojiao Yang a,
Xuelian Wub,
Jun Lia and
Ying Liu
a,
Xuelian Wub,
Jun Lia and
Ying Liu *a
*a
aCollege of Materials Science and Engineering, Sichuan University, No. 24 South Section 1, Yihuan Road, Chengdu 610065, P. R. China. E-mail: liuying5536@scu.edu.cn
bInternational Collaborative Laboratory of 2D Materials for Optoelectronics Science and Technology of MOE, Institute of Microscale Optoelectronics, Shenzhen University, Shenzhen 518060, China
First published on 17th September 2019
Abstract
TiO2-based materials for photocatalytic hydrogen (H2) evolution have attracted much interest as a renewable approach for clean energy applications. TiO2–Au composite nanofibers (NFs) with an average fiber diameter of ∼160 nm have been fabricated by electrospinning combined with calcination treatment. In situ reduced gold nanoparticles (NPs) with uniform size (∼10 nm) are found to disperse homogenously in the TiO2 NF matrix. The TiO2–Au composite NFs catalyst can significantly enhance the photocatalytic H2 generation with an extremely high rate of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 μmol g−1 h−1, corresponding to an adequate apparent quantum yield of 5.11% at 400 nm, which is 25 times and 10 times those of P25 (584 μmol g−1 h−1) and pure TiO2 NFs (1254 μmol g−1 h−1), respectively. Furthermore, detailed studies indicate that the H2 evolution efficiency of the TiO2–Au composite NF catalyst is highly dependent on the gold content. This work provides a strategy to develop highly efficient catalysts for H2 evolution.
440 μmol g−1 h−1, corresponding to an adequate apparent quantum yield of 5.11% at 400 nm, which is 25 times and 10 times those of P25 (584 μmol g−1 h−1) and pure TiO2 NFs (1254 μmol g−1 h−1), respectively. Furthermore, detailed studies indicate that the H2 evolution efficiency of the TiO2–Au composite NF catalyst is highly dependent on the gold content. This work provides a strategy to develop highly efficient catalysts for H2 evolution.
Introduction
Photocatalytic hydrogen (H2) evolution has drawn great attention as a promising approach to overcome the energy crisis by converting solar energy to H2 fuel directly.1–3 On the basis of Fujishima's pioneering work,4 numerous semiconductor-based photocatalysts have been developed to produce H2 (such as TiO2,5 CdS,6 ZnO,7 Cu2O,8 etc.). TiO2 has attracted much attention owing to its excellent properties including low cost, environmental friendliness, strong oxidizing power and high stability.9 However, the photocatalytic efficiency of TiO2-based materials is still limited, with a low solar light harvesting capability due to its wide band gap and high charge recombination rate.10 Therefore, it is important to develop TiO2-based photocatalysts for increasing the light harvesting and promoting the photoinduced charge separation during the H2 generation process.Numerous attempts have been devoted to improving the H2 generation efficiency of TiO2 materials including doping,11 metal deposition,10,12 and heterostructures formation.13 Chiu14 has designed a quaternary Ti–Nb–Ta–Zr–O mixed-oxide nanotube arrays; Hsieh15 has conducted compact deposition of In2S3 nanocrystals on the TiO2 nanowire array; Li16 has prepared by electrodepositing a Cu2O layer on the surface of Au particle-coated TiO2 arrays; Chang17 has synthesized CdS/CdSe co-sensitized brookite TiO2 nanostructures with hydrogen doping (H:TiO2/CdS/CdSe) in a facile solution reaction. All these TiO2-based materials were developed to enhance the amount of accessible charge carriers, modify the electronic structure, improve the hole injection kinetics for the photoelectrochemical water splitting.
Combining TiO2 with highly dispersed noble metal nanoparticles (NPs) as cocatalyst is a feasible approach for enhancing the photocatalytic performance.18 Among the noble metal NPs (i.e. Au,19,20 Ag,21 Pt,22 Pd23,24), gold with finite NPs size can spatially separate the photogenerated charge carriers.3,25 Much efforts have been focused on TiO2 NPs as matrix and different size or shape of gold NPs, to understand the fine-tuning the surface plasmon resonance effect.26 Pu and his colleagues27 have reported that the photocatalytic ability of TiO2 nanowires can be effectively enhanced in the entire UV-visible region by adopting different shapes of the decorated Au nanostructures. Compared to other nanostructures, TiO2 nanofibers (NFs) possess additional advantages due to its specific morphologies with large aspect ratio and large specific surface area for photocatalytic activity.28 Yoshikawa29 has proved that the TiO2 NFs catalyst can promote the opportunities for photocatalytic H2 evolution. Xia and Li30,31 successfully fabricated the TiO2 NFs and its composite deposited with gold NPs. However, there are seldom literatures studying the TiO2–Au composite NFs as photocatalyst for H2 generation and the effect of gold content on photocatalytic performance.
Herein, TiO2–Au NFs composites with different gold content were fabricated via electrospinning combined with subsequent calcination. The gold NPs are in situ reduced during the calcination process while the polymer components are decomposed and removed to form TiO2 NFs.32 The gold content is tuned by controlling the amount of gold precursor added during synthesis process, and all the chemicals are mixed homogenously in solution with molecular level. The H2 evolution with TiO2–Au NFs catalyst has been investigated.
Methods
Materials
Polyvinylpyrrolidone (PVP, Mw ∼ 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300
300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and chloroauric acid (HAuCl4·3H2O, ≥99.9%) were purchased from Aldrich (St. Louis, MO, USA). Titanium isopropoxide (TTIP, C12H28O4Ti, ≥99.9%), ethanol (EtOH) and acetic acid (HAc) were obtained from Chengdu Kelong Chemical Reagent Factory (Chengdu, China). All chemical reagents were analytically pure and used directly without further purification.
000) and chloroauric acid (HAuCl4·3H2O, ≥99.9%) were purchased from Aldrich (St. Louis, MO, USA). Titanium isopropoxide (TTIP, C12H28O4Ti, ≥99.9%), ethanol (EtOH) and acetic acid (HAc) were obtained from Chengdu Kelong Chemical Reagent Factory (Chengdu, China). All chemical reagents were analytically pure and used directly without further purification.
Fabrication of TiO2–Au composite NFs with different gold content
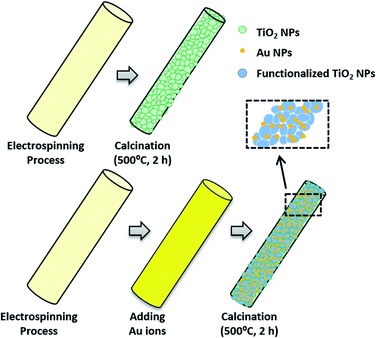
TiO2–Au composite NFs catalysts were synthesized through electrospinning (ES) combined with subsequent calcination process as shown in the schematic diagram (Fig. 1). In a typical process, PVP were dissolved in EtOH to obtain 7 wt% PVP–EtOH polymer solution, and then 1.5 g of TTIP were added into a mixture of 3 mL HAc and 3 mL EtOH, mixing with 6.43 g of the previous PVP–EtOH solution to form PVP–TTIP ES solution.33 A given amount of gold precursor (HAuCl4·3H2O) were added directly into the PVP–TTIP solution under vigorous stirring to form PVP–TTIP–Au ES solution and the mass ratio of Au/TiO2 in the final products were 0 wt%, 3 wt%, 6 wt%, 9 wt%, and 12 wt%, respectively. The as-spun precursor NFs were obtained after a typical ES process, and then calcined at 500 °C for 2 hours in air to get the final products of TiO2 NFs and TiO2–Au composite NFs with different gold content, which were denoted as TiO2–Au-0 wt% (equaled to bare TiO2 NFs), TiO2–Au-3 wt%, TiO2–Au-6 wt%, TiO2–Au-9 wt%, and TiO2–Au-12 wt%, respectively. The commercial titania catalyst of Degussa P25 is chosen as the benchmark.Photocatalyst characterization
The morphologies and structures of the TiO2–Au composite NFs were characterized by scanning electron microscope (SEM, Inspect F50, FEI, USA) and X-ray diffractometer (XRD, DX-2000, HAOYUAN, China), using Cu Kα1 λ = 1.54056 Å radiation. The average fiber diameter and particle size of samples were determined with software of Nano Measurer performed on SEM images. Transmission electron microscope (TEM) and high-resolution transmission electron microscope (HR-TEM) images, as well as energy-dispersive X-ray spectra (EDS) were obtained on a JEOL 2100F microscope to evaluate sample morphology and further confirm crystalline structures and elemental compositions. Photoluminescence (PL) spectra were performed with an excitation wavelength of 320 nm by a spectrofluorometer (FluoroMax-4, Horiba, Japan). UV-visible diffuse reflectance spectra (UV-vis) were obtained on a Shimadzu UV 3600 spectrophotometer.Photocatalytic activity evaluation
The photocatalytic H2 generation performances of all the samples were evaluated under full spectrum irradiation (a 300 W xenon arc lamp) for 3 hours. In a typical procedure, 30 mg of each prepared catalysts was dispersed in 150 mL of water/methanol mixture with the volume ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 under ultrasonic. In order to avoid the influence of air, argon was purged into the system half an hour to remove the air. And then the as-prepared suspension solution was irradiated under light (xenon lamp, 300 W) with magnetic stirring, and a water jacket was used to remove the thermal catalytic effect. The irradiation intensity for photocatalytic experiment was 380 mW cm−2. During the photocatalytic H2 generation process, the evolved gas was analyzed by using a gas chromatography (GC, 8A, Shimadzu) with an interval of 30 min.34 Apparent quantum yield (AQY) of H2 evolution were determined by using the equation of AQY (%) = (number of reacted electrons/number of incident photons) × 100%.35
1 under ultrasonic. In order to avoid the influence of air, argon was purged into the system half an hour to remove the air. And then the as-prepared suspension solution was irradiated under light (xenon lamp, 300 W) with magnetic stirring, and a water jacket was used to remove the thermal catalytic effect. The irradiation intensity for photocatalytic experiment was 380 mW cm−2. During the photocatalytic H2 generation process, the evolved gas was analyzed by using a gas chromatography (GC, 8A, Shimadzu) with an interval of 30 min.34 Apparent quantum yield (AQY) of H2 evolution were determined by using the equation of AQY (%) = (number of reacted electrons/number of incident photons) × 100%.35
Results and discussion
Gold content effects on morphologies and structures
The morphologies of TiO2 NFs and TiO2–Au composite NFs with different gold content were investigated by SEM as shown in Fig. 2. It can be seen that the TiO2 NFs (average diameter of 160 nm) possess a rough surface while the TiO2–Au composite NFs still have fibrous morphology, but with well dispersed bright dots, that were identified as gold NPs by XRD and TEM results. The gold NPs disperse homogeneous in all the TiO2–Au composite NFs with different gold content. In order to check the effects of gold content on the particle size, the average size of gold nanoparticles for each sample were determined from measurements of SEM images performed on 100 particles using the software Nano Measurer. The gold nanoparticles can be divided in two types: one is uniform gold particles, another is the nonuniform gold nanoparticles on the surface of TiO2 fiber. Firstly, by analyzing the SEM images carefully, it can be seen that the gold nanoparticles with uniform size were of ∼10 nm, which is contributed to the crystal of TiO2 that preventing the growth of gold. And this comments also are confirmed by the related TEM images as shown in Fig. 4. | ||
| Fig. 2 SEM images of TiO2–Au composite NFs with different gold content calcined at 500 °C in air for 2 h: (a) 0 wt%; (b) 3 wt%; (c) 6 wt%; (d) 9 wt% and (e) 12 wt%. | ||
Secondly, the gold nanoparticles with nonuniform size and disturbing by the gold contents on the surface of TiO2 NFs. The nanoparticles are more easily to migrate and aggregate to form larger nanoparticles with increasing of the gold content, that can overcome the inhibition from TiO2 crystals. So the higher gold content the larger particle size should make sense in logical. However, the size of particle on the surface didn't have the direct relationship with gold content, it was random form tens of nanometer. The seed formation and particle growth are complicated and influenced by many factors of thermodynamics and kinetics, especially during the calcination process. Thus the size of gold nanoparticle on the surface of TiO2–Au NFs with different contents were nonuniform. As increasing the gold content, the number of gold NPs increase, and the gold content in the final product can be varied precisely by the amount of gold precursor added in the ES solution.
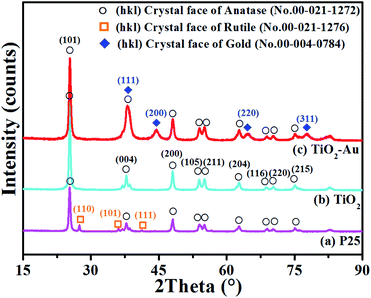
It has been figured out by Wang et al.34 that the intensity of the diffraction peaks of gold grows as increasing the gold content. Thus the sample with highest gold content of 12 wt% has been chosen and analyzed to check the difference between TiO2 NFs and TiO2–Au composite NFs more clearly. The crystal structures of P25, pure TiO2 NFs and TiO2–Au composite NFs (with gold content of 12 wt%) were determined by XRD as shown in Fig. 3. The XRD patterns of P25 and TiO2 can be well-indexed to anatase (JCPDS card no. 00-021-1272). To check carefully, there are three others peaks for P25 could be found at 2θ = 27.4°, 36.1°, and 41.2° indexed as the (110), (101), and (111) planes of rutile (JCPDS card no. 00-021-1276), respectively.32 Comparing to pure TiO2, the XRD patterns of TiO2–Au composite NFs are also well-indexed to anatase and there is no peaks related to rutile phase. Meanwhile, there are three additional peaks located at 2θ = 44.4°, 64.6°, and 77.5°, that correspond to the (200), (220), and (311) planes of the gold cubic phase (JCPDS card no.00-004-0784).32 The commercial Titania Degussa P25 is the composite of anatase and rutile, while the sample of TiO2 NFs and TiO2–Au composite NFs are mainly of anatase. These results confirm that the TiO2–Au composite NFs are composed of anatase and gold, and the crystal sizes were determined to be 20 nm and 8 nm based on the XRD patterns by using Scherrer's equation.
From TEM images (Fig. 4a), it can be seen that the NFs is well decorated with spherical NPs, where the average size is ∼10 nm. The crystallographic (111) plane of gold and (101) plane of anatase are observed clearly with the corresponding lattice distance of 2.2 Å and 3.3 Å in the HRTEM images shown in Fig. 4b. Hence, the HRTEM results of TiO2–Au composite NFs confirm that the gold NPs are well dispersed into TiO2 NFs matrix, which is in accordance with the XRD results. Furthermore, the added elemental composition of gold and titanium (during the preparation of the photocatalysts) were matching with the composition of elements analyzed by EDS shown in Fig. 4c. Murdoch et al.36 have reported that gold NPs with the size range of 3–30 nm deposited on TiO2 are highly active, whereas there is no influence on the H2 generation rate over the 3–12 nm range. Moreover, gold NPs with similar size has better functionalization effect on anatase than rutile NPs. Thus, the TiO2–Au composite NFs with gold NPs (∼10 nm) and anatase matrix of TiO2 NFs are supposed to show good photocatalytic performance for H2 evolution.
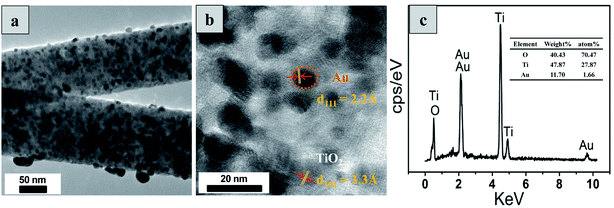 | ||
| Fig. 4 (a) TEM image of TiO2–Au-12 wt% composite NFs; (b) high-resolution TEM image of Au NPs embedded TiO2 NFs showing the Au and TiO2 lattices; (c) EDS spectrum of TiO2–Au-12 wt% composite NFs. | ||
Gold content effects on optical properties
Photoluminescence (PL) measurement was conducted on all the samples with an excitation wavelength of 320 nm, to evaluate the effects of gold content on the photoinduced charge transfer and charge recombination loss.37 As shown in Fig. 5, the PL spectra of each sample have three main emission peaks located at 394 nm, 413 nm and 467 nm within a board emission band ranging from 375 nm to 500 nm. Comparing the PL intensity of all the samples, P25 possesses the highest intensity which is followed by TiO2. After doping with gold NPs, the PL intensity of TiO2–Au is lower than TiO2, and the intensity gradually decreases as the gold content increasing. Au as an electron acceptor had a quantitative effect on the mediation of interfacial charge transfer. When the Au content increased, the emission lifetime was reduced, suggesting the significant decrease in the charge carrier separation at the interface.38 It is reported that a higher PL peak intensity corresponds to a higher incidence of photogenerated carriers recombination within titanium powders.39 The lower PL intensity for TiO2–Au samples is attributed to the electrons transfer from TiO2 conduction band to gold NPs; in this case the electron–hole recombination is restricted. Thus the photoexcited electrons are confined within gold NPs while holes remain at the TiO2 valence band.40 Consequently, TiO2–Au composite NFs catalyst can significantly inhibit the recombination of photogenerated charge carriers.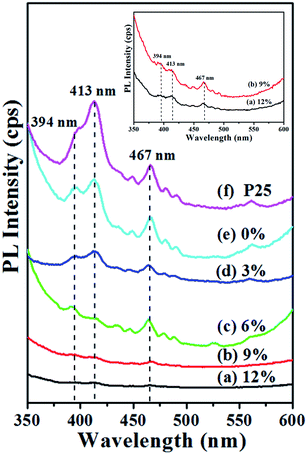 | ||
| Fig. 5 Photoluminescence spectra of P25, TiO2 NFs, and TiO2–Au composite NFs with different gold content. | ||
The UV-vis absorption spectra of all the samples are shown in Fig. 6. Comparing with the pure TiO2 NFs and P25 that can only absorb UV light, the TiO2–Au composite NFs have significant light absorption in the visible region (∼380–780 nm) due to the surface plasmon resonance (SPR) effect of gold. In general, the semiconductor band gap is related to the light absorption capability where a decreased band gap indicates the increasing of absorption edge.41 The absorbance curve of TiO2–Au composite NFs exhibited an absorption edge red shift from ultraviolet to visible region compared with that of TiO2 NFs. As increasing the gold content, the intensity of the SPR absorption peak in the visible region is dramatically increased, while the position of the SPR absorption peak shifted non-monotonically. The position and intensity of SPR absorbance peak are strongly depended on multiple factors, such as environment dielectric properties, the particle size, shape, size distribution and contents of gold NPs.42 The gold NPs of TiO2–Au composite NFs with different contents have different average size and distribution that was discussed in detail at the SEM parts. This could be the possible explanation for the non-monotonically shift.
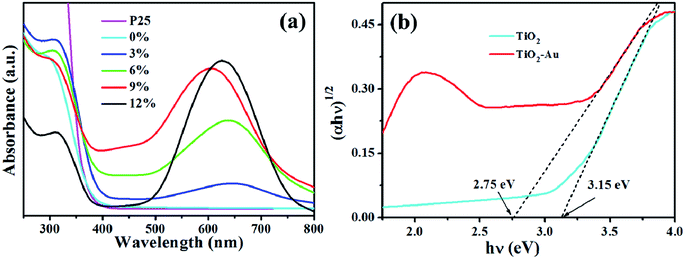 | ||
| Fig. 6 UV-visible absorption spectra of (a) P25, pure TiO2 and TiO2–Au composite with different gold content; (b) Tauc plots of TiO2 and TiO2–Au-9 wt%. | ||
As shown in Fig. 6b, the Tauc plots based on the UV-vis curves determine the band gaps, that are 3.15 eV and 2.75 eV for TiO2 NFs and TiO2–Au composite NFs, respectively.43,44 The reduced band gap of TiO2–Au composite NFs suggests that the presence of gold NPs can effectively increase the absorption edge and enhance the photocatalytic efficiency. It is also beneficial for extending the light absorption to the visible light range, that is one of the vital factors to improve the photocatalytic H2 generation performance.45 Therefore, TiO2–Au composite NFs photocatalyst can be excited by visible light, resulting in an enhancement of H2 generation.
Gold content effects on photocatalytic H2 evolution
The H2 evolution activity was compared among the P25, TiO2 NFs, TiO2–Au composites NFs with different gold content, and the H2 evolution rate is compared by normalized as per gram of catalyst and per hour of irradiation time (as shown in Fig. 7). The linear increase of all the samples in the evolved H2 indicates the stability of the catalyst. The lowest H2 evolution activity of 584 μmol g−1 h−1 was obtained from commercial P25 with the specific surface area of 53.2 m2 g−1, while the activity increases more than 2 times of TiO2 NFs (1254 μmol g−1 h−1) with the specific surface area of 18.7 m2 g−1. The photocatalytic efficiency were promoted for TiO2–Au composite NFs, attributing to the excitation of surface plasmon band by Au NPs. The excess of Au NPs loading on TiO2 can enhance the local electromagnetic field and generate high-energy hot electrons.46 As the gold content increasing from 3 wt% to 6 wt%, while the specific surface area decreased from 11.9 m2 g−1 to 9.5 m2 g−1, leading to a higher H2 generation. The TiO2–Au composite NFs with the gold content of 9 wt%, showing a specific surface area of 8.4 m2 g−1, possesses the best photocatalytic performance of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 μmol g−1 h−1, which is almost 21 times higher than P25 and nearly 10 times of TiO2. However, further increase of the gold content to 12 wt% resulted in the H2 production of 1605 μmol g−1 h−1, which is comparable with TiO2–Au-3 wt% (1602 μmol g−1 h−1). Au with relatively high content may function as recombination centers to invoke interfacial charge recombination instead of promoting charge separation.46
440 μmol g−1 h−1, which is almost 21 times higher than P25 and nearly 10 times of TiO2. However, further increase of the gold content to 12 wt% resulted in the H2 production of 1605 μmol g−1 h−1, which is comparable with TiO2–Au-3 wt% (1602 μmol g−1 h−1). Au with relatively high content may function as recombination centers to invoke interfacial charge recombination instead of promoting charge separation.46
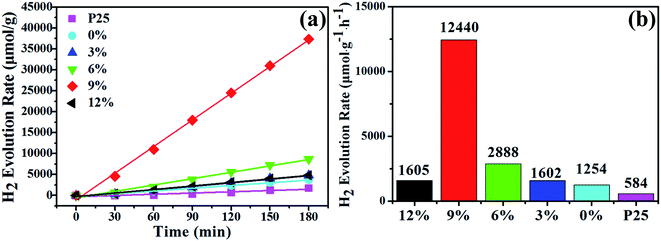 | ||
| Fig. 7 H2 evolution of P25, TiO2 NFs and TiO2–Au composite NFs catalyst with different gold content under light irradiation. | ||
In order to exclude the contribution of specific surface area, the H2 evolution rate was normalized for each sample. As shown in Table 1, the specific surface areas were decreased from 18.7 m2 g−1 to 7.2 m2 g−1, as the gold contents increased from 0 wt% to 12 wt%. It had the same results that TiO2–Au-9 wt% was the optimal sample of 1481 μmol h−1 m−2, which was 135 times higher than P25 (11 μmol h−1 m−2) and 22 times better than TiO2 (67 μmol h−1 m−2). It indicates that gold content is the predominant factor rather than the specific surface area for the photocatalytic efficiency. Meanwhile, the apparent quantum yield (AQY) of H2 evolution for all the catalysts have been determined and provided in Table 1. The highest hydrogen generation rate of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 μmol g−1 h−1, corresponding to the AQY of 5.11% at 400 nm, is achieved for TiO2–Au composite NFs with the gold content of 9 wt%. And the AQY are 0.24%, 0.52%, 0.66%, 1.19%, and 0.66% for P25, 0%, 3%, 6%, and 12%, respectively. When Au NPs with a suitable amount (9 wt%) were loading on TiO2, the interfacial charge transfer of NFs was highly mediated to render the facilitated electron scavenging from TiO2, giving rise to an increased amount of photoexcited free electrons that could be extracted for further utilization.47 These results indicate that the doping of gold NPs plays a positive role in increasing the H2 generation efficiency, and the photocatalytic performance also has direct relation with the gold content.
440 μmol g−1 h−1, corresponding to the AQY of 5.11% at 400 nm, is achieved for TiO2–Au composite NFs with the gold content of 9 wt%. And the AQY are 0.24%, 0.52%, 0.66%, 1.19%, and 0.66% for P25, 0%, 3%, 6%, and 12%, respectively. When Au NPs with a suitable amount (9 wt%) were loading on TiO2, the interfacial charge transfer of NFs was highly mediated to render the facilitated electron scavenging from TiO2, giving rise to an increased amount of photoexcited free electrons that could be extracted for further utilization.47 These results indicate that the doping of gold NPs plays a positive role in increasing the H2 generation efficiency, and the photocatalytic performance also has direct relation with the gold content.
| Sample | P25 | 0% | 3% | 6% | 9% | 12% |
|---|---|---|---|---|---|---|
| H2 evolution rate/μmol g−1 h−1 | 584 | 1254 | 1602 | 2888 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 440 |
1605 |
| Apparent quantum yield at 400 nm | 0.24% | 0.52% | 0.66% | 1.19% | 5.11% | 0.66% |
| Specific surface area/m2 g−1 | 53.2 | 18.7 | 11.9 | 9.5 | 8.4 | 7.2 |
| H2 evolution rate normalized with specific surface area/μmol h−1 m−2 | 11 | 67 | 135 | 304 | 1481 | 223 |
Photocatalytic mechanism
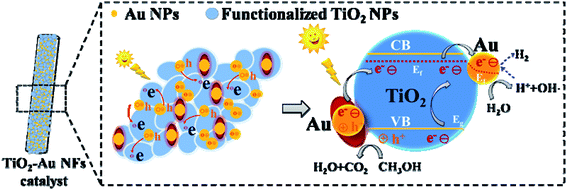
The possible photocatalytic H2 generation mechanism of TiO2–Au composite NFs from water/methanol under light irradiation is proposed (Fig. 8). The TiO2–Au composite NFs catalyst is photoexcited under light irradiation to generate the electron–hole pairs. The excited electrons from the valence band are transferred to conduction band, and then be more easily immigrate to the gold NPs band, due to the positive potential of gold compared with the conduction band level of TiO2. Thus, the gold NPs act as efficient sinks for the excited electrons, whereby it can prolong the lifetime of the charge carriers.19 Meanwhile, the gold NPs can also generate hot electrons owing to the surface plasmon resonance effect.32 The hot electrons can be transferred to the adjacent TiO2, greatly enhancing the potential light-harvesting capabilities of photocatalytic devices. Ratchford has been reported that the electron-injection efficiency from Au NPs to the TiO2 ranges from about 25% to 45%, and the hot electrons spatial distribution is affected by the plasmonic field, with a higher generation rate for stronger electric field intensities.48 Due to electromagnetic wave is transverse wave, the mostly incident light propagating approximately along the interface normal that are absorbed, so the optical electric field will be almost parallel to the interface. The relative weak light absorption efficiency and near field enhancement may be not beneficial to generation of a large number of hot electrons.49 Thus the content of gold should have an optimal value. The adsorbed H2O molecules react with the electrons on gold NPs to form the hydroxyl radicals (OH˙) and hydrogen ion (H+), and then the hydrogen ions (H+) are reduced and converted into hydrogen molecules (H2) by the photogenerated electrons trapped on the gold NPs. Meanwhile, the methanol (CH3OH) molecular as hole scavenger, react with the holes left on the valence band of TiO2 and to be further oxidized to CO2 and H2O.34 The gold NPs play two roles during the photocatalytic H2 evolution process, one is to enhance the light harvesting of TiO2–Au composite materials from UV region to visible region and generate more charge carriers under light irradiation by surface plasmon resonance effect, and another one is to work as the electron sink to reduce the recombination rate of electron–hole pairs. This proposed mechanism demonstrates that the TiO2–Au composite NFs catalyst can enhance the photocatalytic performance for H2 production.Conclusions
In summary, TiO2–Au composite NFs were successfully fabricated by electrospinning combined with subsequent calcination. Gold NPs with uniform size (∼10 nm) have been dispersed homogenously in TiO2 NFs matrix (with average fiber diameter of 160 nm). The TiO2–Au composite NFs photocatalyst showed excellent photocatalytic H2 generation from a water/methanol mixture under light irradiation. The H2 production rate of the TiO2–Au composite NFs catalyst with the gold content of 9 wt% was dramatically enhanced to 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 440 μmol g−1 h−1, corresponding to an adequate apparent quantum yield of 5.11% at 400 nm, which is 21 times and 10 times higher than that of P25 (584 μmol g−1 h−1) and TiO2 NFs (1254 μmol g−1 h−1), respectively. These results illustrate that gold NPs can facilitate the generation and transfer of photoinduced electron–hole pairs and play a vital role in boosting the H2 production efficiency, which could be achieved by tuning the gold content to an optimal value. Furthermore, such TiO2–Au composite NFs catalysts have significant potential for photocatalytic H2 evolution and also provide a new strategy in solar energy conversion.
440 μmol g−1 h−1, corresponding to an adequate apparent quantum yield of 5.11% at 400 nm, which is 21 times and 10 times higher than that of P25 (584 μmol g−1 h−1) and TiO2 NFs (1254 μmol g−1 h−1), respectively. These results illustrate that gold NPs can facilitate the generation and transfer of photoinduced electron–hole pairs and play a vital role in boosting the H2 production efficiency, which could be achieved by tuning the gold content to an optimal value. Furthermore, such TiO2–Au composite NFs catalysts have significant potential for photocatalytic H2 evolution and also provide a new strategy in solar energy conversion.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been financed by National Natural Science Foundation of China (Grant No. 51702224), the program of Sichuan Province Science and Technology Project (Grant No. 2017GZ0416) and the program of Postdoctoral Science Foundation of Sichuan University (Grant No. 2018SCU12001).References
- M. Zhao, H. Xu, S. Ouyang and H. Tong, Fabricating a Au@TiO2 plasmonic system to elucidate alkali-induced enhancement of photocatalytic H2 evolution: surface potential shift or methanol oxidation acceleration?, ACS Catal., 2018, 8(5), 4266–4277 CrossRef CAS.
- A. Fujishima, X. Zhang and D. Tryk, TiO2 photocatalysis and related surface phenomena, Surf. Sci. Rep., 2008, 63(12), 515–582 CrossRef CAS.
- X. Chen, S. Shen, L. Guo and S. Mao, Semiconductor-based photocatalytic hydrogen generation, Chem. Rev., 2010, 110, 6503–6570 CrossRef CAS PubMed.
- E. Photocell, A. Fujishima and K. Kohayakawa, Hydrogen production under sunlight with an electrochemical photocell, J. Electrochem. Soc., 1975, 122(11), 1487–1489 CrossRef.
- M. Altomare, N. Nguyen, S. Hejazi and P. Schmuki, A cocatalytic electron-transfer cascade site-selectively placed on TiO2 nanotubes yields enhanced photocatalytic H2 evolution, Adv. Funct. Mater., 2018, 28(2), 1–9 CrossRef.
- J. Xu, W. Yang, S. Huang and H. Yin, CdS core-Au plasmonic satellites nanostructure enhanced photocatalytic hydrogen evolution reaction, Nano Energy, 2018, 49, 363–371 CrossRef CAS.
- X. Lu, G. Wang, S. Xie and J. Shi, Efficient photocatalytic hydrogen evolution over hydrogenated ZnO nanorod arrays, Chem. Commun., 2012, 48, 7717–7719 RSC.
- Z. Li, J. Liu, D. Wang and Y. Gao, Cu2O/Cu/TiO2 nanotube ohmic heterojunction arrays with enhanced photocatalytic hydrogen production activity, Int. J. Hydrogen Energy, 2012, 37(8), 6431–6437 CrossRef CAS.
- F. Pellegrino, F. Sordello, M. Minella and C. Minero, The role of surface texture on the photocatalytic H2 production on TiO2, Catalysts, 2019, 9(32), 1–28 Search PubMed.
- A. S. Hainer, J. S. Hodgins, V. Sandre and M. Vallieres, Photocatalytic hydrogen generation using metal-decorated TiO2: sacrificial donors vs. true water splitting, ACS Energy Lett., 2018, 3(3), 542–545 CrossRef CAS.
- Y. Yang, K. Ye, D. Cao and P. Gao, Efficient charge separation from f-selective etching and doping of anatase–TiO2{001} for enhanced photocatalytic hydrogen production, ACS Appl. Mater. Interfaces, 2018, 10(23), 19633–19638 CrossRef CAS PubMed.
- R. Su, R. Tiruvalam, A. J. Logsdail and Q. He, Designer titania-supported Au–Pd nanoparticles for efficient photocatalytic hydrogen production, ACS Nano, 2014, 8(4), 3490–3497 CrossRef CAS PubMed.
- X. An, C. Hu, H. Liu and J. Qu, Hierarchical nanotubular anatase/rutile/TiO2(B) heterophase junction with oxygen vacancies for enhanced photocatalytic H2 production, Langmuir, 2018, 34(5), 1883–1889 CrossRef CAS PubMed.
- Y. H. Chiu, T. H. Lai, C. Y. Chen and P. Y. Hsieh, Fully depleted Ti–Nb–Ta–Zr–O nanotubes: interfacial charge dynamics and solar hydrogen production, ACS Appl. Mater. Interfaces, 2018, 10(27), 22997–23008 CrossRef CAS PubMed.
- P. Y. Hsieh, Y. H. Chiu, T. H. Lai and M. J. Fang, TiO2 nanowire-supported sulfide hybrid photocatalysts for durable solar hydrogen production, ACS Appl. Mater. Interfaces, 2019, 11(3), 3006–3015 CrossRef CAS PubMed.
- J. M. Li, C. W. Tsao, M. J. Fang and C. C. Chen, TiO2–Au–Cu2O photocathodes: Au-mediated Z-scheme charge transfer for efficient solar-driven photoelectrochemical reduction, ACS Appl. Nano Mater., 2018, 1(12), 6843–6853 CrossRef CAS.
- Y. S. Chang, M. Choi, M. Baek and P. Y. Hsieh, CdS/CdSe co-sensitized brookite H:TiO2 nanostructures: charge carrier dynamics and photoelectrochemical hydrogen generation, Appl. Catal., B, 2018, 225, 379–385 CrossRef CAS.
- A. Tanaka, S. Sakaguchi, K. Hashimoto and H. Kominami, Preparation of Au/TiO2 with metal cocatalysts exhibiting strong surface plasmon resonance effective for photoinduced hydrogen formation under irradiation of visible light, ACS Catal., 2013, 3(1), 79–85 CrossRef CAS.
- C. Marchal, T. Cottineau, M. G. Méndez-Medrano and C. Colbeau-Justin, Au/TiO2–gC3N4 nanocomposites for enhanced photocatalytic H2 production from water under visible light irradiation with very low quantities of sacrificial agents, Adv. Energy Mater., 2018, 8, 702142 Search PubMed.
- Y. H. Chiu, K. D. Chang and Y. J. Hsu, Plasmon-mediated charge dynamics and photoactivity enhancement for Au-decorated ZnO nanocrystals, J. Mater. Chem. A, 2018, 6(10), 4286–4296 RSC.
- K. H. Chen, Y. C. Pu, K. D. Chang and Y. F. Liang, Ag-nanoparticle-decorated SiO2 nanospheres exhibiting remarkable plasmon-mediated photocatalytic properties, J. Phys. Chem. C, 2012, 116(35), 19039–19045 CrossRef CAS.
- X. Liu, H. Lai, J. Li and G. Peng, Polyaniline sensitized Pt@TiO2 for visible-light-driven H2 generation, Int. J. Hydrogen Energy, 2018, 44(10), 4698–4706 CrossRef.
- J. He, M. Wang, X. Wu and Y. Sun, Influence of controlled Pd nanoparticles decorated TiO2 nanowire arrays for efficient photoelectrochemical water splitting, J. Alloys Compd., 2019, 785, 391–397 CrossRef CAS.
- F. Xue, C. Chen, W. Fu and M. Liu, Interfacial and dimensional effects of Pd co-catalyst for efficient photocatalytic hydrogen generation, J. Phys. Chem. C, 2018, 122, 25165–25173 CrossRef CAS.
- M. J. Li, H. Y. Cheng, Y. H. Chiu and Y. J. Hsu, ZnO–Au–SnO2 Z-scheme photoanodes for remarkable photoelectrochemical water splitting, Nanoscale, 2016, 8(34), 15720–15729 RSC.
- Z. W. Seh, S. Liu, M. Low and S. Zhang, Janus Au–TiO2 photocatalysts with strong localization of plasmonic near-fields for efficient visible-light hydrogen generation, Adv. Mater., 2012, 24, 2310–2314 CrossRef CAS PubMed.
- Y. C. Pu, G. M. Wang, K. D. Chang and Y. C. Ling, Au nanostructure-decorated TiO2 nanowires exhibiting photoactivity across entire UV-visible region for photoelectrochemical water splitting, Nano Lett., 2013, 13(8), 3817–3823 CrossRef CAS PubMed.
- M. Ge, J. Cai, J. Iocozzia and C. Cao, A review of TiO2 nanostructured catalysts for sustainable H2 generation, Int. J. Hydrogen Energy, 2016, 42(12), 8418–8449 CrossRef.
- S. Chuangchote, J. Jitputti, T. Sagawa and S. Yoshikawa, Photocatalytic activity for hydrogen evolution of electrospun TiO2 nanofibers, ACS Appl. Mater. Interfaces, 2009, 1(5), 1140–1143 CrossRef CAS PubMed.
- D. Li and Y. Xia, Electrospinning of nanofibers: reinventing the wheel?, Adv. Mater., 2004, 16(14), 1151–1170 CrossRef CAS.
- D. Li, J. T. McCann, M. Gratt and Y. Xia, Photocatalytic deposition of gold nanoparticles on electrospun nanofibers of titania, Chem. Phys. Lett., 2004, 394, 387–391 CrossRef CAS.
- X. Yang, Synthesis and characterization of hybrid metal–metallic oxide composite nanofibers by electrospinning and their applications, Materials and structures in mechanics [physics.class-ph], PhD thesis, Université de Lyon, 2016.
- D. Li and Y. Xia, Fabrication of Titania Nanofibers by Electrospinning, Nano Lett., 2003, 3(4), 555–560 CrossRef CAS.
- F. Wang, Y. Jiang, A. Gautam and Y. Li, Exploring the origin of enhanced activity and reaction pathway for photocatalytic H2 production on Au/B–TiO2 catalysts, ACS Catal., 2014, 4, 1451–1457 CrossRef CAS.
- Y. H. Chiu, S. B. Naghadeh, S. A. Lindley and T. H. Lai, Yolk-shell nanostructures as an emerging photocatalyst paradigm for solar hydrogen generation, Nano Energy, 2019, 62, 289–298 CrossRef CAS.
- M. Murdoch, G. I. N. Waterhouse, M. A. Nadeem and J. B. Metson, The effect of gold loading and particle size on photocatalytic hydrogen production from ethanol over Au/TiO2 nanoparticles, Nat. Chem., 2011, 3(6), 489–492 CrossRef CAS PubMed.
- X. Wang, H. Wang, H. Zhang and W. Yu, Dynamic interaction between methylammonium lead iodide and TiO2 nanocrystals leads to enhanced photocatalytic H2 evolution from HI splitting, ACS Energy Lett., 2018, 3, 1159–1164 CrossRef CAS.
- Y. H. Hsu, A. T. Nguyen, Y. H. Chiu and M. J. Li, Au-decorated GaOOH nanorods enhanced the performance of direct methanol fuel cells under light illumination, Appl. Catal., B, 2016, 185, 133–140 CrossRef CAS.
- L. Kernazhitsky, V. Shymanovska, T. Gavrilko and V. Naumov, Room temperature photoluminescence of anatase and rutile TiO2 powders, J. Lumin., 2014, 146, 199–204 CrossRef CAS.
- W. H. Saputera, J. Scott, N. Ganda and G. K. C. Low, The role of adsorbed oxygen in formic acid oxidation by Pt/TiO2 facilitated by light pre-treatment, Catal. Sci. Technol., 2016, 6(17), 6679–6687 RSC.
- Q. Wang, N. An, Y. Bai and H. Hang, High photocatalytic hydrogen production from methanol aqueous solution using the photocatalysts CuS/TiO2, Int. J. Hydrogen Energy, 2013, 38(25), 10739–10745 CrossRef CAS.
- T. Hendel, M. Wuithschick, F. Kettemann and A. Birnbaum, In situ determination of colloidal gold concentrations with UV-vis spectroscopy: limitations and perspectives, Anal. Chem., 2014, 86, 11115–11124 CrossRef CAS PubMed.
- M. Aiempanakit, T. Tabtimsri, N. Triamnak and C. Suwanchawalit, Curcumin modified titanium dioxide nanotubes with enhanced visible light photocatalytic performance, Int. J. Electrochem. Sci., 2019, 14, 1954–1967 CrossRef CAS.
- A. A. Ismail and D. W. Bahnemann, Synthesis of TiO2/Au nanocomposites via sol–gel process for photooxidation methanol, J. Adv. Oxid. Technol., 2009, 12(1), 9–15 CAS.
- A. Madhumitha, V. Preethi and S. Kanmani, Photocatalytic hydrogen production using TiO2 coated iron-oxide core shell particles, Int. J. Hydrogen Energy, 2018, 43(8), 3946–3956 CrossRef CAS.
- W. H. Lin, Y. H. Chiu, P. W. Shao and Y. J. Hsu, Metal-particle-decorated ZnO nanocrystals: photocatalysis and charge dynamics, ACS Appl. Mater. Interfaces, 2016, 8(48), 32754–32763 CrossRef CAS PubMed.
- Y. C. Chen, Y. C. Pu and Y. J. Hsu, Interfacial charge carrier dynamics of the three-component In2O3–TiO2–Pt heterojunction system, J. Phys. Chem. C, 2012, 116(4), 2967–2975 CrossRef CAS.
- D. C. Ratchford, A. D. Dunkelberger, I. Vurgaftman and J. C. Owrutsky, Quantification of efficient plasmonic hot-electron injection in gold nanoparticle-TiO2 films, Nano Lett., 2017, 17(10), 6047–6055 CrossRef CAS.
- X. Ma, Y. Dai, L. Yu and B. Huang, New basic insights into the low hot electron injection efficiency of gold-nanoparticle-photosensitized titanium dioxide, ACS Appl. Mater. Interfaces, 2014, 6, 12388–12394 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2019 |